ABSTRACT
The expansion of natural crop protection products as alternatives to the use of synthetic fungicides is currently popular. In this project the effects of essential oils to control Penicillium sp. in vitro and in vivo on grapevine (Vitis vinifera L.) fruit. In the present study, the inhibitory effects of anise, chamomile, black caraway, and marjoram essential oils against Penicillium sp. tested at various concentrations (0, 200, 400, 600, and 800 μL L−1) in vitro and in vivo. The in vitro results showed that the growth of Penicillium sp. was completely inhibited by the application of anise oil at concentrations of 800 μL L−1. The present results showed that the highest anthocyanin contents, total soluble solids, and pH related to the grapes treated with black caraway, chamomile, and marjoram essential oils and the lowest values belonged to the grapes treated with chamomile, marjoram, and chamomile essential oils (respectively). As well, the highest anthocyanin contents, total soluble solids, and pH associated with the control treatment, 600 and 400 μL L−1 and the lowest amounts related to a concentration of 200, 200, and 800 μL L−1 (respectively). This research confirms the antifungal effects of anise, chamomile, black caraway, and marjoram essential oils both in vitro and in vivo on grapevine fruit postharvest. Therefore, these essential oils could be an alternative to chemicals to control postharvest phytopathogenic fungi on grapevine fruit.
KEYWORDS:
Introduction
Grapevine (Vitis vinifera L.) belongs to the vine family (Vitaceae) and is one of the oldest cultivated plants, known for almost 9000 years. Currently, the global area of grape cultivation is ca. 7.5 million ha and the annual production exceeds 75 million tonnes. The main producers are China, Italy, the United States, France, Spain, and Turkey. The preventive role of biologically active compounds present in grape berries has described for cardiovascular diseases, atherosclerosis, inflammations, cancers, eye disorders, and diabetes, obesity, and nervous system dysfunctions. Considering the processing qualities and high prohealth potential, winemaking consumes about 50% of the global grape production, and the remaining half accounts for raisins, juices, musts, direct consumption, and jams. Furthermore, seeds account for approximately 15% of the wine industry waste, and they can be used to produce oils or pharmaceuticals rich in oligomeric proanthocyanidins (Bucić-Kojić et al., Citation2009; FAO, Citation2016).
Blue mold and green mold caused by Penicillium italicum and Penicillium digitatum, respectively, are common postharvest diseases of fruit and vegetables (Gatto et al., Citation2011; Solaimani et al., Citation2009). These Penicillium species are strict wound pathogens, ubiquitous, and produce the profuse amount of asexual conidia that are readily dispersed by air circulation (Boubaker et al., Citation2009; Holmes and Eckert, Citation1995). Penicillium sp. of Korea is insufficiently known. The published Korean literature on the genus is scattered and fragmentary and pertains to about 60 species (Cho et al., Citation2005; Cho and Shin, Citation2004; Lee et al., Citation2003). Most of them are soil-borne, and only 10 species have reported associated with storage diseases of plant products (Cho and Shin, Citation2004; Kim et al., Citation2002; Oh et al., Citation1999). Fruit and vegetables are often subject to varying levels of microbial decay during storage. Pathogenic fungi usually infect the host through wounds and cause significant economic losses in the commercialization stage (Gatto et al., Citation2011). The pre and postharvest losses in world crops due to fungal disease may amount to more than 12% in developing countries (Al-Reza et al., Citation2010). Therefore, they can infect the fruit in the glade, packing houses, and marketing through wounds that occurred during harvest and subsequent handling (Brown and Miller, Citation1999). During the storage period, the fruit of grape may undergo various attacks from insects and microorganisms like fungi, yeasts, and bacteria. Penicillium species are a major cause of deterioration and decay amongst a wide range of postharvest plant products, particularly fruit such as grape, oriental pear (Pyrus pyrifolia), and citrus (Citrus sinensis) (Kim et al., Citation2007).
Essential oils are a complex mix (Holmes and Eckert, Citation1999) of volatile compounds with a strong odor that are synthesized in several plant organs, including buds, flowers, leaves, stems, twigs, seeds, fruit, roots, wood or bark, and stored in secretory cells, cavities, canals, epidermic cells or glandular trichomes (Bakkali et al., Citation2008; Franz and Novak, Citation2010). Plant secondary metabolites, such as flavonoids and essential oils, have been widely studied for their antifungal, antibacterial, antimicrobial, insecticidal, and cytotoxic activities (Faleiro et al., Citation1999). Additionally, the use of essential oils is becoming popular to increase the shelf-life of food products, since consumers are more conscious about the health problems caused by several synthetic preservatives (Gómez-Estaca et al., Citation2010; Holley and Patel, Citation2005). Essential oils represent a defense mechanism against pathogens and pests, produced in different plant section, and they also have been shown to own antimicrobial and anti-fungicidal properties (Znini et al., Citation2011). The latter are plant aromatic substances composed of hydrocarbon mixtures including, terpenes, alcohols, phenols, esters, and organic acids. The role played by these substances in the plant is not known; they likely involved in transpiration control and host defense against pathogens. In particular, several essential oils have shown to affect the growth and multiplication of fungi (Arras, Citation1988) and bacteria (Deans and Svoboda, Citation1989). Several factors influence the chemical composition of plant essential oils, including the species, part of the plant, season of harvesting, geographical origin, and also the extraction method, and consequently their bioactive properties (Bakkali et al., Citation2008; Jordán et al., Citation2006; Mejri et al., Citation2010; Müller-Riebau et al., Citation1997; Viljoen et al., Citation2005).
Several studies have investigated the antifungal properties of essential oils against postharvest pathogens (Bagamboula et al., Citation2004; Bouchra et al., Citation2003; Giamperi et al., Citation2002). Antifungal effects of plant essential oils to control food spoilage fungi in vitro and in vivo study in apple (Malus domestica) (Amiri et al., Citation2008), mango (Mangifera indica) (Dubey et al., Citation2008; Regnier et al., Citation2008), citrus (Du Plooy et al., Citation2009), tomato (Solanum lycopersicum) (Omidbeygi et al., Citation2007), apple (Malus domestica) (Shahi et al., Citation2003), avocado (Persea americana) (Sellamuthu et al., Citation2013), and plum (Prunus salicina cv. Shiro) (Aminifard and Mohammadi, Citation2013a).
Recently, the use of essential oils for biocontrol of postharvest diseases caused by Penicillium digitutum and Aspergillus sp. on stored citrus fruit has reported (Abdollahi et al., Citation2011; Solaimani et al., Citation2009). Moreover, postharvest application of Thymus vulgaris and Carum copticum oils toward Penicillium digitatum and Alternaria alternata rots on artificially inoculated tomato fruit have been studied (Abdolahi et al., Citation2010). Plaza et al. (Citation2004) identified several essential oils able to inhibit Penicillium species in vitro, but none of these oils were effective against the pathogens in vivo.
The present study aimed to evaluate the in vitro and in vivo antifungal efficacy of the essential oils of several plants (anise (Pimpinella anisum), chamomile (Matricaria chamomilla), black caraway (Carum carvi), and marjoram (Origanum majorana), at different concentrations) to control the growth of Penicillium sp. on grapevine fruit.
Materials and Methods
Plant Materials and Extraction of Essential Oils
In this study, essential oil of black caraway obtained from Mashhad Golfa Shafa Company and Chamomile and marjoram oil from the Gorgan Essential Oil Company. Air-dried seeds of anise supplied from agricultural research fields of the University of Birjand, Iran. After the plant seeds, parts had authenticated, a 100 g portion of each subjected to hydrodistillation for 3 h in a Clevenger-type apparatus. The resulting oils dried over anhydrous Na2SO4 and preserved in sealed vials at 4°C for future analysis (Fatemi et al., Citation2013).
Design of Experiments and Treatments
All the experiments (in vitro and in vivo) carried out in a randomized factorial design with two factors; including four essential oils (anise, chamomile, black caraway, and marjoram) and five concentrations (0, 200, 400, 600, and 800 μL L−1) with three replications.
In Vitro Experiment
Antifungal Effects of the Essential Oils on Mycelial Radial Growth in Vitro Conditions
Antifungal acting studied using a contact assay in vitro that produced hyphal growth inhibition. The test was previously used for essential oil treatment on potato dextrose agar (PDA) medium by the “solution method” (SM) (Özden and Bayindirli, Citation2002). In this method, each essential oil dissolved in 5% (v/v) Tween 80 and the required amount added to each 9 cm Petri plate containing 20 mL PDA-agar at 45ºC. A 0.5 mm disc of Penicillium sp. mycelium placed on the treated PDA medium, and the plate incubated at 24ºC. Radial mycelial growth determined each day (up to ten days). The inhibitory percentage (IP) determined using the formula:
IP = [(dc × dt)/dc] × 100
Where dc was the mycelium diameter in a control Petri dish, and dt was the mycelium diameter in the essential oil-treated Petri dish measured daily (Aminifard & Mohammadi, Citation2013a).
In Vivo Experiment
Effect of Essential Oils on Postharvest Some of the Quality Factors of Penicillium Sp. Inoculation on Grape Fruit
Th5e experiment carried out using grape cultivar Rish Baba prepared from the Birjand Bar field. First, remove the grapes from the cluster with scissors with a 1 cm of the tail, then rinse the surface and spread on sterile paper to dry. Infected grape fruit selected and collected from storage to isolate Penicillium sp. The culture maintained on PDA at 4°C. Fresh cultures grown on PDA plates before usage. Spore suspensions collected by removing spores from the sporulation edges of a 7–8-day-old culture with a bacteriological loop and suspending them in sterile distilled water. Spore concentration determined with a hemocytometer and adjusted as required with sterile distilled water (105 spore’s mL−1). Before infection, fruit treated with sodium hypochlorite (100 μL L−1) for 5 minutes. They were then sprayed in the prepared suspension and stored at room temperature for 2 h to fix the fungal inoculation (Asghari Marjanlo et al., Citation2009). In this experiment, three replicates used for each treatment and 20 experimental units (fruit) for each replicate. We then put the grapes in a zipper pack and sprayed the essential oils on various concentrations (0, 200, 400, 600, and 800). We prepared the essential oil solution from the essential oil mixed with acetone and tween 80 (0.05%) for better solubility and uptake by the fruit. Of course, the solvents selected according to the experiments performed with acetone, since it did not affect the growth of the fungus. Samples placed in disposable containers and refrigerated and stored at 4°C for 14 days.
Anthocyanin Contents
Total anthocyanin contents determined by the differential pH method (Rapisarda et al., Citation2000). A 1.0 mL aliquot of each sweet cherry fruit extract diluted to 10 mL with a pH 1.0 solution made from 125 mL of 0.2 M KCl plus 375 mL of 0.2 M HCl. A second 1.0 mL aliquot of fruit extract diluted to 10 mL with a pH 4.5 solution made from 400 mL of 1 M sodium acetate, 240 mL 1 M HCl, and 360 mL H2O. The absorbance of each solution measured at 510 nm using a UV spectrophotometer (BioQuest CE 2502; Cecil Instruments Ltd., Cambridge, UK), and the concentration of anthocyanins calculated using the equation:
Cmg 100 g–1 = [(ApH1.0 – ApH4.5) × 484.82 × 1,000/24,825] × DF
Where the period in parentheses was the difference in absorbance at 510 nm among the pH 1.0 and pH 4.5 solutions, 484.82 was the molecular mass of cyanidin-3- glucoside chloride, 24,825 was its molar absorption at 150 nm in the pH 1.0 solution, and DF was the dilution factor (Aminifard & Mohammadi, Citation2013b).
Total Soluble Solids
Total soluble solids (TSS) determined at 20°C using a refractometer (RF 10, 0–32° Brix, Extech Co., USA) and reported as °Brix.
pH
The pH of fruit juices measured at 20°C using a pH meter (Metro model, manufactured by the Swiss Metro Company).
Weight Loss Percentage
Weight loss determined by weighting the whole grape before and after the storage period. Weight loss expressed as the percentage of loss of weight to the initial weight in the formula:
WL = (Initial weight – Secondary weight)/(Initial weight) × 100 (Hosseini and Moradinezhad, Citation2018).
Statistical Analysis
The experiment conducted in a completely randomized factorial design with three replications consisting of twenty fruit each. Data analyzed using SAS Version 9.1. (SAS Institute, Cary, NC, USA) and means compared by Duncan’s multiple range test at 1 and 5% level of confidence.
Results
In Vitro Experiment
Effect of Essential Oils on Radial Growth of Penicillium Sp. In Vitro Conditions
showed that the fungus treated with marjoram essential oil had the highest growth rate, and the fungus treated with anise essential oil had the lowest growth rate. According to , the minimum growth rate of the fungus was 800 μL L−1, and the highest growth rate observed in the control treatment, which on the 9th day filled the total control plate level. Generally, the highest growth rate of fungi observed in the control treatment and the lowest values in 800 μL L−1 concentration anise, chamomile, black caraway, and marjoram oils ().
Figure 1. Effect of four essential oils on radial growth (mm) of Penicillium sp. in in vitro conditions
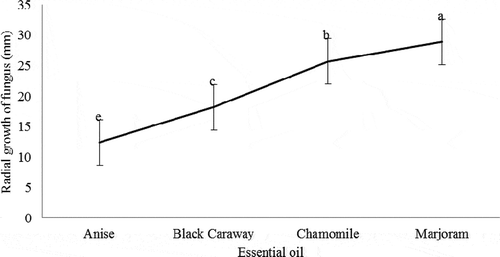
Figure 2. Effect of different concentrations of essential oils on radial growth (mm) of Penicillium sp. in in vitro conditions
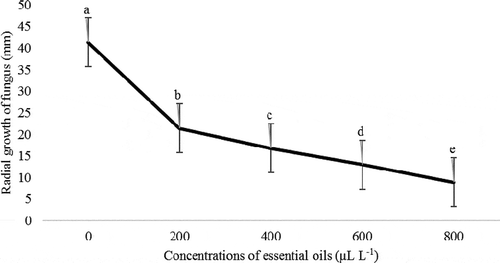
Figure 3. Interaction effect of different concentrations of four essential oils on radial growth (mm) of Penicillium sp. in in vitro conditions
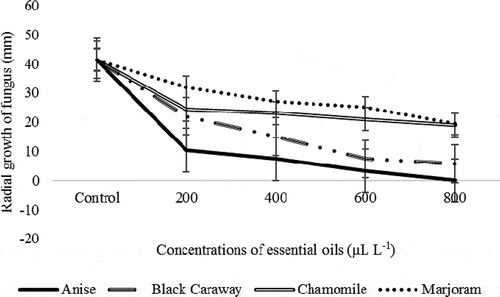
The growth rate of Penicillium sp. fungus on the 9th day showed that there was a significant difference between control and all concentrations of essential oils. So that the highest growth of fungi related to control treatment, and the lowest amount of fungal growth related to the amount of 800 μL L−1 of anise essential oil. Also, there were no significant differences between 600 and 800 μL L−1 of black caraway oil, but both of them showed a significant difference with control treatment and 200 and 400 μL L−1 concentration of black caraway oil. Although the levels of 600 and 800 μL L−1 of black caraway oil is statistically significant at one level. Besides, the amounts of 400 and 600 μL L−1 of marjoram essential oil was not significantly different, but both of them showed a significant difference with control and concentration of 200 and 800 μL L−1 of marjoram essential oil. The highest and lowest growth rates of the fungus were for the control treatment and 800 μL L−1 for marjoram essential oil, respectively.
In Vivo Experiment
Anthocyanin Contents
Comparison of the mean the effects of essential oils on Penicillium sp. inoculated grape showed that the anthocyanin content in type and concentrations of essential oils was different so that the anthocyanin content increased in the treatment of black caraway essential oil (1717.13 mg 100 g−1) and the lowest level of anthocyanin content obtained in chamomile essential oil (516.06 mg 100 g−1) (). Moreover, the highest anthocyanin associated with the control treatment (1319.2 mg 100 g−1) and the lowest amount related to a concentration of 200 μL L−1 (521.09 mg 100 g−1) ().
Table 1. Means Comparison for the effect of type and concentration of essential oil on quality factors for Penicillium sp. fungi treatments in vivo conditions
Total Soluble Solids
The results in showed that the effect of type and concentrations of essential oils on the total soluble solids of grape fruit infected with Penicillium sp. fungi were significant. The lowest amount of total soluble solids in grapes treated with marjoram essential oil (20.52%) and the highest amount in grape treated with chamomile essential oil (22.26%). Moreover, the lowest total soluble solids content was at 200 μL L−1 (20.67%), and the highest value was at 600 μL L−1 (22.70%) ().
pH
The results of the mean comparison of grapes treated with Penicillium sp. fungus of type and concentrations of essential oils showed a significant difference in grape pH. The highest amount of pH obtained from marjoram essential oil (4.42) and the lowest value was the essential oil of chamomile (4.36). Besides, the lowest pH amount observed at 800 μL L−1 concentration (4.34) and the highest value was 400 μL L−1 (4.43) ().
Weight Loss Percentage
There were no significant differences in weight loss percentage between oil-treated and control fruit ().
Discussion
In recent years, enhancement interest has generated in the improvement of healthy and natural antifungal agents such as plant-based essential oils and extracts to control phytopathogens in agriculture (Costa et al., Citation2000). In this study, we investigated the antifungal effects of four essential oils including, anise, chamomile, black caraway, and marjoram on the major agricultural pathogen (Penicillium sp.) and determined their mycelial growth inhibition rate, minimum inhibitory concentration, and minimum fungicidal concentration. Our results showed that the essential oil from anise has more acceptable antifungal properties on Penicillium sp. than other oils in vitro. The efficacy of these essential oils was positively correlated with the concentration and by increasing concentrations from 200 to 800 μL L−1 in essential oils, antifungal activity increased substantially. Fatemi et al. (Citation2013) reported that the examined black caraway and anise essential oils had a fungicidal effect at higher concentrations, especially anise (Pimpinella anisum) essential oil. So, Sams et al. (Citation2003) treated strawberries (Fragaria × ananassa) and grapes with chitosan and chitosan combination with anise oil, and placed in a temperature of 4°C for 18 days and compared to untreated fruit. In fruit that treated with anise essential oil and chitosan until 13 days, no mildew has grown on the fruit. The grapes that treated with chitosan appeared after 9 days of mildew, while untreated fruit appeared on the mildew from the moment they infected with the fungus. Chitosan treatment with anise essential oil was able to prevent the growth of B. cinerea fungus in strawberries put in storage at 4°C. Furthermore, Huang et al. (Citation2010) reported that the antifungal activity of the star anise essential oil to the most important except for its essential oil, Trans Antoul. Ćosić et al. (Citation2010) in the study of antifungal activity, several essential oils such as cloves (Eugenia caryophyllus), peppermint (Mentha piperita L.), salvia (Salvia officinalis L.), thyme (Thymus vulgaris L.), cinnamon (Cinnamomum verum Presl.), anise, black caraway (Carum carvi L.), orange (Citrus aurantium L. ssp. amara Engl.), rosemary (Rosmarinus officinalis L.), lavender (Lavandula angustifolia Mill. ssp. angustifolia), and pine (Pinus mugo) against some fungal phytopathogens including Colletotrichum coccodes, etc., the most antifungal activity observed in the essential oils of black caraway, thyme, cloves, peppermint, cinnamon, and anise. Lopez-Reyes et al. (Citation2010) reported that essential oils from oregano (Origanum vulgare), savory (Saturega montana), and thyme showed significant efficacy against postharvest pathogens B. cinerea and Penicillium expansum. Arras and Usai (Citation2001) reported that the four essential oils distilled from plants growing wild in Sardinia (E. caryophyllata, O. vulgare, T. herba-barona, and T. capitatus) found to have strong fungi toxic activity against P. digitatum in vitro. The report of Mohajeri et al. (Citation2012) also indicates the inhibitory effect of essential oil of Shiraz thyme on the growth of some species of Penicillium in laboratory conditions. Besides, Dauferra et al. (Citation2000) stated that the essential oils of thyme, oregano, and marjoram (Origanum majorana) are effective in stopping germination and the growth of molds from the mold that causes the mildew of Penicillium digitatum. They thought it may be the result of phenolic compounds of essential oils that cause an altering of microbial cell permeability by interaction with membrane proteins. This would cause deformation in cell structure and functionality, and permit the loss of macromolecules from their interior (Rattanapitigorn et al., Citation2006). Moreover, each of the essential oil components has its contribution to the biological activity of the oil. For example, anethole found in anise as the main compound, and these compounds have a more fungicidal effect (Sekine et al., Citation2007).
The present results showed that the highest anthocyanin contents, total soluble solids, and pH related to the grapes treated with black caraway, chamomile, and marjoram essential oils and the lowest values belonged to the grapes treated with chamomile, marjoram, and chamomile essential oils (respectively). As well, the highest anthocyanin contents, total soluble solids, and pH associated with the control treatment, 600, and 400 μL L−1, and the lowest amounts related to a concentration of 200, 200, and 800 μL L−1 (respectively). Aminifard et al. (2013) reported that black caraway oil, at 800 μL L−1 maintained significantly higher anthocyanin contents than all other treatments. Furthermore, the anthocyanin content of the treated peach fruit had significantly different among essential oils and control (Mohammadi and Aminifard, Citation2012). The results were in agreement with those of Asghari Marjanlo et al. (Citation2009), who reported that TSS of strawberry infected with B. cinerea increased with the application of cumin (Cuminum cyminum L.) oil. Mahmoud and El-Salam (Citation2014) worked on peach fruit to evaluate the potential application of essential oils of celery (Apium graveolens), cinnamon, and coriander (Coriandrum sativum) to determine the antifungal effects of the essential oils against gray mold disease of peach fruit and control postharvest decay under shelf conditions. Results showed that these entire essential oils, positively, affected postharvest total soluble solids compared with control. Moreover, Abd El Wahab (Citation2015) worked on ‘Florda 7/2ʹ nectarine to tested some essential oils to maintain postharvest fruit quality and reported that TSS increased with increasing storage and marketing periods, moreover, Coriander oil during cold storage and market life periods delayed the changes in total soluble solids compared with control. Our results disagree with Rattanapitigorn et al. (Citation2006) and Serrano et al. (Citation2005) previous experiments using natural antifungal compounds (eugenol, thymol, and menthol vapors) revealed benefits due to reduced weight loss percentage in cherry and grape. Similar weight loss results obtained when eucalyptus (Eucalyptus camadulensis Dehnh) and cinnamon oils applied to strawberry and tomato (Tian et al., Citation2011). Weight loss is one of the most important quality parameters of table grapes, which affect fruit susceptibility to fungal decay (Valverde et al., Citation2005).
Conclusion
Considering the reduction in mycelial growth of Penicillium sp. in vitro, the reduced incidence of disease symptoms on essential oil-treated grape fruit, we can conclude that anise essential oil could be used as possible bio fungicides, as an alternative to synthetic fungicides, against phytopathogenic fungi on grape fruit. Expression pattern of genes play an important part in glycolysis and energy metabolism indicated that anise essential oil can influence the normal physiology activity of fungal pathogens, probably through inhibiting glycolysis which in turn influenced cell energy metabolism. This means that the original balance of Penicillium sp. is broken. Furthermore, the anise essential oil had characteristics of high activity and low toxicity, therefore, it could be developed as an ideal pesticide for the agricultural industry. Anise essential oil could have direct potential as a green biopesticide because of its high content of antifungal components. However, more studies are required before these essential oils can be recommended as commercial and natural antifungal agents to increase the postharvest storage life of other horticultural crops.
References
- Abd El Wahab, S. 2015. Maintain postharvest quality of nectarine fruit by using some essential oils. Middle East J. Appl. Sci. 5(4):855–868.
- Abdolahi, A., A. Hassani, Y. Ghosta, T. Javadi, and M.H. Meshkatalsadat. 2010. Essential oils as control agents of postharvest Alternaria and Penicillium rot on tomato fruit. J Food Saf 30(2):341–352. doi: 10.1111/j.1745-4565.2009.00211.x.
- Abdollahi, M., H. Hamzehzarghani, and M. Saharkhiz. 2011. Effects of the essential oil of Zataria multiflora Boiss, a thyme-like medicinal plant from Iran on the growth and sporulation of Aspergillus niger both in vitro and on lime fruit. Food Saf. 31:424–432. doi: 10.1111/j.1745-4565.2011.00317.x.
- Al-Reza, S., A. Rahman, Y. Ahmed, and S. Kang. 2010. Inhibition of plant pathogens in vitro and in vivo with essential oil and organic extracts of Cestrum nocturnum L. Pestic Biochem. Physiol. 96:86–92. doi: 10.1016/j.pestbp.2009.09.005.
- Aminifard, M., and S. Mohammadi. 2013a. Efficacy of plant essential oils to control postharvest decay of sweet cherry (Prunus avium L.) fruit. J. Hortic. Sci. Biotechnol. 88(1):79–84. doi: 10.1080/14620316.2013.11512939.
- Aminifard, M.H., and S. Mohammadi. 2013b. Essential oils to control Botrytis cinerea in vitro and in vivo on plum fruit. J. Sci. Food Agric. 93:348–353. doi: 10.1002/jsfa.5765.
- Amiri, A., R. Dugas, A. Pichot, and G. Bompeix. 2008. In vitro and in vivo activity of eugenol oil (Eugenia caryophylata) against four important postharvest apple pathogens. Int. J. Food Microbiol. 126:13–19. doi: 10.1016/j.ijfoodmicro.2008.04.022.
- Arras, G. 1988. Antimicrobial activity of various essential oils against some citrus fruit disease agents. Paper presented at the Proc. 6th Int. Citrus Congr. Middle East, Tel Aviv, Israel.
- Arras, G., and M. Usai. 2001. Fungitoxic activity of 12 essential oils against four postharvest citrus pathogens: Chemical analysis of Thymus capitatus oil and its effect in subatmospheric pressure conditions. J. Food Prot. 64(7):1025–1029. doi: 10.4315/0362-028x-64.7.1025.
- Bagamboula, C., M. Uyttendaele, and J. Debevere. 2004. Inhibitory effect of thyme and basil essential oils, carvacrol, thymol, estragole, linalool, and p-cymene towards Shigellasonnei and Shigellaflexneri. J. Food Microbiol. 21:32–42. doi: 10.1016/s0740-0020(03)00046-7.
- Bakkali, F., S. Averbeck, D. Averbeck, and M. Idaomar. 2008. Biological effects of essential oils – A review. Food Chem. Toxicol. 46:446–475. doi: 10.1016/j.fct.2007.09.106.
- Boubaker, H., B. Saadi, E.H. Boudyach, and A. Ait Benaoumar. 2009. The sensitivity of Penicillium digitatum and P. italicum to Imazalil and Thianbendazole in Morocco. J. Plant Pathol. 8:152–158. doi: 10.3923/ppj.2009.152.158.
- Bouchra, C., M. Achouri, L.I. Hassani, and M. Hmamouchi. 2003. Chemical composition and antifungal activity of essential oils of seven Moroccan Labiatae against Botrytis cinerea Pers: Fr. J. Ethnopharmacol. 89(1):165–169. doi: 10.1016/s0378-8741(03)00275-7.
- Brown, G., and W. Miller. 1999. Maintaining fruit health after harvest. Citrus Health Manage. 1(1):175–188.
- Bucić-Kojić, A., M. Planinić, S. Tomas, L. Jakobek, and M. Šeruga. 2009. Influence of solvent and temperature on extraction of phenolic compounds from grape seed, antioxidant activity, and color of extract. Int. J. Food Sci. Technol. 44:2394–2401. doi: 10.1111/j.1365-2621.2008.01876.x.
- Cho, H.S., S.B. Hong, and S.J. Go. 2005. First report of Penicittium brasilianum and P. daleae isolated from soil in Korea. Mycobiology 33(2):113–117. doi: 10.4489/myco.2005.33.2.113.
- Cho, W.-D., and H.-D. Shin. 2004. List of plant diseases in Korea: Korean society of plant pathology. Korean Society of Plant Pathology Korea Republic.
- Ćosić, J., K. Vrandečić, J. Postić, D. Jurković, and M. Ravlić. 2010. In vitro antifungal activity of essential oils on the growth of phytopathogenic fungi. Poljoprivreda 16(2):25–28.
- Costa, T.R., O.F. Fernandes, S.C. Santos, C.L.M. Oliveira, L.M. Lião, P.H. Ferri, JR Paula, HD Ferreira, BHSales, RS. Maria do Rosário. 2000. Antifungal activity of volatile constituents of Eugenia dysenteric leaf oil. J. Ethnopharmacol. 72(1–2):111–117. doi:10.1016/s0378-8741(00)00214-2.
- Dauferera, D.J., B.N. Ziogas, and M.G. Polissiou. 2000. GC-MS analysis of essential oils from some Greek aromatic plants and their fungitoxicity on Penicillium digitatum. J. Agric. Food Chem. 48(6):2576–2581. doi: 10.1021/jf990835x.
- Deans, S.G., and K.P. Svoboda. 1989. Antibacterial activity of summer savory (Satureja hortensis L.) essential oil and its constituents. Hortic. Sci. 64:205–210. doi: 10.1080/14620316.1989.11515946.
- Du Plooy, W., T. Regnier, and S. Combrinck. 2009. Essential oil amended coatings as alternatives to synthetic fungicides in citrus postharvest management. Postharvest Biol. Technol. 53:117–122. doi: 10.1016/j.postharvbio.2009.04.005.
- Dubey, R.K., R. Kumar, J.P.N. Jaya Chansouria, and N.K. Dubey. 2008. Evaluation of Amomum sub ulatumRoxb oil as a source of botanical fungi toxicant for the protection of mango fruit from fungal rotting. J Food Saf. 28:400–412. doi: 10.1111/j.1745-4565.2008.00108.x.
- Faleiro, L., G.M. Miguel, C.A.C. Guerrero, and J.M.C. Brito. 1999. Antimicrobial activity of essential oils of Rosmarinus officinalis L., Thymus mastichina (L) L. ssp. Mastichina, and Thymus albicans. Proceedings of the II WOCMAP congress on medicinal and aromatic plants, part 2: pharmacognosy, pharmacology, phytomedicine, toxicology. doi: 10.17660/actahortic.1999.501.4.
- FAO. 2016. FAOSTAT, FAO March 21, 2018 statiscal databases. http://faostat.fao.org.
- Fatemi, H., M.H. Aminifard, and S. Mohammadi. 2013. Efficacy of plant essential oils on postharvest control of rot caused by Botrytis cinerea on kiwi fruit. Arch. Phytopathol. Plant Prot. 46(5):536–547. doi: 10.1080/03235408.2012.749687.
- Franz, C., and J. Novak. 2010. Sources of essential oils, p. 39–82. In: K.H.C. Bas¸ Er and G. Buchbauer (eds.). Handbook of Essential Oils: Science, Technology, and Applications. CRC Press/Taylor & Francis Group, Boca Raton. doi: 10.1201/9781420063165-c3.
- Gatto, M., Ippolito, A., Linsalata, V., Cascarano, N., Nigro, F. 2011. The activity of extracts from wild edible herbs against postharvest fungal diseases of fruit and vegetables. Postharvest Biol. Technol. 61(1):72–82. doi:10.1016/j.postharvbio.2011.02.005.
- Giamperi, L., D. Fraternale, and D. Ricci. 2002. The in vitro action of essential oils on different organisms. J. Essent. Oil Res. 14(4):312–318. doi: 10.1080/10412905.2002.9699865.
- Gómez-Estaca, J., A.L.D. Lacey, M.E. López-Caballero, M.C. Gómez-Guillén, and P. Montero. 2010. Biodegradable gelatin-chitosan films incorporated with essential oils as antimicrobial agents for fish preservation. Int. J. Food Microbiol. 27(7):889–896. doi: 10.1016/j.fm.2010.05.012.
- Holley, R.A., and D. Patel. 2005. Improvement in shelf-life and safety of perishable foods by plant essential oils and smoke antimicrobials. Int. J. Food Microbiol. 22:273–292. doi: 10.1016/j.fm.2004.08.006.
- Holmes, G.J., and J.W. Eckert. 1995. Relative fitness of imazalil-resistant and-sensitive biotypes of Penicillium digitatum. Plant Dis. 79:1068–1073. doi: 10.1094/pd-79-1068.
- Holmes, G.J., and J.W. Eckert. 1999. The sensitivity of Penicillium digitatum and P. italicum to Postharvest Citrus Fungicides in California. Phytopathology. 89:716–721. doi: 10.1094/phyto.1999.89.9.716.
- Hosseini, A., and F. Moradinezhad. 2018. Effect of short-term high CO2 treatment on quality and shelf life of button mushroom (Agaricus bisporus) at refrigerated storage. J. Hortic. Postharvest Res. 1(1):37–48.
- Huang, Y., J. Zhao, L. Zhou, J. Wang, Y. Gong, X. Chen, Z. Guo, Q. Wang, W. Jiang. 2010. Antifungal activity of the essential oil of Illicium verum Fruit and is main component trans-Anethole. Molecules. 15(11):7558–7569. doi:10.3390/molecules15117558.
- Jordán, M.J., R.M. Martínez, K.L. Goodner, E.A. Baldwin, and J.A. Sotomayor. 2006. Seasonal variation of Thymus hyemalis Lange and Spanish Thymus vulgaris L. essential oils composition. Ind. Crops Prod. 24(3):253–263. doi: 10.1016/j.indcrop.2006.06.011.
- Kim, J.-H., W.-H. Lee, -S.-S. Cheong, J.-S. Choi, J. Ryu, and Y.-G. Choi. 2002. Identification and characteristics of Penicillium spp. isolated from postharvest decay of pear. Res. Plant Dis. 8(2):107–112. doi: 10.5423/rpd.2002.8.2.107.
- Kim, W.K., H.K. Sang, S.K. Woo, M.S. Park, N.C. Paul, and S.H. Yu. 2007. Six species of Penicillium associated with the blue mold of grape. Mycobiology 35(4):180–185. doi: 10.4489/myco.2007.35.4.180.
- Lee, S., S.-B. Hong, and C.-Y. Kim. 2003. Contribution to the checklist of soil-inhabiting fungi in Korea. Mycobiology 31(1):9–18. doi: 10.4489/myco.2003.31.1.009.
- Lopez-Reyes, J., D. Spadaro, M. Gullinoa, and A. Garibaldia. 2010. Efficacy of plant essential oils on postharvest control of rot caused by fungi on four cultivars of apples in vivo. Flavor Fragrance J. 25:171–177. doi: 10.1002/ffj.1989.
- Mahmoud, G.A., and H. El-Salam. 2014. USE of some essential oils as safe alternatives to conserve peach fruit quality during shelf life. Egypt. J. Hortic. 41(2):313–324. doi: 10.21608/ejoh.2014.1372.
- Marjanlo, A., Y. Mostofi, S. Shoeibi, and M. Fattahi. 2009. Effect of cumin essential oil on postharvest decay and some quality factors of strawberry. J. Med. Plants 3(31):25–43.
- Mejri, J., M. Abderrabba, and M. Mejri. 2010. Chemical composition of the essential oil of Ruta chalepensis L: Influence of drying, hydro-distillation duration, and plant parts. Ind. Crops Prod. 32:671–673. doi: 10.1016/j.indcrop.2010.05.002.
- Mohajeri, F.A., A. Misaghi, A. Akhondzadeh, H. Gheisari, A. Khosravi, H. Gandomi H. Ebrahimnejad. 2012. Growth inhibition and morphological alterations to Penicillium citrinium in response to Zataria multiflora Boiss. essential oil. J. Vet. Res. 67(4):307–312.
- Mohammadi, S., and M.H. Aminifard. 2012. Effect of essential oils on postharvest decay and some quality factors of peach (Prunus persica var. Redhaven). J. Biol. Environ. Sci. 6(17):147–153.
- Müller-Riebau, F.J., B.M. Berger, O. Yegen, and C. Cakir. 1997. Seasonal variations in the chemical compositions of essential oils of selected aromatic plants growing wild in Turkey. J. Agric. Food Chem. 45:4821–4825. doi: 10.1021/jf970110y.
- Oh, S., I. Chung, S. Paik, and S. Yu. 1999. Survey and control of the occurrence of mycotoxins from postharvest fruit. 1. Mycotoxins produced by isolates from apple, pear, citrus, and grape. Plant Dis. Agric. 5:100–104.
- Omidbeygi, M., M. Barzegar, Z. Hamidi, and H. Naghdibadi. 2007. Antifungal activity of thyme, summer savory, and clove essential oils against Aspergillus flavus in liquid medium and tomato paste. Food Control. 18:1518–1523. doi: 10.1016/j.foodcont.2006.12.003.
- Özden, Ç.A., and L. Bayindirli. 2002. Effects of combinational use of controlled atmosphere, cold storage, and edible coating applications on shelf life and quality attributes of green peppers. Eur Food Res. Technol. 214(4):320–326. doi: 10.1007/s00217-001-0448-z.
- Plaza, P., R. Torres, J. Usall, N. Lamarca, and I. Vinas. 2004. Evaluation of the potential of commercial postharvest application of essential oils to control citrus decay. J. Hortic. Sci. Biotechnol. 79(6):935–940. doi: 10.1080/14620316.2004.11511869.
- Rapisarda, P., F. Fanella, and E. Maccarone. 2000. Reliability of analytical methods for determining anthocyanins in blood orange juices. J. Agric. Food Chem. 48(6):2249–2252. doi: 10.1021/jf991157h.
- Rattanapitigorn, P., M. Arakawa, and M. Tsuro. 2006. Vanillin enhances the antifungal effect of plant essential oils against Botrytis cinerea. Int. J. Aromather. 16:193–198. doi: 10.1016/j.ijat.2006.09.003.
- Regnier, T., W. Du Plooy, S. Combrinck, and B. Botha. 2008. Fungi toxicity of Lippia scaberrima essential oil and selected terpenoid components on two mango postharvest spoilage pathogens. Postharvest Biol. Technol. 48:254–258. doi: 10.1016/j.postharvbio.2007.10.011.
- Sams, T., J. Noh, S. Zivanovic, F.A. Draughon, J.R. Mount, and C. Sams. 2003. Extension of fresh produce shelf- life with novel chitosan coatings. Department of Food Science and Technology. National Institute of Food and Agriculture, U.S.A.
- Sekine, T., M. Sugano, A. Majid, and Y. Fujii. 2007. Antifungal effects of volatile compounds from black zira (Bunium persicum) and other spices and herbs. J. Chem. Ecol. 33:2123–2132. doi: 10.1007/s10886-007-9374-2.
- Sellamuthu, P.S., D. Sivakumar, P. Soundy, and L. Korsten. 2013. Enhancing the defense-related and antioxidant enzymes activities in avocado cultivars with essential oil vapors. Postharvest Biol. Technol. 81:66–72. doi: 10.1016/j.postharvbio.2013.02.007.
- Serrano, M., D. Martinez-Romero, S. Castillo, F. Guillen, and D. Valero. 2005. The use of natural antifungal compounds improves the beneficial effect of MAP in sweet cherry storage. Innovative Food Sci. Emerging Technol. 6:115–121. doi: 10.1016/j.ifset.2004.09.001.
- Shahi, S.K., M. Patra, A.C. Shukla, and A. Dikshit. 2003. Use of essential oil as a botanical pesticide against postharvest spoilage in Malus pumilo fruit. Biocontrol. 48:223–232. doi: 10.1023/a:1022662130614.
- Solaimani, B., M. Ramezani, and M. Saharkiz. 2009. Biological control of postharvest disease caused by Penicillium digitatum and P. italicum on stored citrus fruit by Shiraz thyme essential oil. Adv. Environ. Biol. 3:249–254.
- Tian, J., X. Ban, H. Zeng, J. He, B. Huang, and Y. Wang. 2011. Chemical composition and antifungal activity of essential oil from Cicuta virosa L. var. latisecta Celak. Int. J. Food Microbiol. 145:464–470. doi: 10.1016/j.ijfoodmicro.2011.01.023.
- Valverde, J.M., F. Guillén, D. Martínez-Romero, S. Castillo, M. Serrano, and D. Valero. 2005. Improvement of table grapes quality and safety by the combination of modified atmosphere packaging (MAP) and eugenol, menthol, or thymol. J. Agric. Food Chem. 53(19):7458–7464. doi: 10.1021/jf050913i.
- Viljoen, A.M., S. Subramoney, S.F.V. Vuuren, K.H.C. Bas¸ Er, and B. Demirci. 2005. The composition, geographical variation and antimicrobial activity of Lippia javanica (Verbenaceae) leaf essential oils. Ethnopharmacology. 96:271–277. doi: 10.1016/j.jep.2004.09.017.
- Znini, M., G. Cristofari, L. Majidi, H. Mazouz, P. Tomi, J. Paolini, J. Costa. 2011. Antifungal activity of essential oil from asteriscus graveolens against postharvest phytopathogenic fungi in apples. Nat. Prod. Commun. 6(11):1934578X1100601147. doi:10.1177/1934578x1100601147.
