?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Plants and insects are members of diverse ecological communities. Feeding of herbivore insects may damage the host plants indirectly by altering the photosynthetic activity. The objective of this study was to evaluate the physiological changes in citrus plants due to citrus leafminer (CLM) Phyllocnistis citrella Stainton (Gracillariidae: Lepidoptera) larval feeding under field condition. Plants from four economically important citrus cultivars, Citrus reticulata (Kinnow and seedless Kinnow), Citrus tangerines (Fairchild), and Citrus paradisi Macfad (Grapefruit), were selected randomly and leaf damage was assessed through an image analysis technique. The results showed that percent leaf damage was higher at 44.2% on Fairchild compared to other cultivars at 19th day of post-feeding. The reduction in leaf photosynthesis (Pn), stomatal conductance (C), and transpiration rate (E) rates due to larval feeding through 19th day was found consistent in all cultivars except Grapefruit. After 13 days, Pn and gaseous exchange rates in damaged leaves of Grapefruit recovered to some extent but remained significantly lower than in control through 19th day. Reduction in all three physiological parameters in infested leaves was ranged as Fairchild > Kinnow > seedless Kinnow > Grapefruit compared to their level in control. Percent of leaf damage had a strong and negative relation with Pn, C, and E in all citrus cultivars except Grapefruit in which all variables showed a weak relation. Conclusively, P. citrella may cause a serious threat to citrus plants even at lower levels of infestation, and these physiological impairments could be more intense in regions with high P. citrella population.
Introduction
To understand the plant–insect interaction and to develop more accurate economic injury levels, the determination of physiological response in plants due to insect attack is a major component (Peterson and Higley, Citation2001). Herbivore insects cause damage to photosynthetic tissues, affecting the optimal growth of plants, and this indirect effect can be more harmful to plants than the direct removal of plant parts (Sinclair and Hughes, Citation2010). There could be a great variation in the impact of insect feeding on photosynthetic rate depending on the type of insect damage (piercing or chewing) and level of defense mechanism in the host plants (Nabity et al., Citation2009; Tatagiba et al., Citation2015). The direct loss of foliage due to insect feeding activities generally leads to a reduction in photosynthetic rate (Nabity et al., Citation2009); however, the mechanism involved in photosynthesis reduction is very complex. The insect feeding may affect the electron transportation in the chloroplasts of remaining leaf tissues, reducing the tissue’s photosynthetic activity (Aldea et al., Citation2005). Reduction in photosynthesis due to insect feeding may also impact the plant–water relation (Peterson et al., Citation2004). The stomata control the water loss from leaves while allowing photosynthesis (Cowan, Citation1972). Stomatal closure prevents the water loss from leaves, whereas its opening favors the uptake of CO2 for fixation in leaf tissues (Ziegler, Citation1987) and leaf temperature maintenance (Nobel, Citation1999). The herbivores may affect the water status of leaves and disturb the integrity in leaves from cut edges due to the water loss (Hoad et al., Citation1998; Ostlie and Pedigo, Citation1984; Welter, Citation1989; Wilson, Citation1980). However, the leafminer, an endophagous insect, does not interrupt the leaf integrity and lives in the leaf tissues by generating a special structure called mines. The only possibility of water loss is through the stomatal opening, the epidermis layer of the mine (Pincebourde et al., Citation2006; Raimondo et al., Citation2013).
Citrus leafminer (CLM), Phyllocnistis citrella Stainton (Gracillariidae: Lepidoptera) is known to feed and damage all citrus cultivars and some plants related to the Rutaceae family (Wagner et al., Citation2008). The adult females lay egg near midrib, and neonate larvae start feeding on leaf tissues through making serpentine mines on the leaf surface. Due to the mining activity, it destroys the epidermis layer of leaf, and the damaged leaves become curl and necrotic (Uygun et al., Citation2000). Association of citrus canker disease with P. citrella damage is also well known (Raga et al., Citation2001; Rogers and Stansly, Citation2007) because it provides a pathway for a bacterium called Xanthomonas axonopodis pv. citri (Belasque et al., Citation2005). The P. citrella feeding reduces the fruit excellence and may affect the photosynthetic activity in the leaf (Abdella and Mohammed, Citation2004). The impact of P. citrella feeding on the plant leaves was investigated by measuring the photosynthetic rate and gaseous exchange. We hypothesized that increasing the percent leaf damage caused by P. citrella affects the photosynthesis, transpiration, and stomatal conductance in four citrus cultivars.
Materials and Methods
Citrus Plants
A field experiment was performed in citrus nursery plantations located at the College of Agriculture, University of Sargodha (32º07ʹ52.5ʺN, 72º41ʹ03.0ʺE), Pakistan. Four citrus cultivars, Citrus reticulata (Kinnow and seedless Kinnow), Citrus tangerines (Fairchild), and Citrus paradisi Macfad (Grapefruit), were selected to determine the role of larval feeding of P. citrella on plant physiology. These selected cultivars play an important role in the economy of Pakistan and other citrus-producing countries and are also susceptible to P. citrella. Each cultivar was planted in six rows with 5-in. spacing. There were about 85–90 plants in each row at 6-in. spacing. Irrigation was done equally in each plot at a 10-day interval. From each cultivar, ten 1-year-old healthy plants were selected. No insecticide application was done throughout the experimental period. From each plant, three young leaves containing newly hatched P. citrella larvae were selected. The selection was done from upper, middle, and lower strata (one from each section) of the plant. On a daily basis, the selected leaves were examined for the removal of any additional larvae, if observed. Normally, the P. citrella infestation is very low in this region, rarely reaching two larvae per leaf. From each cultivar, two plants were selected to serve as control plants and were covered with fine mesh cages to avoid P. citrella infestation (Arshad et al., Citation2018a). The cages were ventilated to keep more than 90% transmission of light to leaves and did not affect the gas exchange compared to unprotected leaves (Delaney and Higley, Citation2006). Three leaves from each control plant were selected at the same height as corresponding infested plants to measure the physiological parameters. Data were recorded from selected leaves of each plant at 1, 7, 13, and 19 days of post-feeding by P. citrella larvae.
Percent of Leaf Damage
To assess the leaf damage caused by P. citrella larval feeding, image analysis method was used. On each observation day, the image of each selected leaf was captured by the digital camera (DSC-WX60, 16.2MP HD, China). The total leaf area and mine area of each experimental leaf were calculated using SigmaScan Pro 5.0 (Point Richmond, CA, USA) software (Richardson et al., Citation2001). The percent leaf damage was calculated by the formula suggested by Raimondo et al. (Citation2013):
Photosynthesis and Gaseous Exchange Rates
The data for the selected physiological parameters such as photosynthetic rate (Pn), transpiration rate (E), and stomatal conductance (C) were recorded using CI-340 portable photosynthesis system (CID, Inc., Camas, WA, USA). Each selected leaf was placed carefully in a gas exchange cuvette for a single minute, which was sufficient to record the data. Measurements were done at a photosynthetically active radiation of 1,000 μmol m−2 s−1.
Data Analysis
A one-way analysis of variance (ANOVA) was performed by keeping the cultivar as a main factor to determine the percent leaf damage and the difference in gas exchange parameters in control and damaged leaves. Means were separated by a post hoc Tukey’s multiple comparison tests. Pearson’s correlation was done to check the relationships between percent leaf damage in each cultivar with Pn rate, C, and E. All the statistical analyses were done using Minitab 16.1 software (Minitab Ltd, Brandon Court, UK).
Results
Percent Leaf Damage by P. citrella Larval Feeding
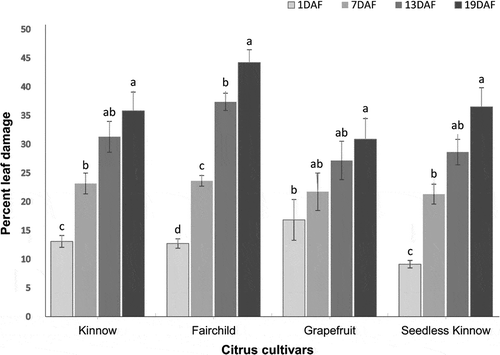
Phyllocnistis citrella larval feeding caused significant leaf damage on Kinnow (F = 17.9, P< .001), Fairchild (F = 90.5, P< .001), Grapefruit (F = 3.18, P< .05), and seedless Kinnow (F = 27.8, P < .001) cultivars. With the passage of time, percent leaf damage increased in all citrus cultivars; however, the leaf damage level of 44.2% was higher on Fairchild compared to other cultivars at the last day of observation. From 1st to 19th days of post-feeding, the range of percent leaf damage was 12.7–44.2% on Fairchild, 9.2–36.5% on seedless Kinnow, 13.1–35.8% on Kinnow, and 16.8–30.9% on Grapefruit ().
Impact of P. citrella Larval Feeding on Photosynthesis and Gas Exchange
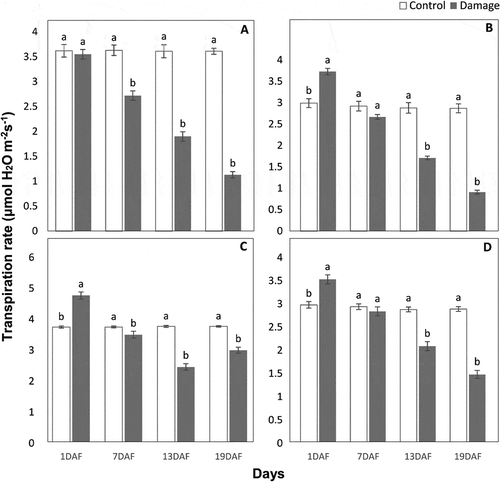
In the case of damage vs. control, photosynthesis was significantly reduced in all citrus cultivars at different days of larval feeding except in Grapefruit, where no significant difference of Pn rate was found at 7th (F = 0.03, P> .05) and 19th (F = 2.75, P > .05) days. At 19th day of post-feeding, Pn rate (μmol CO2 m−2 s−1) remained considerably lower in leaves fed by P. citrella larvae than leaves of control plants protected from feeding. The larval feeding reduced Pn activity to 94.6% in Fairchild, 89.1% in Kinnow, and 88.4% in seedless Kinnow cultivars at 19th day of post-feeding. With the passage of time, the Pn rate reduced consecutively in damaged leaves of all citrus cultivars except Grapefruit, in which the Pn rate reduced at 13th day of post-feeding and then increased at 19th day. Thus, a total reduction in Pn rate in damaged leaves of Grapefruit at 19th day of post-feeding was only 14.8% (). A similar trend of reduction in stomatal conductance (C) and transpiration rate (E) was found in all citrus cultivars (). A significant difference of C was found in damaged and control leaves of all citrus cultivars at different post-feeding periods except in Kinnow (F = 0.24, P > .05) and Grapefruit (F = 0.34, P > .05) after 1st day. However, C (μmol H2O m−2 s−1) was reduced by 64.5% in leaves fed by P. citrella larvae in Fairchild followed by 56.5% in Kinnow, 56.3% in seedless Kinnow, and 45.8% in Grapefruit when we compared with control leaves at 19th day of post-feeding (). Similarly, in the case of damage vs. control, a significant difference of E was found in all citrus cultivars except in Kinnow at 1st day (F = 0.62, P > .05), in Fairchild at 7th day (F = 0.063, P > .05), and in seedless Kinnow at 7th day (F = 0.66, P > .05) of post-feeding. Most reduction of 68.6% in E (μmol H2O m−2 s−1) was observed in Kinnow followed by 68.3% in Fairchild and 48.7% in seedless Kinnow. In Grapefruit, E was reduced by 34.7% at 13th day and then increased the rate of E up to 19th day, and the reduction was found only 20.4% on the last day of observation (), a response similar to Pn and C.
Figure 2. Photosynthetic rate (μmol CO2 m−2 s−1) (means ± SE) of damaged leaves due to feeding of Phyllocnisits citrella larvae in comparison with control leaves of Kinnow (a), Fairchild (b), Grapefruit (c), and seedless Kinnow (d) cultivars at different post-feeding periods; means sharing similar letters are not significantly different at P > .05. DAF = day after feeding
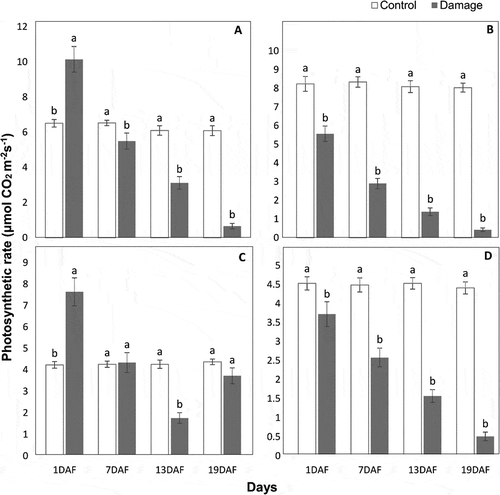
Figure 3. Stomatal conductance (μmol H2O m−2 s−1) (means ± SE) of damaged leaves due to feeding of Phyllocnisits citrella larvae in comparison with control leaves of Kinnow (a), Fairchild (b), Grapefruit (c), and seedless Kinnow (d) cultivars at different post-feeding periods; means sharing similar letters are not significantly different at P > .05. DAF = day after feeding
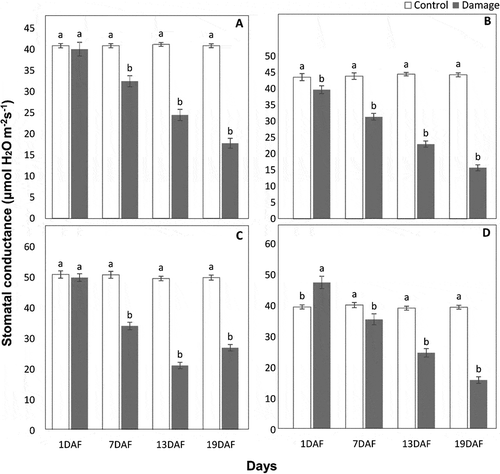
Figure 4. Transpiration rate (μmol H2O m−2 s−1) (means ± SE) of damaged leaves due to feeding of Phyllocnisits citrella larvae in comparison with control leaves of Kinnow (a), Fairchild (b), Grapefruit (c), and seedless Kinnow (d) cultivars at different post-feeding periods; means sharing similar letters are not significantly different at P > .05. DAF = day after feeding
Phyllocnistis citrella Larval Feeding – Photosynthesis and Gas Exchange Relationship
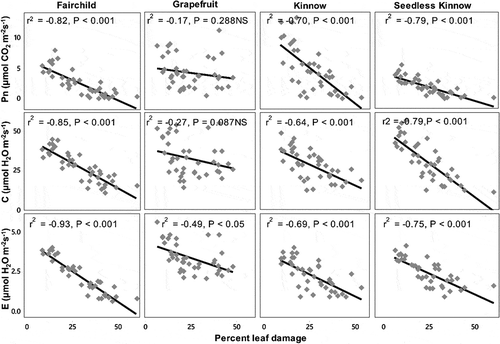
Percent leaf damage due to P. citrella larval feeding had a negative effect on Pn rate, C, and E of all four citrus cultivars. As leaf damage increased, the effect on all three physiological parameters was also significantly increased except in the case of Pn (r2 = −0.17, P > .05) and C (r2 = −0.27, P > .05) in Grapefruit. A weak relation of Pn, C, and E with leaf damage was observed in the case of Grapefruit. In Fairchild cultivar, Pn (r2 = −0.82, P < .001), C (r2 = −0.85, P < .001), and E (r2 = −0.93, P < .001) were strongly and negatively related to percent leaf damage. A similar trend of relation was observed in seedless Kinnow and Kinnow cultivars; however, the relation of all three parameters with leaf damage was little lower in Kinnow as in Fairchild and seedless Kinnow ().
Figure 5. Photosynthetic rate (Pn), stomatal conductance (C), and transpiration rate (E) in relation to percent leaf damage by feeding of Phyllocnistis citrella larvae on different citrus cultivars, r2 = Pearson’s correlation coefficients; P < .05 and P < .001 show the level of significance, NS = nonsignificant
Discussion
The leaf damage by the mining activity of P. citrella larvae was higher in Fairchild compared to other cultivars. Our results are supported by Arshad et al. (Citation2018b) who also reported the higher infestation level of P. citrella larvae in nursery plantations of Fairchild in this region. Photosynthetic rate is a top-down factor to evaluate the effect of herbivore damage on plants (Neves et al., Citation2006). The effect of P. citrella larval feeding on photosynthesis and the gaseous exchange rate was higher on Fairchild followed by Kinnow and seedless Kinnow cultivar. After 1st day of the post-feeding period, all three physiological parameters were found lower in damaged leaves than control in all citrus cultivars that we tested. As the leaf damage increased with a post-feeding period of P. citrella larvae, the effects on photosynthetic rate, transpiration, and stomatal conductance also increased in all citrus cultivars except Grapefruit. The physiological parameters decreased through 13th day in damaged leaves of Grapefruit, and then, the level increased through 19th day but remained significantly lower compared to control. The increase in Pn rate might be due to the fact that intrinsic factor like the photosynthesis in the undamaged portion of leaves increased to compensate the sink demand (Welter, Citation1989).
The reduction in photosynthesis could be due to the failure of stomata to normally open on the mined leaves (Raimondo et al., Citation2013). The reduction in photosynthetic rate could also be ascribed to reduced light absorption due to callus tissue production or peridermal injury in response to the pest feeding or due to dehydration of mesophyll cell caused by rupture of epidermis layer (Achor et al., Citation1997; Connor and Taverner, Citation1997; Nardini et al., Citation2010; Raimondo et al., Citation2013).
In previous studies, there are many variations in response to impairment of Pn rate in leaves which might be due to many factors like measurement on newly emerged leaves or measurement of an injured or uninjured portion of leaves only (Delaney and Macedo, Citation2001; Zangerl et al., Citation2002). There might be another possibility that the defense mechanism of plant inhibited due to the injecting of insect saliva (Kessler and Baldwin, Citation2002; Korth and Dixon, Citation1997), which resulted in more Pn reduction (Tang et al., Citation2006). The effect of insect feeding on Pn rate may vary depending on the feeding behavior like feeding on xylem/phloem portion, the stem, or leaf fluids (Haile and Higley, Citation2003; Heng-Moss et al., Citation2006; Macedo et al., Citation2003, Citation2005, Citation2007; Wagner et al., Citation2008).
The decrease in transpiration rate suggests that the stomatal closure occurs which restricts the evaporation of water and alternatively decreases the Pn rate (Nabity et al., Citation2009). Leaf stoma is closely associated with leaf transpiration in response to plant’s water status. In the photosynthesis process, stomata must be open so that it can take in CO2 and evaporate the water (Neves et al., Citation2006). Additionally, leaves must have the capacity to preserve and provide the sufficient amount of water used in the photosynthesis process as well as to maintain the turgor pressure (Neves et al., Citation2006). By P. citrella larval feeding, the transpiration rate affected and caused impairment in the Pn rate. Rate of transpiration and stomatal conductance were less in P. citrella-affected leaves as compared to control. This could be due to the CO2 released by a larva within the mine or due to high radiation levels inside the feeding window of the mine. Higher CO2 inside the intercellular spaces and higher radiations reduced the stomatal conductance (Pincebourde et al., Citation2006). Reduced stomatal conductance resulted in the reduced movement of moisture from the leaves and hence reduced the transpiration rate. It is also possible that the reduction of leaf water content directly affects the biochemical reactions within the leaf and slows down the rate of photosynthesis (Meyer, Citation1998).
There was a great relation of percent leaf damage due to the mining activity of P. citrella larvae with photosynthesis and gaseous exchange parameters. All the physiological parameters were strongly and negatively related to the percent leaf damage in all tested citrus cultivars except in Grapefruit, in which the relationship was weak. Neves et al. (Citation2006) also reported the negative relation of leaf damage due to Orthezia praelonga Douglas (Hemiptera: Ortheziidae) on rangpur lime, Citrus limonia Osbeck.
In conclusion, our study suggests the negative impact of P. citrella larvae on the photosynthetic rate of citrus cultivars, and it could be even more intense to impact the plant health in the regions with high infestation level of this insect. The stomatal conductance and transpiration rate of plants were also negatively affected by the feeding of P. citrella larvae. The results also explained why P. citrella is more damaging to citrus cultivars (Fairchild, Kinnow, and seedless Kinnow) and their effects persisted for a long period. Furthermore, the photosynthesis is a very important and adequate variable to determine the damage level in citrus cultivars due to P. citrella larvae because it is a primary physiological assay for plant growth. It can be considered as a new parameter in the IPM program to identify the damage level or control level.
Acknowledgments
We thank the authorities of the Department of Agronomy and Horticulture at the University of Sargodha for the laboratory facilities and for the citrus cultivars.
Disclosure Statement
The authors declare no conflict of interest.
Additional information
Funding
References
- Abdella, T.E., and E.S.I. Mohammed. 2004. Guidelines for testing insecticides against the citrus leafminer. Proceedings of 70th Pests and Diseases Committee. Crop Protection Centre. ARC, Wad Madani.
- Achor, D.S., H. Browning, and L.G. Albrigo. 1997. Anatomical and histochemical effects of feeding by citrus leafminer larvae (Phyllocnistis citrella Stainton) on citrus leaves. J. Am. Soc. Hortic. Sci. 122:829–836. doi: 10.21273/JASHS.122.6.829.
- Aldea, M., J.G. Hamilton, J.P. Resti, A.R. Zangerl, M.R. Berenbaum, and E.H. DeLucia. 2005. Indirect effects of insect herbivory on leaf gas exchange in soybean. Plant Cell Environ. 28:402–411.
- Arshad, M., M.I. Ullah, M. Afzal, H.M. Aatif, Y. Iftikhar, J. Molina-Ochoa, and J.E. Foster. 2018b. Association of citrus leafminer Phyllocnistis citrella (Lepidoptera: Gracillariidae) damage with physiological parameters and larval weight in Citrus reticulata. Int. J. Trop. Insect Sci. 38:26–32. doi: 10.1017/S1742758417000273.
- Arshad, M., M.I. Ullah, J.A. Qureshi, and M. Afzal. 2018a. Physiological effects of citrus leafminer Phyllocnistis citrella (Lepidoptera: Gracillariidae) larval feeding on photosynthetic and gaseous exchange rates in citrus. J. Econ. Entomol. 111:2264–2271. doi: 10.1093/jee/toy150.
- Belasque, J.J., A.L. Parra-Pedrazzoli, J. RodrigouesNeto, P.T. Yamamoto, M.C.M. Chagas, J.R.P. Parra, B.T. Vinyrad, and J.S. Hratung. 2005. Adult citrus leafminer (Phyllocnistis citrella) are not efficient vectors for Xanthomonas axonopodis pv citri. Plant Dis. 89:594. doi: 10.1094/PD-89-0590.
- Connor, E.F., and M.P. Taverner. 1997. The evolution and adaptive significance of the leaf-mining habit. Oikos. 79:6–25. doi: 10.2307/3546085.
- Cowan, I.R. 1972. Oscillations in stomatal conductance and plant functioning associated with stomatal conductance: Observations and a model. Planta. 106:185–219. doi: 10.1007/BF00388098.
- Delaney, K.J., and L.G. Higley. 2006. An insect countermeasure impacts plant physiology: Midrib vein cutting, defoliation and leaf photosynthesis. Plant Cell Environ. 29:1245–1258. doi: 10.1111/j.1365-3040.2006.01504.x.
- Delaney, K.J., and T.B. Macedo. 2001. The impact of herbivory on plants: Yield, fitness, and population dynamics, p. 135–160. In: R.K.D. Peterson and L.G. Higley (eds.). Biotic stress and yield loss. CRC, Boca Raton, FL.
- Haile, F.J., and L.G. Higley. 2003. Changes in soybean gas-exchange after moisture stress and spider mite injury. Environ. Entomol. 32:433–440. doi: 10.1603/0046-225X-32.3.433.
- Heng-Moss, T., T. Macedo, L. Franzen, F. Baxendale, L. Higley, and G. Sarath. 2006. Physiological responses of resistant and susceptible buffalo grasses to Blissus occiduus (Hemiptera: Blissidae) feeding. J. Econ. Entomol. 99:222–228. doi: 10.1603/0022-0493(2006)099[0222:PRORAS]2.0.CO;2.
- Hoad, S.P., A. Marzoli, J. Grace, and C.E. Jeffree. 1998. Response of leaf surfaces and gas exchange to wind stress and acid mist in birch (Betula pubescens). Trees Struct. Funct. 13:1–12.
- Kessler, A., and I.T. Baldwin. 2002. Plant responses to insect herbivory: The emerging molecular analysis. Annu. Rev. Plant Biol. 53:299–328. doi: 10.1146/annurev.arplant.53.100301.135207.
- Korth, K.L., and R.A. Dixon. 1997. Evidence for chewing insect-specific molecular events distinct from a general wound response in leaves. Plant Physiol. 115:1299–1305. doi: 10.1104/pp.115.4.1299.
- Macedo, T.B., C.S. Bastos, L.G. Higley, K.R. Ostlie, and S. Madhavan. 2003. Photosynthetic responses of soybean to soybean aphid (Homoptera: Aphididae) injury. J. Econ. Entomol. 96:188–193. doi: 10.1093/jee/96.1.188.
- Macedo, T.B., R.K.D. Peterson, D.K. Weaver, and W.L. Morrill. 2005. Wheat stem sawfly, Cephus cintus Norton, impact on wheat primary metabolism: An ecophysiological approach. Environ. Entomol. 34:719–726. doi: 10.1603/0046-225X-34.3.719.
- Macedo, T.B., D.K. Weaver, and R.K.D. Peterson. 2007. Photosynthesis in wheat at the grain filling stage is altered by the wheat stem sawfly (Hymenoptera: Cephidae) injury and reduced water availability. J. Entomol. Sci. 42:228–238. doi: 10.18474/0749-8004-42.2.228.
- Meyer, G.A. 1998. Pattern of defoliation and its effect on photosynthesis and growth of Goldenrod. Funct. Ecol. 12:270–279. doi: 10.1046/j.1365-2435.1998.00193.x.
- Nabity, P.D., J.A. Zavala, and E.H. Delucia. 2009. Indirect suppression of photosynthesis on individual leaves by arthropod herbivory. Ann. Bot. 103:655–663. doi: 10.1093/aob/mcn127.
- Nardini, A., F. Raimondo, M.A. Lo Gullo, and S. Salleo. 2010. Leafminers help us understand leaf hydraulic design. Plant Cell Environ. 33:1091–1100. doi: 10.1111/j.1365-3040.2010.02131.x.
- Neves, A.D., R.F. Oliveira, and J.R.P. Parra. 2006. A new concept for insect damage evaluation based on plant physiological variables. An. Acad. Bras. Cienc. 78:821–835. doi: 10.1590/S0001-37652006000400015.
- Nobel, P.S. 1999. Physicochemical & environmental plant physiology. Second ed. Academic Press, New York, USA.
- Ostlie, K.R., and L.P. Pedigo. 1984. Water loss from soybeans after simulated and actual insect defoliation. Environ. Entomol. 13:1675–1680. doi: 10.1093/ee/13.6.1675.
- Peterson, R.K.D., and L.G. Higley. 2001. Illuminating the black box: The relationship between injury and yield, p. 1–12. In: R.K.D. Peterson and L.G. Higley (eds.). Biotic stress and yield loss. CRC, Boca Raton, FL.
- Peterson, R.K.D., C.L. Shannon, and A.W. Lenssen. 2004. Photosynthetic responses of legume species to leaf-mass consumption injury. Environ. Entomol. 33:450–456. doi: 10.1603/0046-225X-33.2.450.
- Pincebourde, S., E. Frak, H. Sinoquet, J.L. Regnard, and J. Casas. 2006. Herbivory mitigation through increased water use efficiency in a leaf mining moth–apple tree relationship. Plant Cell Environ. 29:2238–2247. doi: 10.1111/j.1365-3040.2006.01598.x.
- Raga, A., M.E. Satol, M.F. Souza, and R.C. Siloto. 2001. Comparison of spray insecticides against citrus leafminer. Arq. Inst. Biol. 68:77–82.
- Raimondo, F., P. Trifilò, and M.A.L. Gullo. 2013. Does citrus leafminer impair hydraulics and fitness of citrus host plants? Tree Physiol. 33:1319–1327. doi: 10.1093/treephys/tpt094.
- Richardson, M.D., D.E. Karcher, and L.C. Purcell. 2001. Quantifying turfgrass cover using digital image analysis. Crop Sci. 41:1884–1888. doi: 10.2135/cropsci2001.1884.
- Rogers, M.E., and P.A. Stansly. 2007. Florida citrus pest management guidelines: Asian citrus psyllid and citrus leafminer. Florida Cooperative Extension Service, Institute of Food and Agriculture Sciences, University of Florida. ENY-734. EDIS 2007 (10). https://journals.flvc.org/edis/article/view/116574.
- Sinclair, R.J., and L. Hughes. 2010. Leaf miners: The hidden herbivores. Austral. Ecol. 35:300–313. doi: 10.1111/j.1442-9993.2009.02039.x.
- Tang, J.Y., R.E. Zielinski, A.R. Zangerl, A.R. Crofts, M.R. Berenbaum, and E.H. DeLucia. 2006. The differential effects of herbivory by first and fourth instars of Trichoplusia ni (Lepidoptera: Noctuidae) on photosynthesis in Arabidopsis thaliana. J. Exp. Bot. 57:527–536. doi: 10.1093/jxb/erj032.
- Tatagiba, S.D., F.M. Damatta, and F.A. Rodrigues. 2015. Leaf gas exchange and chlorophyll a fluorescence imaging of rice leaves infected with Monographella albescens. J. Phytopathol. 105:180–188. doi: 10.1094/PHYTO-04-14-0097-R.
- Uygun, N., D. Senal, I. Karaca, and N.Z. Elekcioglu. 2000. Effect of citrus leafminer, Phyllocnistis citrella Stainton (Lepidoptera: Gracillariidae) on citrus fruit yield, p. 1–12. In: Proceedings of the 4th Turkish national congress of entomology, Aydın, Turkey, 12–15 September.
- Wagner, D., L. DeFoliart, P. Doak, and J. Schneiderheinze. 2008. Impact of epidermal leaf mining by the aspen leaf miner (Phyllocnistis populiella) on the growth, physiology and leaf longevity of quaking aspen. Oecologia. 157:259–267. doi: 10.1007/s00442-008-1067-1.
- Welter, S.C.1989. Arthropod impact on plant gas exchange. p. 135–150. In: ed. E.A. Bernays. Insect-plant interactions. Vol. 1, CRC, Boca Raton, FL.
- Wilson, J. 1980. Macroscopic features of wind damage to leaves of Acer pseudoplatanus L. and its relationship with season, leaf age and wind speed. Ann. Bot. 46:303–311. doi: 10.1093/oxfordjournals.aob.a085921.
- Zangerl, A.R., J.G. Hamilton, T.J. Miller, A.R. Crofts, K. Oxborough, M.R. Berenbaum, and E.H. deLucia. 2002. Impact of folivory on photosynthesis is greater than the sum of its holes. Proc. Natl. Acad. Sci. 99:1088–1091. doi: 10.1073/pnas.022647099.
- Ziegler, H. 1987. The evolution of stomata, p. 29–58. In: E. Ziegler, G.D. Farquhar, and I.R. Cowan (eds.). Stomatal function. Stanford University Press, Stanford, CA.