 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Multi-target preservation involving pH, water activity, mild preservative, thermal treatment, and an effective packaging was used in combination to stabilize fresh pineapple cubes for room temperature storage up to two months. Bromelain, total phenolics, and flavonoid contents were quantified from different parts of pineapple and from three varieties Mauritius, Kew, and Queen. The highest quantity of bromelain was determined in pineapple crown (50.00 ± 0.6 GDU/g), followed in pulp (48.32 ± 1.00 GDU/g) for the Kew variety. Pineapple peel had the highest phenolic and flavonoid contents among all the varieties studied. The stabilized product was microbiologically safe, and the sensory analysis indicated an acceptable product. The economics of establishing a small processing plant was also studied to prove the profitability.
Introduction
India produces approximately 1.53 million tonnes of pineapple with a productivity of about 15.3 tonnes/hectare and ranks sixth in the world. Pineapple is also a commercially important fruit crop of India with around 90,000-hectare area under this crop. (Priya Devi et al., Citation2013). In India, the types of pineapples grown are popularly known as Mauritius, Kew, and Queen. West Bengal, Kerala, Assam, Meghalaya, Karnataka are the most pineapple producing states in India. Pineapple (Ananus comosus) belongs to the family Bromeliaceae. Pineapple has exceptional juiciness, typical flavor and has immense health benefits. Pineapple contains a good amount of fiber, calcium, potassium, high amounts of vitamin C, bromelain and is a common ingredient for making canned slices, juices, chunks, jams, jellies, pickles, squashes, essence, etc.
Vitamin C is a primary water-soluble vitamin responsible for antioxidant properties in the human body and is often used as an indicator of vitamin stability in fruits and vegetables subjected to various processing techniques. A variety of factors like conditions of growth, maturity, storage, and processing conditions influence the content of vitamin C in fruits and vegetables. Bromelain is an enzyme, cysteine protease, present in all parts of pineapple plant and has been reported to aid digestion (Balakrishnan et al., Citation1981; Knill-Jones et al., Citation1970), reduce inflammation (Tassman et al., Citation1964, Citation1965), swelling (Masson, Citation1995), modulate T cell responses (Engwedra et al., Citation2001) and possess other immunomodulatory properties (Hale et al., Citation2002, Citation2005). It is currently the 13th widely used herbal medicine in the world. Pineapple is the most abundant source of bromelain and bromelain is present in all parts of the fruit (stem, crown, peel, pulp, and core). The enzyme purified from the stem is called stem bromelain (EC 3.4.22.32) and its isoelectric point is 9.5, and the enzyme purified from the fruit is called fruit bromelain (EC3.4.22.33) with an isoelectric point of 4.6. In the human intestine, bromelain is absorbed without losing its biological activity and without degradation. Processed pineapple is however known to be devoid of all enzymes because of the harsh conditions of heat, precipitation, and autodigestion (Bhattacharya and Bhattacharya, Citation2009), and studies are lacking in identifying the processing steps which lead to significant losses of these enzymes and vitamins from pineapple.
With the changing consumer perception and demands, processing of foods should place special emphasis on the maximum retention of phytonutrients, minimal usage of preservatives and have sensory appeal very close to fresh produce. These often conflicting requirements necessitate the intelligent use of existing technologies of food preservation or adoption of newer methods of food processing technologies. Multi-target preservation, involving the use of a combination of different preservation factors or techniques (‘hurdles’), tries to achieve these requirements, to delay the growth of microorganisms and also prevent the undue losses of phytonutrients present. Hurdle technology relies on the use of different hurdles in a food which have an additive effect on microbial stability. However, multi-target preservation of foods goes a step further in ensuring that the hurdles in a food hit, act at the same time on different targets within the microorganisms and thus disturb the homeostasis of the microorganisms in several aspects making the repair of homeostasis more difficult. The deliberate and intelligent application of hurdles in this technology allows a gentle, efficient post-harvest preservation of fruits with its associated functional ingredients and the processed product bears the closest resemblance to fresh produce. The word intelligent in the current context means to be able to anticipate the targets in microorganisms for different preservative factors and be able to effectively use them all, together at a smaller intensity, rather than use a single factor at a larger intensity. This makes the food products safe for consumption and their shelf life is extended. This technology is aptly suited for fruits and vegetables and is more effective in the preservation of acidic or low pH fruits like pineapple.
Multi-target or hurdle technology preservation of pineapple has been studied extensively for its effect on mass fluxes, water loss, solid uptake, weight reduction (Lombard et al., Citation2008; Ramallo and Mascheroni, Citation2005), and the diffusion coefficients mathematically modeled (Rastogi and Raghavarao, Citation2004). However, reports are scarce on the chemical and microbiological stability of pineapple cubes subjected to hurdle technology especially with reference to changes in bromelain, phenolics, total carotenoids, and flavonoid contents during processing and subsequent storage.The current work aimed to preserve fresh pineapple using multi-target preservation technology and identify the processing steps where losses of enzymes, vitamins, and phytonutrients occur. Three varieties of pineapple typical to the sub-continent viz., Mauritius, Kew, and Queen were studied. The pineapple was stabilized with potassium metabisulphate, sugar, pH, and temperature as hurdles and the loss of major ingredients like vitamin C, bromelain, total carotenoids, total phenolics, and flavonoids were monitored during different steps of processing and during storage. Since the process is not capital intensive and can be carried out on a small scale, the technology offers an easy opportunity for farmers to extend the shelf life, buy some time, and get a better price for their raw produce. The economics of establishing a small processing plant was also studied to prove the profitability.
Materials and Methods
Raw Materials
Raw materials such as pineapple and sugar used in the present investigation were purchased from the local market of Mysore, India. The study was conducted with the following varieties of pineapple (A. comosus) Mauritius, Kew, and Queen. The different varieties were identified by a botanist and according to the technical bulletin published by the Indian Council of Agricultural Research, ICAR (New Delhi, India) (Priya Devi et al., Citation2013).
Multi-target Preservation Technology for Pineapple
Fresh pineapple of all the three varieties was processed as shown in . A total of eight samples (1–8) per variety were drawn of different parts of pineapple fruit and at various stages of processing and analyzed for physicochemical parameters. About 20 fruits per variety were processed and samples 1–8 were randomly drawn, in triplicate at each stage. Bromelain, polyphenol, and flavonoid contents were quantified in different parts of pineapple as per the samples. Storage of processed samples was done in an insulated wooden closed cabinet and temperature was monitored periodically using a thermometer. Stored samples of individual pineapple varieties were also analyzed at 20 d intervals for 2 months. The entire study was repeated three times and the mean results for the three varieties are presented here.
Figure 1. Flow Chart for Multi-target Preservation Technology for Pineapple (1–8 samples were taken for analysis at different processing stages)

Experimental Design
The study was conducted in two parts for three varieties of pineapple (Mauritius, Kew, and Queen) with 20 pineapples for each variety. The first part was to quantify key ingredients in various parts of pineapple and study the losses of key ingredients of pulp at various stages of processing. For the first part, 8 samples at different processing stages were drawn for the three varieties in triplicate. The second part was to study the losses during the extended period of storage and establish the shelf stability of the hurdle technology product. A full factorial design was followed for the second part with pineapple varieties and storage temperature as independent variables and physicochemical parameters like moisture content, pH, total soluble solids (TSS), water activity, pigment total carotenoids, bromelain, ascorbic acid, total phenolics, and flavonoids as dependent variables.
Sample Preparation
Each of the eight representative pineapple samples collected during processing and after storage was macerated in a pestle and mortar (50 g in 100 ml distilled water) made into a thick slurry and taken for all the chemical analysis unless mentioned otherwise.
Analysis of Physicochemical Parameters of Pineapple
Various physicochemical parameters like moisture content, pH, total soluble solids (TSS), water activity, total carotenoids, bromelain, ascorbic acid, total phenolics, and flavonoids were monitored for fresh pineapple, after each step of multi-target preservation () and also during storage (28 ± 5°C) for a period of 60 days.
Moisture content was estimated by vacuum oven method (AOAC, Citation1990). pH was measured using a pH meter (Cyber Scan, Eutech Instruments, India; Accuracy ±0.01). Total soluble solids (TSS) content was determined using a digital refractometer (PAL-I, ATAGO, Tokyo, Japan; Accuracy ± 0.2%).
Water Activity
Three gram of macerated sample (25 g) was spread in a water activity cup. The sample was placed in the water activity chamber, water activity values were directly read from water activity meter, a dew point hygrometer at 25° C (Du Pont equipment, Aqualab, Decagon Devices Inc., Pullman, WA, USA).
Estimation of Total Carotenoids
Total carotenoids were quantified based on the method by Ranganna (Citation1986). Five gram macerated sample was extracted repeatedly with acetone and hexane (40:60) and the carotenoid was estimated spectrophotometrically (UV 1800 Shimadzu Corporation, Kyoto, Japan) by measuring the absorbance at 450 nm.
Quantification of Bromelain
Total bromelain content was quantified based on the method by Moodie (Citation2001). Twenty-five ml substrate (25 g gelatin boiled in 375 ml hot water) was taken in each of two 100 ml beakers containing stir bars and placed in a water bath at 45° C for 5 minutes, (one for the test solution and another for the blank solution). One ml of crude pineapple extract (macerated sample) was pipetted into beaker designated as the test solution and 0.1 ml of 3% Hydrogen peroxide was pipetted to the beaker designated as blank. Both were incubated at 45° C for 20 minutes. After exactly 20 minutes 0.1 ml of 3% hydrogen peroxide was added to the test solution and 1 ml of crude sample to the blank solution and again incubated for an additional 5 minutes. The beakers were removed from the water bath. Both the test and blank were adjusted to pH 6.0 with 0.1 N NaOH. Then, 10 ml of 37% formaldehyde was added to the beakers. Both were titrated to pH 9.0 with 0.1 N NaOH. The volume of NaOH for both test and blank solutions were recorded.
Where:
T = Test titer, B = Blank titer, 14 = mg nitrogen per millimole nitrogen, N = Normality of standardized NaOH, Wt (g) = Initial weight of enzyme.
One GDU is that amount of enzyme which will liberate, after 20 minutes digestion at 45°C, 1 mg of amino nitrogen from a standard gelatin solution at specific pH.
Estimation of Ascorbic Acid (2, 6, -dichloro Indophenol Titrimetric Method)
Titrimetric method was used for the estimation of ascorbic acid (Ranganna, Citation1986). An aliquot (2–10 ml) of the metaphosphoric acid, HPO3 extract (10 g of sample blended with 3% HPO3and made up to 100 ml with 3% HPO3) of the sample was taken and titrated with the standardized dye 2, 6, -dichloroindophenol to a pink end-point which persisted for at least 15 seconds. The aliquot of the sample taken was such that the titer did not exceed 3 to 5 ml.
Sulfur dioxide, SO2, when present in the sample, reduces the 2, 6, -dichloroindophenol dye and thus interferes in the ascorbic acid analysis. Since the sample contains SO2, interference was eliminated following the formaldehyde condensation procedure given below.
Ten ml of the filtrate was taken in a test tube, and 1 ml of 40% formaldehyde was added along with 0.1 ml of HCl, kept for 10 min and titrated as before. Ascorbic acid content was estimated based on the titer value for standard dichloroindophenol dye and the results were expressed as mg ascorbic acid/100 g sample.
Estimation of Total Phenols-Folin-Ciocalteu Assay
Total phenolic content was determined by the method given by Singleton and Rossi and expressed as mg gallic acid equivalents (GAE) (Singleton and Rossi, Citation1965). A 1 ml aliquot of the macerated extract was taken for analyzing the total phenols by the Folin-Ciocalteu phenol reagent method spectrophotometrically at 750 nm with a UV-visible spectrophotometer.
Estimation of Total Flavonoids – Aluminum Chloride, AlCl3 Colorimetric Assay
An aliquot (1 ml) of the macerated sample was taken for analyzing the flavonoid content spectrophotometrically at 510 nm (Zhishen. et al., Citation1999). The total flavonoid content was expressed as mg catechin equivalents (CE).
Statistical Data Analysis
The data were subjected to two-way and one-way analysis of variance (ANOVA) and significant differences between means (P < .05) were determined by Duncan’s Multiple Range Test (DMRT). Stastitica 7.1 (Stat Soft Inc. OK, USA) was used for data analysis.
Sensory Analysis
The sensory panel consisted of 10 volunteers who were trained for the sensory attributes in a preparatory session. Sensory analysis of the multi-target preserved pineapple samples of the three varieties was conducted using the following descriptors: physical integrity, sour to sweet taste, fibrous nature, juiciness, flavor retention, color retention, overall acceptability and evaluated using 100 mm graphical nonstructured abscissas with the description of extreme points.
Microbiological Analysis
Total bacterial counts, coliform, yeast, and mold counts were determined by serially diluting the sample in 0.85% physiological saline (w/v) and pour plating on plate count agar, Mac Conkey agar, and potato dextrose agar, respectively (Himedia Laboratories, Mumbai, India). The plates were incubated at 37° C for the bacteria and 30° C for the yeast and mold counts and the number of colonies counted after 48 h. The microbial counts were expressed as mean colony forming units (cfu) per gram of the sample.
Economics of the Process
In order to be able to study the economics of the process before it can be recommended to the farmers, a small basic processing plant capable of handling a variety of fruits and vegetables like pineapple, tomatoes, ginger, kiwi, and oranges was set up at Salari, West Kameng district of Arunachal Pradesh, India under Programme Arunodaya, a flagship program under the Life Sciences wing of Defense Research Development Organization (DRDO), India. The commercial aspects of this plant are discussed with reference to profitability.
Results and Discussion
The present study was conducted to find out the effect of multi-target preservation of three varieties of pineapple on functional components like vitamin C, bromelain, total carotenoids, total phenolics, and flavonoids during processing as well as storage.
Water activity, pH, mild preservative, thermal treatment, and packaging were some of the targets identified and effectively used as hurdles for the long-term preservation of pineapple in a fresh-like form. Dipping in hypertonic sugar solution (70%) enabled partial removal of water during the osmotic dehydration process. This process achieves a water activity of 0.94 ± 0.1 () which is not sufficient to prevent the growth of bacteria, yeasts, and molds. The sulfur dioxide released by the addition of potassium metabisulphite (KMS) at a concentration of 0.2% achieves this and KMS also serves to prevent enzymatic browning of the product. The pineapple chunks are packed in paper foil aluminate laminate pouches (PFP) which exhibits superior barrier function against the migration of moisture, oxygen, and other gases, preserves the volatile aroma of pineapple as well as protects it against the impact of light. PFP also permits in-pack thermal processing when the packed pouches are exposed to heat treatment of 80°C. Hence, lower levels of many preservation techniques were effectively used in combination to achieve shelf life extension, retention of total phenolics, flavonoids, carotene, vitamin C, etc., thereby preserving the fresh-like appearance of pineapple and offsetting the deleterious effects the very same preservation technique could have had, if it were to be used alone at a much higher level.
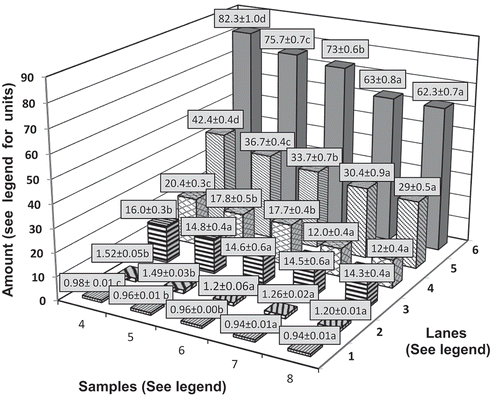
Figure 2. The water activity (Lane 1), total flavonoids (mg catechin equivalents/100 g, Lane 2), total phenolics (mg gallic acid equivalents/100 g, Lane 3), β-carotene (mg/100 g, Lane 4), vitamin C (mg/100 g, Lane 5), and moisture (%, Lane 6) contents of multi-target preserved pineapple (mean of three varieties Mauritius, Kew, and Queen) for fresh pineapple (Sample 4), after boiling in sugar syrup (Sample 5), after adding potassium meta bisulfate (Sample 6), after osmosis (Sample 7) & cooled sample before packaging (Sample 8). Mean values ± standard deviation with small letters (a-d) for differences between samples are significantly different (P < .05) as measured by Duncan’s Multiple Range Test (DMRT)
Distribution of Bromelain, Polyphenol, and Flavonoids in Pineapple
The distribution of bromelain, polyphenol, and flavonoids in different parts of pineapple was studied () for three varieties. The highest quantity of bromelain was determined from pineapple crown (50.00 ± 0.6 GDU/g), followed by pulp (48.32 ± 1.00 GDU/g) for the Kew variety whereas the Mauritius variety had high bromelain content in core (38.00 ± 0.91 GDU/g) and peel (35.00 ± 1.02 GDU/g) as compared to the other two varieties. The Queen variety had relatively higher total soluble solid content of 15–16° Brix among the varieties.
Table 1. Bromelain, polyphenol, and flavonoid contents of different parts of pineapple
Pineapple peel had the highest and pineapple core the lowest phenolic and flavonoid content among all the varieties studied (). Mulyono et al. (Citation2013) extracted bromelain from different parts of the pineapple of Indonesian origin and reported maximum amount of bromelain in the flesh part of pineapple while the crown contained maximum amounts of protein. Babagana and Bala (Citation2015) did a comparative study on the extraction and purification of the stem and fruit bromelain from pineapple and also reported higher amounts of bromelain in fruit portion as compared to the stem. Gautam et al. (Citation2010) however noted better bromelain activity in stem extracts compared to fruit while pineapple peel, pulp, stem, and leaves were reported to contain bromelain in decreasing concentrations by Krishnan and Gokulakrishnan (Citation2015). However, with respect to extraction and purification, the method used by these authors showed higher recoveries of bromelain from stem as compared to fruit.
Processing Induced Losses of Bromelain
Bromelain content present in fresh pineapple pulp for all varieties was totally lost during further processing, i.e.,, after boiling in sugar solution for 2–3 minutes (data not shown). In the current study temperatures of 80°C were reached in the pulp during processing and it is but natural for the enzyme to be degraded at this temperature as sulphydryl groups in cysteine proteases are readily oxidized and might account for the denaturation of bromelain at elevated temperatures (Poh and Abdul Majid, Citation2011). Bromelain-polyphenol complexes as seen in the case when pineapple juices are mixed with other juices like apple were shown to enhance bromelain thermal stability especially at lower temperatures of 50°C (Majid et al., Citation2008) while at higher temperatures (>70°C) such an effect was not seen. Poh and Abdul Majid (Citation2011) studied the thermal stability of free bromelain in pineapple juice and noted a decrease in enzymatic activity gradually when pineapple juice was heated from 25°C to 95°C. Thermal inactivation kinetics of bromelain in pineapple juice with an intention to optimize heat treatment conditions for minimum loss of bromelain has also been studied by Sriwatanapongse et al. (Citation2000).
Processing Induced Changes in Physicochemical Parameters
Other parameters like water activity, total flavonoids, total phenolics, total carotenoids, vitamin C, and moisture contents were monitored during the processing of different varieties of pineapple. shows the average values for the three varieties of processed pineapple (Lanes 1–6 depth axis). During the processing of pineapple, eight samples were collected at different processing stages (). Water activity and moisture content decreased after each processing step ( Lane 1 and 6, respectively, on depth axis). Water activity of fresh pineapple which was 0.98 ± 0.01 reached 0.94 ± 0.01 at the end of processing as moisture content decreased from 82% to 62%.
Total carotenoids and vitamin C (Lanes 4 and 5, respectively, on depth axis, ) values decreased after each processing step as these components are water soluble and heat labile. Fresh pineapple of Mauritius variety contained a higher amount (42.4 ± 0.4 mg/100 g) of ascorbic acid. At the end of processing, 30% of ascorbic was lost, but nevertheless at the end of 2 months, storage around 12.7 ± 0.6 mg/100 g was still retained ( and ). Uckia et al. (Citation2009) studied the vitamin C content during processing and storage of pineapple. They reported that fresh peeled pineapple fruit contains an average ascorbic acid content of 24.8 mg/100 g of fruit. These authors reported that during the juice making process, peeling led to the highest percentage loss of vitamin C (41.8%) followed by exhausting (23.7%). Processing of pineapples into jam revealed to be most destructive toward ascorbic acid (a loss of 46.8%) as compared to juice making (38.5%) and sorbet preparation (15.5%). Storage of pineapple in the specific conditions used in the study led to a significant decrease (p < .05) in vitamin C content, and the highest rate of degradation was in pineapple juice (0.6 mg loss per day).
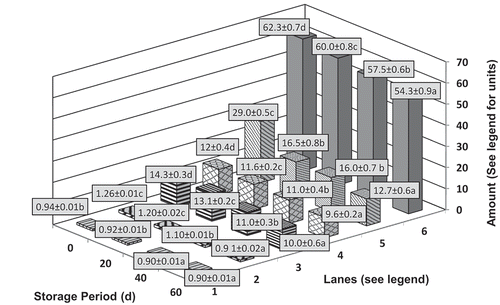
Figure 3. The water activity (Lane 1), total flavonoids (mg catechin equivalents/100 g, Lane 2), total phenolics (mg gallic acid equivalents/100 g, Lane 3), β-carotene (mg/100 g, Lane 4), vitamin C (mg/100 g, Lane 5), moisture (%, Lane 6) contents of multi-target preserved pineapple (mean of three varieties Mauritius, Kew, and Queen) stored for different periods of time. Mean values ± standard deviation with small letters (a-d) for differences between storage periods are significantly different (P < .05) as measured by Duncan’s Multiple Range Test (DMRT)
Fresh pineapple pulp of the Mauritius variety had a phenolic content of 16.0 ± 0.30 mg GAE/100 g and a flavonoid content of 1.52 ± 0.05 mg CE/100 g. The phenolic and flavonoid contents showed minor losses during processing (Lanes 2 and 3, respectively, on depth axis, ). Goh et al. (Citation2012) compared the effect of thermal and UV treatments on the content of antioxidants (phenolic acids, flavonoids, carotenoids, ascorbic acids) and antioxidant capacity of single strength pineapple juice. The antioxidant stability of juices throughout 14 days of refrigerated storage was also studied. The authors noted no significant differences in total phenolics and flavonoids between thermal or UV treated and as compared to the control. However, they noted a slight increase in the flavonoid content which was probably due to the release of more flavonoids from the cell matrix by thermal treatment. However, the contents of all these constituents decreased with storage over a period of 14 days in the above study (). Thermal degradation of phenolics and flavonoids could have happened during the processing stages employed in the current study ().
Storage Induced Changes in Physicochemical Parameters
In our study as well, the parameters water activity, total flavonoids, total phenolics, total carotenoids, vitamin C, and moisture contents were also monitored during the storage of pineapple (Lanes 1–6 depth axis, ). Upon storage, losses in the moisture content of pineapple were noted and the moisture content reached 54.3 ± 0.9% after 2 months of storage. (Lane 6, ). Vitamin C contents also decreased with storage and reached levels of 12.70 ± 0.6 mg/100 g after 60 days of storage (Lane 5, ). Water loss and water activity (aw) of the final product during the osmotic dehydration depend not only on the aw (water activity) of osmotic solution during osmosis but also on the amount of solids in the sample. In addition to this, final product quality also depends on the treatment, solid gain, chemical composition of syrup, and shape of sample (Lerici et al., Citation1985).
The processing of sugar can introduce inorganic minerals (Salles et al., Citation2015) that can be further concentrated in pineapples osmotically treated with sugar. In the current study, although the ascorbic acid content is likely to be lost during the heat treatment, the metal impurities present in sucrose content could also have a role in accelerating the oxidation of ascorbic acid as noted by earlier workers (Halliwell and Foyer, Citation1976; Leal et al., Citation1993). The total flavonoid and phenolic contents, however, showed marginal losses with storage period up to 60 days (Lanes 2 and 3, respectively, ). Auto-oxidation is common among phenolic compounds during storage under the influence of pH, light, and temperature (Irina and Mohamed, Citation2012). The use of paper foil aluminate laminate pouches (PFP) in the current study could have given a certain degree of protection from the deleterious effects of light. Leaching of phenolics and flavonoids into the sugar syrup could also have given lower values for phenolics and flavonoids upon storage of pineapple chunks.
Two levels of packaging, the first with polypropylene and later with paper aluminum foil polyethylene laminate pouch, helped in the retention of almost 50% of the total carotenoids as compared to fresh pineapple (). Paper aluminum foil polyethylene laminate pouches have aluminum foil with a thickness around 20 μ and their water vapor transmission rate (WVTR) is less than 0.01 g/m2/day at 38°C and 90% relative humidity and oxygen transmission rate (OTR) is less than 0.006 ml/m2/day at 23°C. Carotenoids are light sensitive and the double packaging helped in their retention. However, it is important to note that the method employed for the analysis included quantifying the total carotenoids in the samples and not just beta carotene as the wavelength of spectrophotometric detection 450 nm also picks xanthophylls and other carotenoids. The loss of carotenoids because of heat and light is well established. Goh et al. (Citation2012) among others have studied the effect of thermal and UV treatments on the content of carotenoids in pineapple juice and reported lower total carotenoid content in pineapple juice which they attributed to the thermolabile and light-sensitive nature of carotenoids.
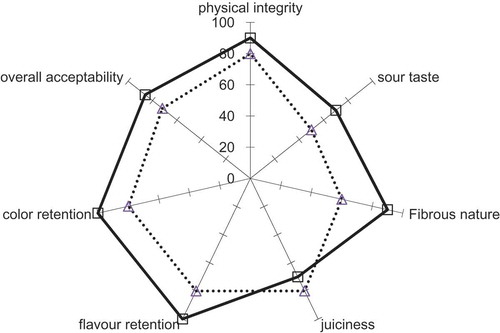
The results of the sensory profile analysis of multi-target pineapple samples at the end of two months of storage are presented in . The different varieties did not have any significant differences in the sensory profile as the process itself results in a uniformity of samples. The samples were evaluated on the following descriptors viz., physical, integrity, sour to sweet taste, fibrous nature, juiciness, flavor retention, color retention, and overall acceptability. A comparison was made with respect to fresh pineapple. Multi-target preserved pineapple was rated higher in juiciness as the infusion of sugar gave a sensory gratification. The values for all other descriptors were lower for multi-target preserved pineapple but the overall acceptability was only about 6 points lower compared to fresh pineapple.
Figure 4. Sensory profile analysis of fresh (□, solid line) and multi-target preserved pineapple after 2 months of storage (Δ, dotted line)
Hurdle technology products come under the category of Fruits and Vegetable products which are thermally processed other than pasteurization at less than 100°C and they have an upper limit of 1 X 102/g for both aerobic plate and yeast and mold count, while Enterobacteriaceae should not be detectable under prescribed methods and Staphylococcus aureus should be absent in 25 g of the sample (https://www.fssai.gov.in/ … /Gazette_Notification_Fruits_Vegetables_04_04_2018.pdf Accessed on 21.12.2018). The microbial quality with reference to total plate count, coliform, and yeast and mold count of all the stored samples monitored was less than 2.0 log10cfu/g, the limit of detection of conventional plating technique at the end of 60 days of storage which indicated the effectiveness of multi-target preservation in ensuring the microbiological safety of pineapple.
Economics of the Process
The processing method mentioned in this study requires very basic equipment. Batch processing is being promoted as there is no requirement for sophisticated equipment or electricity to run them. lists the different types of equipment required for the process.
Table 2. Capital investment of pineapple processing plant
The data on capital investment, variable costs, cost of raw material, marketing cost, and income received are presented. This will in effect give us an idea about the profitability of the unit. The major items of total capital investment were the purchase of land (40%) and construction of building (40%). Machinery and equipment constitute only 2% of the overall expense proving that the technology does not depend on sophisticated equipment especially for a batch type of operation. The total production cost was estimated to be around Rs.1,50,00,000/- for one year (extrapolated from the indicative costs for a three-month period), which included the purchase of raw materials, ingredients, and packing materials. The variable costs like wages, pay and allowances, expenditure incurred for servicing loan, depreciation on building, equipment and machinery, marketing charges, and transport charges accounted to about (Rs. 2,00,00,000). One needs to sell 750,000 units at a price of Rs. 25 per 100 g product in order to cover the fixed costs, i.e.,, the breakeven point. If the anticipated 6,00,000 units are sold, then the profit would be Rs.30,00,000 per annum after a period of 2 years. Hence, the cultivators/farmers themselves can establish these units for increasing their income as these units can be run economically.
Conclusions
In the current study, the acidic nature of pineapple, the reduced water activity as a result of osmosis-driven expulsion of moisture, relatively lower levels of preservatives, mild heat treatment, and an effective packaging (paper aluminum foil polyethylene laminate pouch) were effectively used to provide a reasonable shelf life of two months at ambient temperature for hurdle technology-processed pineapple. Further, the varietal differences in the final product were found to be minimal, indicating that the process has universal adoptability. This process is particularly useful in places where a cold-chain cannot be established for extending the shelf life. Since the process is not demanding on any specialized types of equipment, it offers an easy alternative where farmers themselves can turn into processors which is also economically viable as shown in the economic study undertaken.
Acknowledgments
The corresponding author thanks Defence Research Development Organisation for providing funds for the study under Programme Arunodaya, a flagship program of the life sciences wing.
Additional information
Funding
Literature cited
- Association of Official Analytical Chemists (AOAC). 1990. Official methods of analysis of the Association of Official Analytical Chemists. 15th ed. Association of Official Analytical Chemists, Washington, DC.
- Babagana, K., and M. Bala. 2015. Comparative study on the extraction and purification of the stem and fruit bromelain from pineapple (Ananas comosus). J. Nat. Sci. Res. 5(18):30–34.
- Balakrishnan, V., A. Hareendran, and N.C. Sukumaran. 1981. Double-blind cross-over trial of an enzyme preparation in pancreatic steatorrhea. J. Assoc. Phys. India 29:207–209.
- Bhattacharya, R., and D. Bhattacharya. 2009. Preservation of natural stability of fruit “bromelain” from Ananas comosus (Pineapple). J. Food Biochem. 33:1–19.
- Engwedra, C.R., D. Andrew, A. Ladhams, and T.L. Mynott. 2001. Bromelain modulates T cell and B cell immune responses in vitro and in vivo. Cell. Immunol. 210:66–75. doi: 10.1006/cimm.2001.1807.
- Gautam, S.S., S.K. Mishra, V. Dash, A.K. Goyal, and G. Rath. 2010. Comparative study of extraction, purification and estimation of bromelain from stem and fruit of pineapple plant. Thailand J. Pharm. Sci. 34:67–76.
- Goh, S.G., M. Noranizan, C.M. Leong, C.C. Sew, and B. Sobhi. 2012. Effect of thermal and ultraviolet treatments on the stability of antioxidant compounds in single strength pineapple juice throughout refrigerated storage. Int. Food Res. J. 19:1131–1136.
- Hale, L.P., P.K. Greer, and G.D. Sempowski. 2002. Bromelain treatment alters leukocyte expression of cell surface molecules involved in cellular adhesion and activation. Clin. Immunol. 104:183–190. doi: 10.1006/clim.2002.5254.
- Hale, L.P., P.K. Greer, C.T. Trinh, and M.R. Gottfried. 2005. Treatment with oral bromelain decreases colonic inflammation in the IL-10-deficient murine model of inflammatory bowel disease. Clin. Immunol. 116:135–142. doi: 10.1016/j.clim.2005.04.011.
- Halliwell, B., and C.H. Foyer. 1976. Ascorbic acid, metal ions and the superoxide radical. Biochem. J. 155:697–700. doi: 10.1042/bj1550697.
- Irina, I., and G. Mohamed. 2012. Biological activities and effects of food processing on flavonoids as phenolic antioxidants, advances in applied biotechnology. Prof. Marian Petre (Ed.). In Tech, Croatia. ISBN: 978-953-307-820-5.
- Knill-Jones, R.P., H. Pearce, and J. Batten. 1970. Comparative trial of Nutrizym in chronic pancreatic insufficiency. Br. Med. J. 4:21–24. doi: 10.1136/bmj.4.5726.21.
- Krishnan, A.V., and M. Gokulakrishnan. 2015. Extraction, purification of bromelain from pineapple and determination of its effect on bacteria causing periodontitis. Int. J. Pharm. Sci. Res. 6(12):5284–5294.
- Leal, J.M., P.L. Domingo, B. Garcia, and S. Ibeas. 1993. Alkali-metal ion catalysis of the oxidation of L-ascorbic acid by hexacyanoferrate (III) in strongly acidic media. J. Chem. Soc. Faraday Trans. 89:3571–3577. doi: 10.1039/ft9938903571.
- Lerici, C.R., G. Pinnavala, M. Daliarosa, and L. Bartolucci. 1985. Osmotic dehydration of fruit: Influence of osmotic agents on drying behaviour and product quality. J. Food Sci. 50:1217–1220. doi: 10.1111/j.1365-2621.1985.tb10445.x.
- Lombard, G.E., J.C. Oliveira, P. Fito, and A. Andrés. 2008. Osmotic dehydration of pineapple as a pre-treatment for further drying. J. Food Eng. 85(2):277–284. doi: 10.1016/j.jfoodeng.2007.07.009.
- Majid, F.A.A., M.A. Gani, S.Z. Talib, and K.K. Hasyim. 2008. Stability of bromelain-polyphenol complex in pineapple juice. J. Technol. 49:27–38.
- Masson, M. 1995. Bromelain in blunt injuries of the locomotor system. A study of observed applications in general practice. Fortshcritteder Medizin 113:303–306.
- Moodie, P. 2001. Gelatin digestion unit analytical method. Enzyme Development Corporation, New York. http://www.enzymedevelopment.com/wp-content/uploads/2011/10/Gelatin-Digestion-Unit-GDU.pdf
- Mulyono, N., E. Rosmeilia, Philander, J.G. Moi., B.O. Valentine, and M.T. Suhartono. 2013. Quantity and quality of bromelain in some Indonesian pineapple fruits. Int. J. Appl. Biol. Pharm. Technol. 4(20):235–240.
- Poh, S.S., and F.A. Abdul Majid. 2011. Thermal stability of free bromelain and bromelain- polyphenol complex in pineapple juice. Int .Food Res .J. 18(3):1051–1060.
- Priya Devi, S., M. Thangam, M.S. Ladaniya, and N.P. Singh 2013. Pineapple – a profitable fruit for Goa. Technical Bulletin No. 35, ICAR (RC), Goa, India.
- Ramallo, L.A., and R.H. Mascheroni. 2005. Rate of water loss and sugar uptake during the osmotic dehydration of pineapple. Braz. Arch. Biol. Technol. 48(5):761–770. doi: 10.1590/S1516-89132005000600012.
- Ranganna, S. 1986. Handbook of analysis and quality control for fruit and vegetable products. 2nd ed. Tata McGraw-Hill Publishing Company Limited, New Delhi.
- Rastogi, N.K., and K.S.M.S. Raghavarao. 2004. Mass transfer during osmotic dehydration of pineapple: considering Fickian diffusion in cubical configuration. LWT Food Sci. Technol. 37(1):43–47. doi: 10.1016/S0023-6438(03)00131-2.
- Salles, P.M.B., M.A.B.C. Menezes, R. Jacimovic, and T. Campos. 2015. Inorganic elements in sugar samples consumed in several countries. J. Radioanal. Nucl. Chem. 308:485–493.
- Singleton, V.L., and J.A. Rossi. 1965. Colorimetry of total phenolics with phosphomolybdic, phosphotungstic acid reagent. Am. J. Enol. Vitic. 16:144–158.
- Sriwatanapongse, A., M. Balaban, and A. Teixeira. 2000. Thermal inactivation kinetics of bromelain in pineapple juice. Trans. Am. Soc. Agric. Eng. 43:1703–1708. doi: 10.13031/2013.3071.
- Tassman, G.C., J.N. Zafran, and G.M. Zaynon. 1964. Evaluation of a plant proteolytic enzyme for the control of inflammation and pain. J. Dent. Med. 19:73–77.
- Tassman, G.C., J.N. Zafran, and G.M. Zaynon. 1965. A double-blind crossover study of a plant proteolytic enzyme in oral surgery. J. Dent. Med. 20:51–54.
- Uckia, H.A., Goburdhun, and A. Ruggoo. 2009. Vitamin C content during processing and storage of pineapple. Nutr. Food Sci. 39(4):398–412. doi: 10.1108/00346650910976275.
- Zhishen., J., T. Mengcheng, and W. Jianming. 1999. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 64:555–559. doi: 10.1016/S0308-8146(98)00102-2.
