ABSTRACT
Date fruit contains a variety of polyphenols such as phenolic acids, flavonoid glycosides, and certain hydroxycinnamates for mediating the biological effects. It is important to analyze the degradation of chlorophylls, carotenes, and other pigments in different stages of date fruit ripening by studying the association of date fruit ripening with these pigments. The study has aimed to examine the degradation of pigments during the ripening stages of two selected date fruits. Ten different samples were gathered of Hilwa and Sullaj dates where each sample represented different ripening stages. The samples were collected during July and August, also known as the ripening months. The outcome of the study exhibited degradation of chlorophyll throughout its maturation stages, whereas the carotene degradation was comparatively low. It also demonstrated the degradation of anthocyanins in both Hilwa and Sullaj dates. The study results also implied that the absorption of ultraviolet rays appears to be the same in the rapid degradation of pigments among date fruits.
Introduction
The date palm (Phoenix dactylifera L.) cultivates in different regions including the Middle East, North Africa, South Asia, and the USA (Zhang et al., Citation2015). The date palm is the tallest among the species of Phoenix, which grows up to 30 m and in some regions 100 ft (Lobo et al., Citation2014). It is one of the oldest cultivated trees, which is tied intimately with human civilization history, particularly in Arab, where it has served as a staple food for many decades. The Middle East is still known for the early cultivation of date palm, despite the economic and symbolic importance in their arid region (Tengberg, Citation2012). Date fruit also provides various health benefits and essential nutrients to its consumers; for instance, dietary fibers, carbohydrates, protein, enzymes, vitamins, fats, minerals, carotenoids, and phenolic acids are some of the major chemical components of dates (Tang et al., Citation2013).
In the maturity and ripeness stages, the date fruits undergo some variation in the form of color, texture, taste, and chemical composition (Almeida et al., Citation2015). In all the ripening stages, the change in color results from the degradation of chlorophyll, which marks the transition from one stage to another (Diboun et al., Citation2015). The four different stages of fruit ripening include Kimri (19 weeks), Khalal (29 weeks), Rutab (30 weeks), and Tamar (31 weeks) (Haider et al., Citation2014). In Kimri stage, the date fruit is unripe, whereas, in the Khalal stage, the date becomes slightly crunchy in taste. The third stage is Rutab, in which the date becomes soft and edible. The final stage of ripening is Tamar, in which the fruit is completely ripened, and the amount of moisture is also reduced. The date palm fruit comprises hundreds of commercial cultivars, having distinctive shapes, sizes, and colors of fruits (Mathew et al., Citation2015).
Chlorophyll is one of the most abundant pigments, which is an essential component of photosynthesis, and is usually required for the absorption of sunlight. On the contrary, chlorophyll is considered a hazardous molecule because it possesses light-absorbing properties. The breakdown of chlorophyll seems to occur in different tissues of the green plant during the process of ripening fruits. It is one of the most fundamental processes of physiology in plants, which usually occur during various phases of plant development. The degradation of chlorophyll is generally considered as a biological enigma (Christ and Hörtensteiner, Citation2014). For instance, the ripening of fruits like banana, dates, and tomatoes includes massive degradation of chlorophyll. The step-wise explanation of chlorophyll degradation is paved by identifying first colorless nonfluorescent chlorophyll catabolite. This is likely to lead to the ultimate degradation of chlorophyll resulting in colorless pigmentation.
Carotenes are usually found in the tissues of certain plants and animals, which is an orange-yellow to red crystalline pigment. Carotenoids are a class of natural fat-soluble pigments providing bright color to the plants (Baliga et al., Citation2011). Al-Farsi et al. (Citation2005) investigated carotenoid pigments and the value of pro-vitamin A among three different varieties of palm dates. All these dates were analyzed at three different stages of ripening, which include Khalal, Rutab, and Tamar. The chromatographic analysis evaluated that lutein is the major carotenoid pigment between Khalal and Tamar stages followed by the disappearance of carotenoid during the ripening process. Approximately up to 30% of the total carotenoids are lost from the date fruits upon sun drying. Moreover, the presence of phenolic compounds and carotenoids in date fruits is attributed to their antioxidant effects (Vayalil, Citation2012).
Some pigments, including anthocyanins and carotenoids (carotenes, lycopene, and lutein), are present in the date fruits. The chlorophyll and carotenoids are considered as significant pigments for the maintenance of the nutritional and sensory quality of the date fruits. It is also important to investigate the association of carotenoid and chlorophyll pigments in the ripening of date fruits. In the same context, chlorophyll content has appeared to be a suitable internal marker of fruit ripeness. According to Hasnaoui et al. (Citation2011), different aspects govern the degradation of pigments and colors during the processing and storage of date fruits. High-performance liquid chromatography is used for the identification of pigments in fruits and vegetables. Furthermore, changes in the content of carotenoid and chlorophyll pigments have been used for following the process of ripening in apples (Solovchenko et al., Citation2005).
Agricultural science needs to be more flexible, productive, and adequate to counter the adverse effects as it plays a significant role in the development of the country’s economy. The date fruits comprise a variety of polyphenols, which include phenolic acids, flavonoid glycosides, and certain hydroxycinnamates that help in the mediation of biological effects (Farag et al., Citation2016; Nematallah et al., Citation2018). The compositional quality of vegetables and fruits is likely to be influenced by the ripening stage. Different studies have been conducted to scrutinize the phenolic compounds and biological activities of different date palm fruits (Chaira et al., Citation2009). Previously, four different varieties of date palm in Tunisia were considered to examine the impact of the ripening stage on phytochemical composition and antioxidant activities (Amira et al., Citation2012). Moreover, the information about biochemical changes associated with date fruit is scarce, despite the significance of skin color that defines the quality of fruit. However, the present study aims to examine the pigment degradation during ripening stages. This study has contributed to the comprehensive analysis of the degradation of chlorophylls, carotenes, and other pigments in different stages of date fruit ripening by studying the association of date fruit ripening with these pigments.
Materials and Methods
For the current study, two types of date fruits have been selected, i.e., Hilwa and Sullaj. In total, 10 samples were collected at different ripening stages of date palm fruits from a private farm of Riyadh in Saudi Arabia.
Collection of Sample
A total of 10 different samples were collected at 10 distinctive stages of maturity for each variety of date fruit. The samples were collected from different ripening stages of date palm fruits, i.e., Hilwa and Sullaj from the private farm of Riyadh in Saudi Arabia. The selected fruits were stored at −70°C until required for further investigation. The interval for the collection of Hilwa fruit samples included 2, 14, and 24 June; 3, 18, and 27 July; 10 and 25 Aug.; and 8 Sept. On the other hand, the time interval for Sullaj date samples is 6, 16, and 30 May; 14 and 23 June; 3, 17, and 27 July; and 10 and 25 Aug. However, the same tree was considered for the collection of a variety of date fruits to minimize the variations.
Preparation and Extraction of Sample
The first step in the utilization of phytochemicals to prepare the extract is known as the extraction of bioactive compounds. The extract from plant material is prepared by solvent extractions due to their ease of use, efficiency, and wide applicability (Dai and Mumper, Citation2010). These extractions used various solvents such as methanol, ethanol, acetone, ethyl acetate, or their combinations. The present study has used the methanolic extraction procedure to extract sample for enzymatic assay.
Quantification of Pigments
UV-VIS spectrometer equipped with UVWinLab V 2.85.04 software was used to perform quantification of the pigments. The distinctive spectra of absorption in the pigments of plants were used for quantitation and identification. Two solvents, McIlvaine buffer (pH 6.5, citrate phosphate) and water, were used to extract the pigments (Yahia et al., Citation2010). During various stages of fruit ripening, the absorption spectra of all extracts were recorded in 240–700 nm regions. The absorption spectra were categorized into two regions for the enhancement of the appropriateness of measurements, which are UV-VIS-region (240–500 nm) and the visible region (350–700 nm).
The quantification of anthocyanins in the phenolic extracts was performed using SHIMADZU spectrophotometer (UV-1601PC, Kyoto, Japan) at 535 nm. The total anthocyanin content was quantified using the following formula:
C’ = (A x FD)/(ℇ x b)
where
C’: total anthocyanin content (mg. L-1);
A: absorbance (535 nm);
ℇ: absorbance coefficient of cyanidines (98.2);
b: thickness of the cuvette (1 cm);
FD: dilution factor of the extract.
Determining Polyphenol Oxidase (PPO) Activity
The spectrophotometric method based on an initial increase rate was used to determine the PPO activity in absorbance at 410 nm at 15-s intervals at 30°C. The reaction mixture containing 0.1 ml freshly prepared crude enzyme extract, 1.0 ml of 50 mM catechol, and 3.9 ml of 100 mM phosphate buffer was used for determining PPO activity. The slope of the absorbance vs. time curve was used for calculating the initial velocity. The amount of the enzyme that increased the absorbance by 0.001 min−1 under the conditions of the assay was defined as one unit of PPO activity (Manohan and Wai, Citation2012).
Data Analysis
The study was conducted by following a complete randomized design. The absorption spectra were obtained through the various extracts that were diluted in the corresponding solvent. The formulas used in this study to identify and quantify the pigments (carotenoids and chlorophyll) were proposed by Gupta and Kaul (Citation2012):
[Chlorophyll a], mM = 12.7 × A663 − 2.69 × A645
[Chlorophyll b], mM = 22.9 × A645 − 4.68 × A663
[Carotenoids], mM = (A480 + (0.114 × A663) – (0.638 × A645)) ÷112.5
where
A663, A645, and A480 represent the absorption spectra at 663, 645, and 480 nm, respectively.
Results
Hilwa
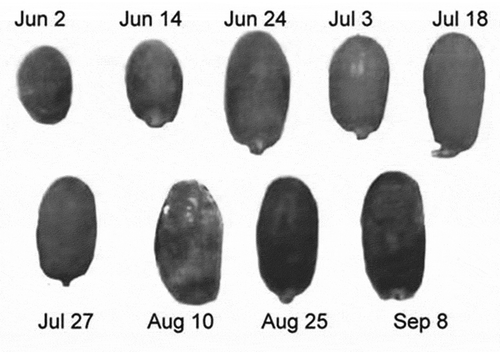
Hilwa date fruit is an excellent type of date, which usually ripens during the period of July and August. The Hilwa fruit is distinguished in contrast with other types of date fruits because of its red color during the ripening stages of Khalal and Rutab. The different ripening stages of Hilwa fruit of all collected samples can be observed in .
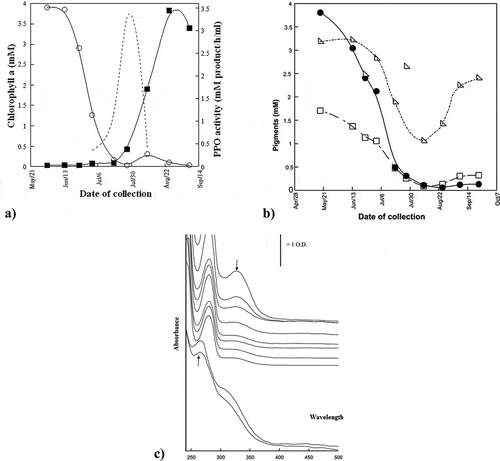
provides the composition of the UV spectra of methanol extracts of all ripening stages of Hilwa fruit. It has also reflected the visible spectra of alcoholic extracts for the samples of Hilwa fruits. It also depicts the decline of 0.013 mM in chlorophyll (a); however, the chlorophyll (b) was decreased to 0.14 mM from 1.8 mM. The quantitative estimation of pigments in Hilwa date fruits is reflected in .
Sullaj
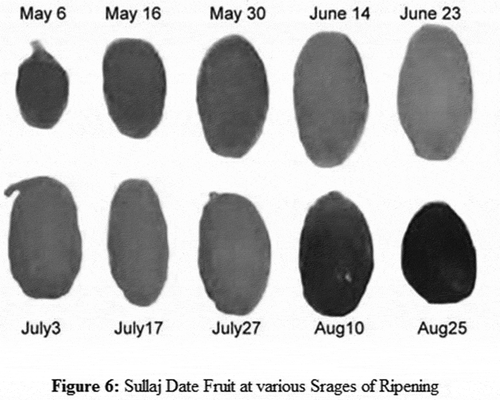
The date fruit Sullaj is usually distributed within the central regions of Saudi Arabia, and it ripens during the period of July and August. The samples collected for Sullaj dates reflected a change of color from green to yellow and then from yellow to brown. The different stages of Sullaj dates have been represented in .
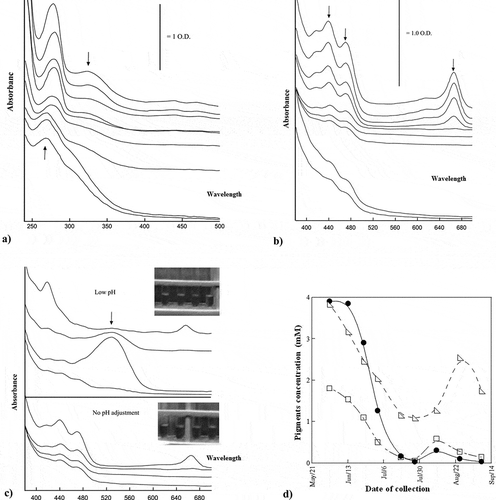
The different stages of ripening of Sullaj dates were measured, which were likely to be represented by the absorption spectra. The composite UV spectra of methanol extracts have been presented in . The visible spectra for the alcoholic extracts of Sullaj dates are also observed. The existence of anthocyanins was evaluated through absorption spectra in the Sullaj date fruit samples, after the acidification of alcoholic extracts. It was observed through outcomes that the ripening of Sullaj dates resulted in a decrease in the fruit content of chlorophyll a and b to negligible levels.
Figure 4. (a) UV spectra of methanol extracts of Sullaj during ripening; (b) visible spectra of methanol extracts of Sullaj date; (c) visible spectra of methanol extracts of Sullaj at different pH; (d) chlorophyll and carotenoid content of Sullaj during ripening
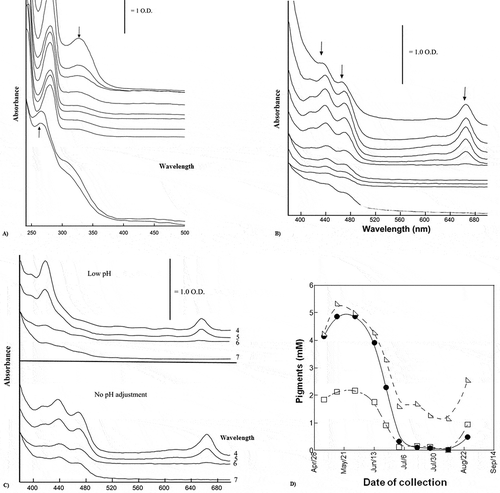
The development of colors can be observed in . It has provided UV spectra for the ripening of Sullaj date fruits. The majority of the molecules were strongly absorbed in this region, particularly the flavones and polyphenols.
Discussion
The study has demonstrated various changes taking place during the ripening stages of Hilwa and Sullaj date fruits. The change in color along with the skin appearance depends on the pigment concentration of chlorophyll and carotenoid. and have illustrated that both the date fruits, Hilwa and Sullaj, have undergone changes during ripening. The major change based on the color of date fruit during ripening is the concentration of chlorophyll that decreases. The carotenoid concentration in the fruits does not improve throughout the ripening stages, which is represented through the presence of yellow color in the dates. This shows that carotenoid masks the chlorophyll at the time of harvest, followed by its unmasking in the form of chlorophyll degradation (Charoenchongsuk et al., Citation2018).
In , the major band during the first stage of ripening was at 280 and two smaller bands were at 330 and 418 nm. However, these bands also represent the extract of content flavanones (280 nm band), chlorophylls (418 nm band), and polyphenols (330 nm band). In one region of Hilwa, there were three distinctive bands at 418, 436, and 469 nm, whereas another region reflected a major band at 665 nm. On the other hand, the alcoholic extracts of Hilwa fruits were made acidic by adding HCl. At the onset of ripening, chlorophyll degradation causes changes in the pigment composition. A similar study showed no significant change in the content of chlorophyll and carotenoids; however, the production of ethylene indicated the stay-green characteristics (Charoenchongsuk et al., Citation2015). The present study showed that the stay-green characteristics do not result from an inability of the fruit to produce ethylene.
The different stages of Sullaj date ripening were represented by the absorption spectra. The composite UV spectra of methanol extracts are presented in . The first stage of ripening of Sullaj extracts exhibited a complex absorption spectrum, having two major bands at 280 and 330 nm. The absorption spectra of extracts were observed to be dominated by shorter-wavelength bands, corresponding to the early ripening stages. All these bands were located at 436, 469, and 665 nm. The date samples at fourth and seventh stages of maturity were analyzed based on the number of anthocyanins. These results have been endorsed by Maria et al. (Citation2015). Pilkington et al. (Citation2012) supported that chlorophyll has decreased in concentration unlike the carotenoid in both the date fruit samples. The study also highlighted the decline in chlorophyll by with the maturity of the fruit. This variation in color is also highlighted in it as the decline in chlorophyll changes the color of the outer pericarp.
The extracts exhibited a complex absorption spectrum in the first stage of ripening of Sullaj and represented two major bands at 280 and 330 nm. The visible spectra for the alcoholic extracts of Sullaj dates were also observed. The absorption spectra of extracts were dominated by shorter-wavelength bands, which are correspondent to the early ripening stages. All these bands were observed to be located at 436, 469, and 665 nm. In the visible region of Hilwa date, the carotenes, anthocyanins, and chlorophylls had strong absorption bands. The visible spectra of alcoholic extracts for the samples of Hilwa fruits can be seen in . In Sullaj, the existence of anthocyanins was evaluated through absorption spectra in the Sullaj date fruit samples, after the acidification of alcoholic extracts. Moreover, the acidification of extracts altered the spectra of absorption; however, the majority of the alterations are dominated by chlorophyll bands at 410–470 nm. On the contrary, the absence of significant bands in 600–400 nm regions suggested that very little quantity of anthocyanins exist in Sullaj dates. This proves that the ripening of Sullaj dates decreases the fruit content of chlorophyll a and b to negligible levels.
The existence of anthocyanins was evaluated through absorption spectra in the Sullaj date fruit samples, after the acidification of alcoholic extracts. The acidification of extracts altered the spectra of absorption; however, most of the alterations were dominated by chlorophyll bands at 410–470 nm regions. On the contrary, the absence of significant bands in 600–400 nm regions suggested that very little quantity of anthocyanins exist in Sullaj dates. It was observed that the ripening of Sullaj dates resulted in a decrease in the fruit content of chlorophyll a and b to negligible levels. A similar study investigated the production of quality date with enhanced shelf life, considering the need for improved dehydration techniques (Haider et al., Citation2012).
The present study has examined that degradation of anthocyanins develops red color in Hilwa date fruits (). The findings are consistent with Diboun et al. (Citation2015), demonstrating the degradation of chlorophyll in dates. The results showed a considerable level of carotenes and chlorophylls in Sullaj date during its early ripening stages. At a spectrum of 272 nm, the emergence of a strong band was recommended by the absorption spectra. Its magnitude enhanced with the procedure of ripening. has provided UV spectra for the ripening of Sullaj date fruits. The majority of the molecules were strongly absorbed in this region, particularly the flavones and polyphenols. Consistent with this, one of the previous studies by Mortazavi et al. (Citation2015) states that the pigment contents are generally responsible for green, yellow, and red color when the date fruit is in its Khalal stage of ripening.
Conclusion
The study has evaluated the degradation of chlorophylls and other pigments such as anthocyanins and and carotenes among date fruits. The Hilwa and Sullaj date fruits were selected as the sample, against which different samples were collected at different time intervals. The results have shown that there is a rapid degradation of chlorophylls and anthocyanins in the samples of both types of dates. On the other hand, it was also evaluated that the degradation of carotenoids seems to be slower, in contrast with the degradation of chlorophylls. Moreover, the degradation of anthocyanins was also examined, which is responsible for the development of red color in Hilwa date fruits. Considering the degradation of anthocyanins, they tend to have measurable levels in Hilwa date fruits only. However, the results of this study are limited as they fail to emphasize on the exact content of pigment at different spectrophotometric absorption. This needs to be focused in the upcoming studies.
Competing Interest
The authors declare no competing interest.
Acknowledgments
The authors are very thankful to all the associated personnel in any reference that contributed to the purpose of this research.
Additional information
Funding
References
- Al-Farsi, M., C. Alasalvar, A. Morris, M. Baron, and F. Shahidi. 2005. Comparison of antioxidant activity, anthocyanins, carotenoids, and phenolics of three native fresh and sun-dried date (Phoenix dactylifera L.) varieties grown in Oman. J. Agric. Food Chem. 53(19):7592–7599. doi: 10.1021/jf050579q.
- Almeida, J., R. Assis, V.N. Molineri, I. Sestari, B.S. Lira, F. Carrari, L.E. Peres, and M. Rossi. 2015. Fruits from ripening impaired, chlorophyll degraded and jasmonate insensitive tomato mutants have altered tocopherol content and composition. Phytochemistry 111:72–83. doi: 10.1016/j.phytochem.2014.11.007.
- Amira, E.A., S.E. Behija, M. Beligh, L. Lamia, I. Manel, H. Mohamed, and A. Lotfi. 2012. Effects of the ripening stage on phenolic profile, phytochemical composition and antioxidant activity of date palm fruit. J. Agric. Food Chem. 60(44):10896–10902. doi: 10.1021/jf302602v.
- Baliga, M.S., B.R. Baliga, S.M. Kandathil, H.P. Bhat, and P.K. Vayalil. 2011. A review of the chemistry and pharmacology of the date fruits (Phoenix dactylifera L.). Food Res. Int. 44(7):1812–1822. doi: 10.1016/j.foodres.2010.07.004.
- Chaira, N., A. Mrabet, and A. Ferchichi. 2009. Evaluation of antioxidant activity, phenolics, sugar and mineral contents in date palm fruits. J. Food Biochem. 33(3):390–403. doi: 10.1111/j.1745-4514.2009.00225.x.
- Charoenchongsuk, N., K. Ikeda, A. Itai, A. Oikawa, and H. Murayama. 2015. Comparison of the expression of chlorophyll-degradation-related genes during ripening between stay-green and yellow-pear cultivars. Sci. Hortic. 181:89–94. doi: 10.1016/j.scienta.2014.10.005.
- Charoenchongsuk, N., D. Matsumoto, A. Itai, and H. Murayama. 2018. Ripening characteristics and pigment changes in russeted pear fruit in response to ethylene and 1-MCP. Horticulturae 4(3):22. doi: 10.1186/s12870-015-0672-5.
- Christ, B., and S. Hörtensteiner. 2014. Mechanism and significance of chlorophyll breakdown. J. Plant Growth Regul. 33(1):4–20. doi: 10.1007/s00344-013-9392-y.
- Dai, J., and R.J. Mumper. 2010. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 15(10):7313–7352. doi: 10.3390/molecules15107313.
- Diboun, I., S. Mathew, M. Al-Rayyashi, M. Elrayess, M. Torres, A. Halama, and K. Suhre. 2015. Metabolomics of dates (Phoenix dactylifera) reveals a highly dynamic ripening process accounting for major variation in fruit composition. BMC Plant Biol. 15(1):291. doi: 10.1186/s12870-015-0672-5.
- Farag, M.A., H. Handoussa, M.I. Fekry, and L.A. Wessjohann. 2016. Metabolite profiling in 18 Saudi date palm fruit cultivars and their antioxidant potential via UPLCqTOF-MS and multivariate data analyses. Food Funct. 7(2):1077–1086. doi: 10.1039/c5fo01570g.
- Gupta, D., and V. Kaul. 2012. Phytochemical screening of bioactive compounds from different populations of Hippophae rhamnoides L. growing in Kargil district (J & K, india). Int. J. Pharm. Bio. Sci. 3:447–455.
- Haider, M.S., I.A. Khan, M.J. Jaskani, S.A. Naqvi, and M.M. Khan. 2014. Biochemical attributes of dates at three maturation stages. Emirates J. Food Agric. 953–962. doi: 10.9755/ejfa.v26i11.18980.
- Haider, M.S., M. Rauf, N. Saleem, K. Jamil, and O. Mukhtar. 2012. Studies on ripening of dates from Rutab stage to ripe dehydrated dates. Pakistan. J. Biochem. Mol. Biol. 45:31–34.
- Hasnaoui, A., A. Elhoumaizi, A. Hakkou, B. Wathelet, and M. Sindic. 2011. Physico-chemical characterization, classification and quality evaluation of date palm fruits of some moroccan cultivars. J. Sci. Res. 3(1):139–149. doi: 10.3329/jsr.v3i1.6062.
- Lobo, M.G., E.M. Yahia, and A.A. Kader. 2014. Biology and postharvest physiology of date fruit. Dates 57–80. doi: 10.1002/9781118292419.ch3.
- Manohan, D., and W.C. Wai. 2012. Characterization of polyphenol oxidase in sweet potato (Ipomoea batatas (L.)). J. Adv. Sci. Arts 3:1.
- Maria, C.R., C.A. De, and F.R. Ribani. 2015. Anthocyanins and phenolic compounds in five ripening stages of Byrsonima ligustrifolia after extraction optimization. J. Food Nutr. Res. 54(4):365–378. (ISSN 1336-8672).
- Mathew, L.S., M.A. Seidel, B. George, S. Mathew, M. Spannagl, G. Haberer, and R.R. Krueger. 2015. A genome-wide survey of date palm cultivars supports two major subpopulations in Phoenix dactylifera. G3: Genes, Genomes, Genetics 5(7):1429–1438. doi: 10.1534/g3.115.018341/-/DC1.
- Mortazavi, S.M., F. Azizollahi, and N. Moallemi. 2015. Some quality attributes and biochemical properties of nine iranian date (Phoenix dactylifera L.) cultivars at different stages of fruit development. Int. J. Hortic. Sci. Technol. 2(2):161–171.
- Nematallah, K.A., N.A. Ayoub, E. Abdelsattar, M.R. Meselhy, M.M. Elmazar, A.H. El-Khatib, and S.A. Mousa. 2018. Polyphenols LC-MS2 profile of Ajwa date fruit (Phoenix dactylifera L.) and their microemulsion: Potential impact on hepatic fibrosis. J. Funct. Foods 49:401–411. doi: 10.1016/j.jff.2018.08.032.
- Pilkington, S.M., M. Montefiori, P.E. Jameson, and A.C. Allan. 2012. The control of chlorophyll levels in maturing kiwifruit. Planta 236(5):1615–1628. doi: 10.1007/s00425-012-1723-x.
- Solovchenko, A.E., O.B. Chivkunova, M.N. Merzlyak, and V.A. Gudkovsky. 2005. Relationships between chlorophyll and carotenoid pigments during on-and off-tree ripening of apple fruit as revealed non-destructively with reflectance spectroscopy. Postharvest Biol. Technol. 38(1):9–17. doi: 10.1016/j.postharvbio.2005.05.004.
- Tang, Z.X., L.E. Shi, and S.M. Aleid. 2013. Date fruit: Chemical composition, nutritional and medicinal values, products. J. Sci. Food Agric. 93(10):2351–2361. doi: 10.1002/jsfa.6154.
- Tengberg, M. 2012. Beginnings and early history of date palm garden cultivation in the Middle East. J. Arid. Environ. 86:139–147. doi: 10.1016/j.jaridenv.2011.11.022.
- Vayalil, P.K. 2012. Date fruits (Phoenix dactylifera Linn): An emerging medicinal food. Rit Rev Food Sci Nutr 52(3):249–271. doi: 10.1080/10408398.2010.499824.
- Yahia, E.M., E. Castellanos, and C. Mondragon-Jacobo. 2010. Identification and quantification of pigments in prickly pear fruit. Acta Hortic. 877(1129):1136.
- Zhang, X., Xin, C., Liu, W., Lin, Q., Cui, P., Li, F., ... & Al-Mssallem, I. S. (2015). Profiling microRNA expression during multi-staged date palm (Phoenix dactylifera L.) fruit development. Genomics 105(4), 242–251.