?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Cordia sinensis
Lam is a multipurpose underutilized species of arid and semiarid regions. It is commonly propagated through seeds, but germination percentage is low (50–60%) and erratic. Therefore, an investigation was undertaken to standardize the propagation technique during year 2016 at CAZRI RRS Bhuj, India. The seeds of Cordia gharaf were treated with gibberellic acid (four levels: 0, 250, 500, and 1000 ppm) and potassium nitrate (four levels: 0%, 0.25%, 0.5%, and 1%) singly or in combination and it was found that combined application of GA3 and KNO3 enhanced seed germination synergistically (p = .05). The treatment consisted of GA3 250 ppm and KNO3 (1%) resulted in earliest seed germination (4.33 ± 0.33 days), maximum seed germination percentage (79.47%), and subsequent seedling growth e.g. highest leaf area (25.52 ± 1.41 cm2), and maximum leaf fresh (1.59 g) and dry weight (0.72 g). However, maximum plant height was observed under GA3 500 ppm and KNO3 (1%) treatment. In conclusion, the combination treatment of GA3 250 ppm and KNO3 1% is an effective and practical method for improving C. sinensis seed germination.
Introduction
Cordia sinensis Lam. (Cordia gharaf (Forssk.) Ehrenb. ex Asch.) is a multi-stemmed tree, growing up to 8 to 12 m height. Cordia sinessis is a potential underutilized plant which is also known as Gondi, narrow-leaved sebastian or Greyleaved saucer-berry and belongs to the family Boraginaceae. The genus Cordia consists of about 250 species and the majority of species are tree or shrub (Barroso and Oliveira, Citation2009). It is well-adapted plant species in arid to semiarid regions of West Africa to East to the Middle East, India, Sri Lanka, and Pakistan due to its various adaptive features e.g. strong tap root system, waxy leaves, hairiness, sunken and covered stomata in leaves, water binding mechanism, moderate tolerance to salinity and alkalinity. These traits make it hardier than Cordia myxa, a popular fruit plant of the Boraginaceae family (Meghwal et al., Citation2014). The fruits of the C. sinensis Lam. are consumed by local inhabitants. The leaves provide the protein (13.12%) rich fodder to cattle (Kuria et al., Citation2005). Various parts of the plant have ethnomedicinal value and are traditionally used to cure different human and livestock ailments (Gumgumjee and Hajar, Citation2015). Farmers of arid regions grow C. sinensis around the farm boundary to protect the main crop from hot and dry wind (Meghwal et al., Citation2014). Therefore, C. sinensis may be a potential species for domestication and utilization in the arid region. The total plant population of C. sinensis was about 864 thousand which share only 0.32% of the total plant population of the Gujarat state (Pradeep and Singh, Citation2010). In Kachchh region of Gujarat, C. sinensis was distributed among the 29.09% of surveyed sites (Dev et al., Citation2016). While, it is reported to be threatened plant in other regions of India (e.g., western and southern Haryana) (Singh, Citation2018). Generally, Corida sinensis is propagated through the seeds but germination percentage is poor (50–60%) and erratic (Meghwal, Citation2007). Various germination studies conducted in the other Cordia species viz. Cordia boissieri reveals conflicting information with regard to breaking seed dormancy through stratification (Schuch et al., Citation2001). The study conducted in Cordia myxa and C. gharaf indicated seed treatment with gibberellic acid (GA3) (250, 500 mg L−1) promotes germination significantly (Meghwal, Citation2006)) Similarly, about 50–60% seed germination could be obtained with fresh seeds of C. sinensis, but the germination percentage is low and not uniform (Maundu et al., Citation2005; Meghwal et al., Citation2014). However, another study in Cordia biosseri showed high percentage of seed dormancy in the newly produced seeds (Fulbright, Citation1992; Schuch et al., Citation2001). Both GA3 and KNO3 stimulate the seed germination of dormant seeds of various plant species (Koyuncu Citation2005; Cárdenas et al., Citation2013; Mello et al., Citation2009). However, the studies carried out by Ding et al. (Citation2007), Gao et al. (Citation2011), Cárdenas et al. (Citation2013) observed the effect of GA3 and KNO3 on seed germination of other species but very few on C. sinensis seed germination.
The proper seedling growth of germinated seeds is the most important factor for successful sapling production under nursery conditions. The earlier studies showed the variable effect of GA3 and KNO3 on growth traits of various plants. Application of GA3 and KNO3 promotes overall plant growth in Brassica oleracea Capitata (Majumdar, Citation2013), Cucumis sativus (Pal et al., Citation2016), and Solanum lycopersicum (Balaguera-Lopez et al., Citation2009). GA3 (250 mg L−1) treatment resulted in maximum seed germination (98.75%), subsequent shoot length, root length, leaf area, shoot, and root dry weight (Shabaq, Citation2013). Similar growth promotion of seedlings was also reported under the GA3 treated seeds of Citrus limon (Dzayi and Rahman, Citation2010), Nothapodytes nimmoniana (Patil et al., Citation2015), and Citrus aurantifolia (Jaiswal et al., Citation2018). Lay et al. (Citation2015), found GA3 and KNO3 presowing treatment to seeds of papaya improves the seedling growth, fresh and dry weight of seedlings. However, very few studies are available with quantitative data on the subsequent growth of treated plants. The C. sinensis is very important species of arid and semiarid regions due to its multiple uses for fodder, fruits, and medicines, yet the multiplication of species through seeds provides poor and erratic germination. Therefore, present investigation attempted to improve the seed germination rate and quantify the subsequent growth for faster multiplication for the Cordia sinensis, an important species of the arid region.
Material and Methods
The study was conducted at Horticulture Nursery, ICAR-Central Arid Zone Research Institute, Regional Research Station, Bhuj Gujarat, India. The seed germination experiment was performed in the laboratory, while, subsequent plant growth study was carried out under the shade net. Cordia sinensis fruits were collected from the native population in the Kachchh region of Gujarat (22°84ʹ624 – 23°56ʹ288 N° latitude and 68°95ʹ730 – 70°73ʹ100 E° longitude). Fresh seeds were extracted from the ripened fruits. The mucilaginous pulp was removed from the seeds by rubbing the seeds with sandy soil followed by washing with tap water. Air dried, cleaned seeds were stored in the paper bag for 2 weekss at the room temperature before use.
Experiment 1. Chemical Treatment of Seeds
Before the seed treatment, seeds were disinfected with fungicide (Carbendazim 2%) for 20–30 minutes and rinsed three times with running tap water. For the treatments, seeds were divided into four groups, the total no. of treatment combinations were 16, four levels of each of GA3 concentration (0, 250, 500, and 1000 ppm) and four KNO3 concentrations (0%, 0.25%, 0.5%, and 1% KNO3) purchased from Sigma Aldrich (90% gibberellin A3 basis) Loba Chemie (99% purity), respectively and were evaluated with 3 replications 1) seeds of first group were transferred into the beaker filled with distilled water and left for 24 h; 2) seeds of second group were soaked in 250, 500, and 1000 mg L−1 (w/v) GA3 solution for 24 h; 3) similarly, seeds of the third group were soaked in 0.25%, 0.5%, and 1% KNO3 solution for 24 h; 4) the seeds of the fourth group were treated with the aqueous solution supplemented different combinations of GA3 and KNO3 ().
Table 1. Test design of chemicals (KNO3, GA3) and combinations of KNO3 and GA3 treatments
The previously treated seeds of Cordia sinensis were taken for germination test. The 16 treatments were arranged in completely randomized design with three replications. The average temperature of laboratory during experiment was about 35°C. A layer of cotton wool was placed in sterilized the Petri dish (150 mm × 15 mm) and covered with equal size of blotting paper. The 20 seeds were wrapped in blotting paper and cotton wool placed in the Petri dishes followed by soaking with distilled water (each treatment with three Petri dishes, e.g., 60 seeds per treatment). The seeds were kept moist uniformly by frequent spraying of water. The initiation of germination was recorded at daily basis and number of germinated seeds on particular day was also noted and percentage of germination was recorded at the weekly interval by unfolding the blotting paper up to 30 days of sowing after which there was no further germination noticed. The germination percentage was calculated as follows (Gashi et al., Citation2012)
Where G = germination percentage, a = the total no. of germinated seeds till 30 days of sowing and N = the total no. of seeds sown.
While, mean germination time was estimated using the following method (Moradi et al., Citation2008).
MGT = ΣDn/Σn
Where n = number of seeds which were germinated on day D,
D = number of days taken for seed germination.
Experiment II. Seedling Growth at Net House
After the 30-day, the germinated plantlets of C. sinensis were shifted from laboratory to green shade net (50% shade and 1.5 mm thickness) conditions. The plantlets were established in the black-colored nursery poly bags (20 × 10 cm) and filled with sterilized soil (sand: soil: farmyardd manner – 2:2:1) potting mixture. The average temperature and relative humidity under shade net house during experiment were about 30°C and 74%. Different plant characteristics like plant height (cm), stem thickness (mm), no. of leaves per plant were measured at 30, 90, 180, 270, and 360 days of seed germination. Leaf area (cm2), leaf fresh weight (g), leaf dry weight (g), leaf nitrogen (%), phosphorous (%), potassium (%), sodium (%) were estimated at the end of the experiment. The stem thickness and leaf area (cm2) were measured using a digital vernier calliper and the leaf area meter (Licor-3100). For recording leaf dry weight, leaf samples were dried in the oven at 72°C for 48 h or until the constant weight was achieved. The leaf nutrients (e.g. (available N, P, K, and Na) were estimated. The collected leaf samples were cleaned by washing in 0.1% detergent solution, followed by single and double distilled water. The cleaned leaf samples were dried in a hot air oven at 72°C for 48 h or until they obtained a constant weight and ground and sieved. The concentration of K and Na was determined using the flame photometer (Model: ESICO1382). While, measurement of leaf P and N content was done through colorimetric and Kjeldahl method, respectively.
Statistical Analysis
The randomized complete block design (RCBD) was used for conducting the experiment in laboratory and shade net. The critical differences (CD) at P < .05 and P < .01 level of probability were determined after analysis of variance (ANOVA) for all treatments. The experiment was laid out with 16 treatments including control with three replications and 60 seeds per replicate were used. The mean and analysis of variance were performed to determine significant differences among treatments (P < .05) using the Statistical Software Package for Agricultural Research Workers (Sheoran et al., Citation1998).
Result and Discussion
Experiment 1. Chemical Treatment of Seeds
Mean Germination Time (MGT) and Germination Percentage (GP)
The different concentrations of GA3 and KNO3 significantly fasten the seed germination as compared to the germination percentage of control seeds. The KNO3 (0.5%) treatment significantly shortens the germination initiation duration (4 days), compared to other single KNO3 applications. Whereas the synergistic effect of GA3 × KNO3 treatments was observed on the shortening of germination time. The seeds treated with aqueous solution supplemented with GA3 250 mg L−1 and 1% KNO3, took minimum time for germination initiation (4.33 ± 0.33 days), which was about 1 week less than germination initiation in control treatment. Among the treatment, maximum time for germination initiation was taken by the seeds soaked in the KNO3 0.25% and 1.0% concentration ().
Table 2. Analysis of variance of gibberellic acid and potassium nitrate effects on seed germination properties for Cordia sinensis.
The result of the GA3 × KNO3 experiment revealed significant enhancement of final germination percentage (). Based on the results for germination percentage, the highly significant difference was noted between seeds treated with a different gibberellic acid concentration (250, 500, and 1000 mg L−1 GA3) and with a combination of the different concentration of potassium nitrate (0.25%, 0.5%, and 1%) and untreated seeds (control). The maximum final germination percentage was noted in the seeds treated with 250 mg L−1 GA3 × 1% KNO3 (79.47%) and 500 mg L−1 GA3 × 0.25% KNO3 (67.17%), as compared to the germination percentage of control (21.97%). In contrast, when seeds treated with a higher GA3 concentration along with higher KNO3 treatment, the final germination percentage reduced drastically. Analysis of data revealed that both single factors (GA3 and KNO3 concentration) affected seed germination percentages (p ≤ 0.05). However, higher germination percentage was reported under the 0.5% KNO3 treatment, which was higher than all other single treatments. However, GA3, treatment singly did not significantly increase the final seed germination percentage. Similar study conducted in Cordia biosseri reveals a high percentage of dormant seeds and GA3 treatment, breaks the seed dormancy (Fulbright, Citation1992; Schuch et al., Citation2001). Similarly, Meghwal (2007) noted 20–30% seed germination in Cordia myxa and up to 60% seed germination in Cordia gharaf with freshly extracted seeds and seed treatment with GA3 and mechanical scarification significantly improve (66.66%) germination of species. On the contrary, Maundu et al. (Citation2005) observed that, seed treatment of fresh seeds of Cordia sinensis generally not necessary but, stored seeds need to be soaked with warm water (40°C) until water is cool. However, Acharya and Purohit (Citation1999) found that GA3 promoted germination of seeds in Cordia gharaf to the maximum extent of 81%. Waman et al. (Citation2017) recommended that presowing treatments with KNO3 and GA3 were effective to produce the healthy seedlings of Semecarpus kurzii. In general, seeds treated with KNO3 (0.5%) had a higher overall percentage seedling emergence than GA3 treatments. The gibberellic acid promotes the germination of dormant seeds of many species such as, Citrullus lanatus (Ding et al., Citation2007), Penstemon digitalis (Mello et al., Citation2009), Tamarindus indica (Patel et al., Citation2018) and Elaeocarpus prunifolius (Iralu and Upadhaya, Citation2018). The KNO3 solution increases the germination percentage of Lilium orientalis seeds (Gao et al., Citation2011), Eremurus spectabilis seeds (Keskiner and Tuncer, Citation2019), and Passiflora edulis f. Flavicarpa (Cárdenas et al., Citation2013). The present results are in agreement with the results of Cárdenas et al. (Citation2013) who reported that increased seed germination was directly proportional to the increase in GA3 concentrations (500 and 1000 mg L–1). However, at higher GA3 concentration (1500 mg L–1), germination of Passiflora species decreased (Cárdenas et al., Citation2013). On the contrary, lower concentration (80–250 mg L–1) promotes the seed germination of Eriobotrya japonica (Al-Hawezy, Citation2013), Nothapodytes nimmoniana (Patil et al., Citation2015) and Citrus aurantifolia (Jaiswal et al., Citation2018). Seed germination was improved due to gibberellic acid (GA) treatment possibly through increased synthesis of hydrolytic enzymes, which were further transported to endosperm. The enzymes break down the stored food to supply the energy required for germination (Chen et al., Citation2008; Varner, Citation1965). GA3 may promote cytokinin action and counteracts the germination inhibitor (ABA) which ultimately stimulate the biochemical processes of seed germination (Chen et al., Citation2008; Cetinbas and Koyuncu, Citation2006; Tuan et al., Citation2018). Potassium nitrate improves the seed germination percentage in the various plants species (Duermeyer et al., Citation2018; Keskiner and Tuncer, Citation2019). A study on the germination of mountain ash seeds showed that germination of the species was affected by both the concentration of KNO3 and the time of KNO3 exposure (Bian et al., Citation2013). Similarly, Gupta et al. (Citation2011) reported that treatment of Hippophae salicifolia seeds with 0.1% KNO3 pretreatment resulted in 96% seed germination. However, a higher concentration of KNO3 negatively affects seed germination. A similar trend of reduction of seed germination on the application of higher KNO3 concentration was reported in Salvia cyanescens (Yücel and Yilmaz, Citation2009) and Gladiolus alatus (Ramzan et al., Citation2010).
Table 3. Mean of germination, growth (MGT, GP, LA, LFW, LDW) and seedling nutrients (NPK and Na) of Cordia sinensis under various levels of GA3 (in ppm) and KNO3 (in %)
According to Gniazdowska et al. (Citation2007), KNO3 possibly stimulates seed germination by the production of nitrous oxide. Nitrous oxide may increase seed germination and break seed dormancy by regulation of endogenous balance of abscisic acid and gibberellic acid. It may influence the expression of enzymes that triggers a nitrate-induced ABA decrease and the biosynthesis of germination promoters (gibberellins) (Duermeyer et al., Citation2018; Finch et al., Citation2007; Sirova et al., Citation2011). The present study shows a synergistic effect on promoting germination of Cordia sinensis seeds when seeds are treated with an aqueous solution of gibberellins supplemented with potassium nitrate solution. Similar study conducted by Meghwal (2007) indicates that merely water imbibition (20–30% germination) was not sufficient to trigger the germination in Cordia myxa but seed treatment with gibberellic acid (GA3) (500 mg L−1) was essential to promote seed germination (66.66%). However, KNO3 did not influence the seed germination, irrespective of treatment duration and chemical concentration. Gashi et al. (Citation2012) found the higher germination percentage (92.26%) in the Ramonda species’ seeds treated with 1000 ppm GA3 + 0.3% KNO3. Treatment of Sorghum bicolor seeds with GA3 (100 ppm) and KNO3 (1%) resulted in increase of germination percentage to a higher extent (94%) (Shanmugavalli et al., Citation2007). Similar synergistic results of KNO3 (1%) and GA3 (500 ppm) seed treatments were reported by Dewir et al. (Citation2011) in Sabal palmetto. Whereas, soaking cracked seeds of Elaeocarpus prunifolius in the solution of GA3 and KNO3 accelerate the germination rate (Iralu and Upadhaya, Citation2018).
Experiment 2. Subsequent Quantification of Growth
Plant Height and Collar Diameter
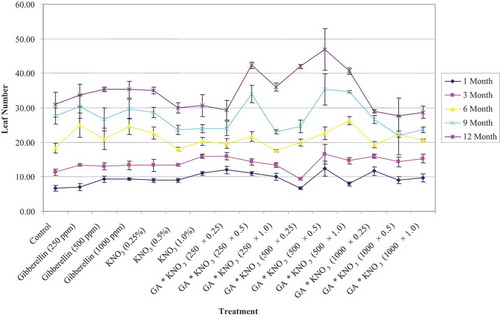
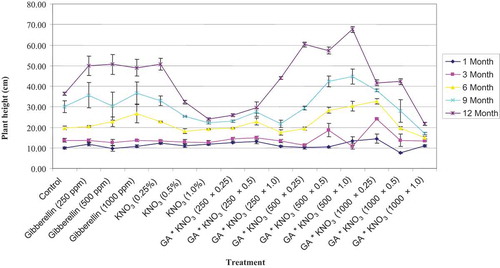
Application of GA3 × KNO3 singly or in combination, significantly (p = .05) affected the plant height (). However, plant height under different single KNO3 applications differed significantly. Lower KNO3 application (0.25% conc.) tends to increase plant height at par to GA3 500 mg L−1, but at higher KNO3 application (1%), there was a reduction in the plant height. The longest plants were observed from the GA3 (500 mg L−1) and KNO3 (1%) applications, followed by combined application of 500 mg L−1 GA3 and 0.5% KNO3. Whereas, the minimum increase in the plant height was seen when plants seeds soaked in the GA3 1000 mg L−1 and KNO3 1% solutions for 24 h.
Figure 1. Plant height of Cordia sinensis, under various levels of GA3 (in ppm) and KNO3 (in %) seed treatment, recorded at three month interval
Data presented in illustrated the effect of GA3 and KNO3 on the collar diameter of C. sinensis. The significant difference was ascertained for C. sinensis between seeds treated with GA3, KNO3 and H2O (control). All GA3 and KNO3 applications had a positive effect on collar diameter. However, single GA3 applications were more effective than different single KNO3 application. Moreover, integrated application of GA3 and KNO3 resulted pronounced improvement in collar diameter of plantlets. The maximum increase in the collar diameter in one-year-old plants was observed with the GA3 500 mg L−1 and KNO3 0.5% treatment followed by GA3 1000 mg L−1 and KNO3 0.25% treatment. On the contrary, the minimum collar diameter was recorded in the plants of control group. The presowing treatment with lower concentration of GA3 and KNO3 improved the seedling height significantly however, reduction in the plant height was observed under the higher concentration of GA3 and KNO3. Treatment of Eriobotrya japonica seeds with lower concentration of GA3 (250 mg L−1) resulted maximum collar diameter and plant height comparing to control (Shabaq, Citation2013). Similarly, maximum plant height and stem diameter of Citrus aurantifolia plantlets were observed with GA3 (200 mg L−1) seed treatment (Meshram et al., Citation2015).
Figure 2. Collar diameter of Cordia sinensis, under various levels of GA3 (in ppm) and KNO3 (in %) seed treatment, recorded at three month interval
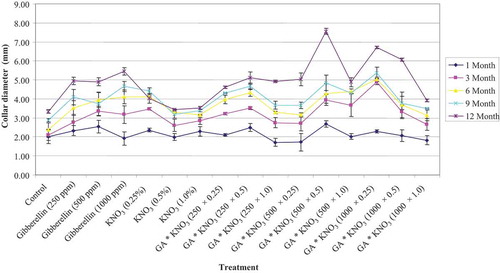
The GA mediated increment in plant height and collar diameter chiefly due to enhancement of cell division in cambium tissues and its prompt cell progeny as supported by the Parab et al. (Citation2017) in Carica papaya and Harsha et al. (Citation2017) in Citrus maxima.
Leaf Area and Leaves Number per Plant
The single factors (GA3 and KNO3 concentration) significantly influenced the leaf area (p ≤ 0.05) (). Seeds treated with the different concentrations of GA3 are found to have more leaf area as compare to KNO3 treated seed and untreated seeds (control). A lower concentration of GA3 (250 mg L−1) had a favorable effect on leaf expansion. While at a higher concentration of GA3 treatment leaf area found to be non-significantly decreased. Similar to GA3, leaf area of treated KNO3 seeds was declined with increases of KNO3 concentration. The GA3 and KNO3 treatments significantly affected the leaf area. The maximum leaf area was recorded in the plants treated with GA3 250 × KNO3 1%, followed by GA3 500 × KNO3 0.5%. While, treatment combination, GA3 1000 mg L−1 × KNO3 0.25% had some adverse effect on leaf expansion, and it resulted in minimum leaf area. Similar, leaf expansion at lower GA3 concentration (250 mg L-1) was observed in Eriobotrya japonica (Shabaq, Citation2013), Citrus limon (Dzayi and Rahman, Citation2010) and Citrus aurantifolia (Meshram et al., Citation2015).
In general, numbers of leaves per plant were not improved by the pretreatment of GA3 and KNO3 (). However, numbers of leaves per plant were significantly improved with combined application of GA3 and KNO3. The presowing treatment of seeds with GA3 500 mg L−1 × KNO3 0.5%, led to maximum increase in the leaf number per plant, followed by seeds treated with GA3 250 mg L−1 × KNO3 0.5%. In contrast, GA3 seeds soaked in higher gibberellin concentration (1000 mg L−1) supplemented with medium or high KNO3 (0.5% or 1%) concentration for 24 h, gave the minimum increase in the leaf numbers per plant. The various previous study reported the opposite relations between leaf growth and plant height increment due to GA3 treatment (Arney and Mancinelli, Citation1966). These trends are concurrent with the present study. GA3 pregermination treatments increase the leaf area and leaf numbers in Carica papaya (Parab et al., Citation2017) and Citrus maxima (Harsha et al., Citation2017). This is possibly due to the stimulation of cell division and enlargement by GA application (Parab et al., Citation2017).
Leaf Fresh and Dry Weight
The result indicated that the highest leaf fresh and dry weight was achieved when the C. sinensis seeds were treated with the aqueous solution of GA3 250 mg L−1, supplemented with the KNO3 1%, followed by the solution containing GA3 500 mg L−1 and KNO3 0.25%. The minimum leaf fresh weight was noted under the highest KNO3 (1% conc.) treatment. In general, fresh weight of leaf was higher when seeds were treated with GA3 than a KNO3 solution; further fresh weight was decreased when GA3 concentration increased from 0.25% to 1%. While the lowest dry weight was found under maximum concentration of GA3 treatment and KNO3. It was observed that higher concentration of both the chemicals (GA3 and KNO3) resulted in the decline in the dry weight of leaves. Cárdenas et al. (Citation2013) found the higher dry weight, total plant weight, specific leaf area and leaf area of KNO3 treated passiflora seedling. Similarly, higher fresh and dry shoot weight of GA3 treated seeds were found in the seedlings of Carica papaya (Meena and Jain, Citation2012 & Parab et al., Citation2017) and Citrus maxima (Harsha et al., Citation2017). Maximum vegetative dry weight and root weight were observed with GA3 (250 mg L−1) treatment in Eriobotrya japonica (Shabaq, Citation2013) and similarly, higher shoot and root dry weight were found due to GA3 treatment in Citrus limon (Dzayi and Rahman, Citation2010) and Citrus aurantifolia (Jaiswal et al., Citation2018). The higher fresh and dry shoot weight in the GA3 presowing treatment chiefly because of increased mobilization of water, membrane permeability, nutrient uptake, and transportation which ultimately leads to higher production of photosynthetic products (Parab et al., Citation2017; Harsha et al., Citation2017). While, higher mobilization of photosynthetic products was also taken place in the KNO3 treated seeds in the early stage of seed germination (Lay et al., Citation2015).
Leaf Nutrient Traits
The different concentration of GA3 × KNO3 affects the leaf N, P, K, and Na content significantly (). The leaf N content was lower under the different single GA3 and KNO3 treatments as compared to control. However, combination treatment of GA3 1000 mg L−1 and KNO3 0.25% resulted in highest leaf N content. In general leaf N content was higher in the plants treated with GA3 as compare to KNO3 treatments. The leaf P content was higher under the GA3 treated plants it was highest under GA3 250 mg L−1 treatment closely followed by GA3 500 mg L−1 × KNO3 1% treatment. The combined application of GA3 1000 mg L−1 × 0.5% KNO3 resulted minimum leaf P content. In general higher leaf P content was achieved when seeds were treated with GA3 for 24 h than KNO3 treatment for the same duration. Percentage leaf K content was lower for GA3 and KNO3 treated seeds as compared to seeds treated with distilled water (control). However, among the treatments, the highest K content was noted when seeds were soaked in GA3 500 mg L−1 followed by KNO3 (0.25%). The lowest leaf K content was reported when the seeds were soaked in the aqueous solution of GA3 1000 mg L−1 supplemented with KNO3 1% concentration. The treatment of GA3 resulted significantly high leaf Na content than control. However, there was no significant difference between seeds soaked in H2O (control) and KNO3 solution for leaf Na content. The seeds soaked in GA3 (500 mg L−1) and KNO3 0.25% had the highest leaf Na content. Whereas, lowest leaf Na content was recorded when seeds were treated with an aqueous solution of GA3 1000 mg L−1 supplemented with the KNO3 0.25% for 24 h.
Increase in leaf nitrogen and phosphorous content with foliar application of GA3 and KNO3 was reported in Cucumis sativus plants (Pal et al., Citation2016). While the higher leaf nitrogen and potassium contents were significantly influenced by the different GA3 application. Maximum leaf nitrogen and potassium were reported under combination of GA3 (100 ppm) and urea (1%) in the Citrus jambhiri (Kant et al., Citation2017). Kazemi (Citation2014) noted that GA3 application did not significantly affect leaf NPK content, but the application of potassium nitrate influenced leaf NPK content in Solanum lycopersicum leaves. The highest increase was reported with 6 mM KNO3 application. A similar effect was observed by Zhang et al. (Citation2002) and Lin and Danfeng (Citation2003). Application of potassium nitrate might increase the plant metabolism and related functions thereby were responsible for increase in leaf NPK contents (Marschner, Citation2012). Leaf sodium content was found to be decreased with combined application of gibberellins and potassium nitrate in the study. The similar antagonistic correlation was also observed by Song and Fujiyama (Citation1996).
Thus, the external application of KNO3 and GA3 showed a synergistic effect in promoting seed germination and seedling vigor of Cordia sinensis seed in the present investigation. In conclusion, it can be stated that the application of GA3 (250 ppm) and KNO3 (1%) is a simple, effective, and practical method for improving seed germination and seedling growth of Cordia sinensis. Although, further work might be useful for in-depth understanding on effects of chemicals in Cordia sinensis.
Acknowledgments
Author is thankful to Director, ICAR-CAZRI for proving necessary facility for conducting research.
Disclosure Statement
The authors declare that they have no conflict of interest.
Literature Cited
- Acharya, J., and S.S. Purohit. 1999. Effect of quality of light and growth regulators on seed germination of Cordia gharaf and Cordia dichotoma. In: Paper presented in national seminar on plant physiol. At interface of Agri Horticulture and Industary, (Vol. 66, 150.), Udaipur, India.
- Al-Hawezy, S.M.N. 2013. The role of the different concentrations of GA3 on Seed Germination and Seedling Growth of Loquat (Eriobotrya japonica L.). IOSR Journal of Agriculture and Veterinary Science 4 (5): 3–6.
- Arney, S.E., and P. Mancinelli. 1966. The basic action of gibberellic acid in elongation of ‘Meteor’ pea stems. New Phytol. 65:161–175.
- Balaguera-Lopez, H.E., J.F. Cardenas-Hernandez, and J.G. Alvarez-Herrera. 2009. Effect of gibberellic acid (GA3) on seed germination and growth of tomato (Solanum lycopersicum L.). Acta Hort. (ISHS). 821:141–148. doi:10.17660/ActaHortic.2009.821.15.
- Barroso, I.C.E., and F.D. Oliveira. 2009. Pharmacognostic diagnosis of fruits of Cordia sellowiana Cham. and Cordia myxa L. (Boraginaceae). Rev. Bras. Farmacogn. 19:458–470. doi:10.1590/S0102-695X2009000300021.
- Bian, L., L. Yang, J. Wang, and H. Shen. 2013. Effects of KNO3 pretreatment and temperature on seed germination of Sorbus pohuashanensis. J. Forest Res. 24:309–316. doi:10.1007/s11676-013-0354-9.
- Çetinbaş, M. and Koyuncu, F. 2006. Improving germination of Prunus avium L. seeds by gibberellic acid, potassium nitrate and thiourea. Horticultural Science - Prague 33(3): 119–123.
- Cárdenas, J., C. Carranza, D. Miranda, and S. Magnitskiy. 2013. Effect of GA3, KNO3, and removing of basal Point of seeds on germination of sweet granadilla (Passiflora ligularis Juss) and yellow Passion fruit (Passiflora edulis f. Flavicarpa). Rev. Bras. Frutic. 35(3):853–859. doi: 10.1590/S0100-29452013000300023.
- Chen, S.Y., S.R. Kuo, and C.T. Chien. 2008. Roles of gibberellins and abscisic acid in dormancy and germination of red bayberry (Myrica rubra) seeds. Tree Physiol. 28(9):1431–1439. doi: 10.1093/treephys/28.9.1431.
- Dewir, Y.H. Mahrouk, M.E. and Naido, Y. 2011. Effect of some mechanical and chemical treatments on seed germination of Sabal palmetto and Thrinax morrisii palms. Australian Journal of Crop Science 5(3): 248-253.
- Dev, R., M.S. Kumar, S. Kumar, and D. Dayal. 2016. Survey, collection and utilization of grewia and cordia species for human and animal nutrition in Arid Kachchh, Gujarat, p.344. In IAC abstracts book. 1st International agrobiodiversity congress. November 6–9, 2016. New Delhi, India.
- Ding, Q.L., X.M. Dang, and Y.F. Zhan. 2007. Effect of potassium nitrate and gibberellin solution soaking on mini-watermelon seed germination. J. South China Uni. Trop. Agric. 13:14–16. in Chinese with English abstract.
- Duermeyer, L., E. Khodapanahi, D. Yan, A. Krapp, S. Rothstein, and E. Nambara. 2018. Regulation of seed dormancy and germination by nitrate. Seed Sci. Res. 28(3):150–157. doi: 10.1017/S096025851800020X.
- Dzayi, F.H., and Rahman. 2010. Effect of GA3 and Soaking time on Seed Germination and Seedling Growth Lemon (Citrus limon L.) High Diploma.Thesis coll.Agric, univ. of Salahaddin – Erbil.
- Finch, W.E., C.S. Cadman, P.E. Toorop, J. Lynn, and H. Hilhorst. 2007. Seed dormancy release in Arabidopsis Cvi by dry after-ripening, low temperature, nitrate and light shows common quantitative patterns of gene expression directed by environmentally specific sensing. Plant Jour. Lond. 51:60–78. doi:10.1111/j.1365-313X.2007.03118.x.
- Fulbright, T. 1992. Temperature Effects on Seed Germination of Cordia boissieri A. DC. (Boraginaceae). Southwest. Nat. 37(2):197–199. doi: 10.2307/3671669.
- Gao, N., G.F. Cui, Y.Q. Lai, S.X. Zhang, J. Li, J.H. Wang, and F.H. Liu. 2011. Effects of Different Treatments on the Germination of Oriental lily Seeds. Acta Agric. Univ. Jiangxi 33:660–664. (in Chinese with English abstract).
- Gashi, B. Abdullai, K. Mata, V and Kongjika, E. 2012. Effect of gibberellic acid and potassium nitrate on seed germination of the resurrection plants Ramonda serbica and Ramonda nathaliae. African Journal of Biotechnology 11(20): 4537-4542.
- Gniazdowska, A., U. Dobrzyjska, T. Babajczyk, and R. Bogatek. 2007. Breaking the apple embryo dormancy by nitric oxide involves the stimulation of ethylene production. Planta. 225:1051–1057. doi:10.1007/s00425-006-0384-z.
- Gumgumjee, N.M., and A.S. Hajar. 2015. Antimicrobial efficacy of Acacia saligna (Labill.) H.L.Wendl. and Cordia sinensis Lam. Leaves extracts against some pathogenic microorganisms. Int. J. Microbiol. Immunol. Res 3(4):51–57.
- Gupta, S.M., P. Pandey, A. Grover, and Z. Ahmed. 2011. Breaking seed dormancy in Hippophae salicifolia, a high-value medicinal plant. Physiol. Mol. Biol. Plants. 17:403–406. doi:10.1007/s12298-011-0082-6.
- Harsha, H.R., R. Venkat, K.J. Dayamani, and M. Shivanna. 2017. Effect of growth regulators and macronutrients on seedling growth of pummelo (Citrus maxima Merill). Int.l J Recent Sci. Res. 8(10):20531–20533. doi: 10.24327/IJRSR.
- Iralu, V., and K. Upadhaya. 2018. Seed dormancy, germination and seedling characteristics of Elaeocarpus prunifolius Wall. ex Müll. Berol.: A threatened tree species of north-eastern India. N.Z. J. Of For. Sci. 48:16. doi:10.1186/s40490-018-0121-y.
- Jaiswal, S.B., R.V. Nainwad, S.J. Supekar, and S.B. Mane. 2018. Effect of growth regulators and chemicals on growth of Kagzi Lime (Citrus aurantifolia Swingle.) seedlings. Int.J.Curr.Microbiol.App.Sci. 6:940–944.
- Kant, G., R.P.S. Dalal, and B.S. Beniwal. 2017. Effect of PGR on leaf nutrients content and root growth in rough lemon (Citrus jambhiri) seedling. Int. J. Pure App. Biosci. 5(4):346–351. doi: 10.18782/2320-7051.5386.
- Kazemi, M. 2014. Effect of gibberellic acid and potassium nitrate spray on vegetative growth and reproductive characteristics of tomato. J. Biol. Environ. Sci. 8(22):1–9.
- Keskiner, K., and B. Tuncer. 2019. Dormancy breaking treatments for wild Eremurus spectabilis M.Bieb seeds. Fresen. Environ. Bull. 28(2A):1167–1173.
- Koyuncu, F. (2005). Breaking seed dormancy in black mulberry (Morusnigra L.) by cold stratification and exogenous application of gibberellic acid. Acta Biol. Cracoviensia Ser. Bot. 47(2):23–26.
- Kuria, S.G., M.M. Wanyoike, C.K. Gachuiri, and R.G. Wahome. 2005. Nutritive value of important range forage species for camels in marsabit district, Kenya. Trop. Subtrop. Agroecosyst. 5:15–24.
- Lay, P., G.V. Basvaraju, V. Pashte, and M. Gowri. 2015. Studies on effect of gibberellic acid (GA3) and potassium nitrate (KNO3) on breaking of seed dormancy of papaya (Carica papaya L.) cv. Surya. The Ecoscan 9:109–113.
- Lin, D., and H. Danfeng. 2003. Effects of potassium levels on photosynthesis and fruit quality of muskmelon in culture medium. Acta Horticulturae Sinica 30(2):221–223.
- Majumdar, F. 2013. Response of gibberellic acid and potash nutrient on growth and yield of late planting cabbage (Brassica oleracea Capitata). MS thesis, Department of Horticulture, Sher-e-Bangla Agricultural University, Dhaka, Bangladesh, pp. 62–84.
- Marschner, P. 2012. Marschner’s mineral nutrition of higher plants. 3rd. Academic Press, London. p. 672 p.
- Maundu, P.M., B. Tengnäs, N. Muema, and A. Birnie. (2005). Cordia sinensis (C. gharaf, C. rothii) in Useful trees and shrubs for Kenya World Agroforestry Centre, Eastern and Central Africa Regional Programme: Nairobi 173–174 Retrieved December 5, 2018, from http://www.worldagroforestry.org/usefultrees/pdflib/Cordia_sinensis
- Meena, R.R., and M.C. Jain. 2012. Effect of seed treatment with gibberellic acid on growth parameters of papaya seedling (Carica papaya L.). Prog. Hort 44(2):248–250.
- Meghwal, P.R. 2006. Propagation study in Lehsua (Cordia myxa), p. 117–119. In: S.N. Ghosh, S.K. Mitra, B.C. Banik, M.A. Hasan, S.K. Sarkar, R.S. Dhua, J. Kabir, and J.K. Hore eds. Proceedings of the national symposium on production, utilization and export of underutilized fruits with commercial potentialities. 22–24 November 2006. Kalyani, Nadia, West Bengal, India.
- Meghwal, P.R. 2007. Propagation studies in lehsua (Cordia myxa). Indian Journal of Agricultural sciences 77 (11) : 765–767
- Meghwal, P.R., S. Akath, P. Kumar, and B.R. Morwal. 2014. Diversity, distribution and horticultural potential of Cordia myxa L.: A promising underutilized fruit species of arid and semi-arid regions of India. Genet. Resour. Crop Evol. 61:1633–1643. doi:10.1007/s10722-014-0161-y.
- Mello, A.M., N.A. Streek, E.E. Blankenship, and E.T. Paparozzi. 2009. Gibberellic acid promotes seed germination in Penstemon digitalis cv. Husker Red. HortSci 44(3):870–873. doi: 10.21273/HORTSCI.44.3.870.
- Meshram, P.C., P.S. Joshi, R.K. Bhoyar, and A.K. Sahoo. 2015. Effect of different plant growth regulators on seedling growth of acid lime (Citrus aurantifolia Swingle). Res. Envrn. Life Sci. 8(4):725–728.
- Moradi, D.P., F. Sharif-zadeh, and M. Janmohammadi. 2008. Influence of priming techniques on seed germination behavior of maize inbred lines (Zea mays L.). J. Agr. Biol. Sci. 3(3):22–25.
- Pal, P., K. Yadav, K. Kumar, and N. Singhm. 2016. Effect of gibberellic acid and potassium foliar sprays on productivity and physiological and biochemical parameters of parthenocarpic cucumber (Cucumis sativus) cv. ‘seven star F1. J. Horticultural Res. 24(1):93–100. doi: 10.1515/johr-2016-0011.
- Parab, A.M., J.C. Mathad, and K.V. Malshe. 2017. Effect of pre-soaking chemicals on germination and subsequent seedling growth of papaya (Carica papaya L.) cv. Solo. Int. J. Chem. Stud. 5(4):1812–1816.
- Patel, M., R.V. Tank, D.R. Bhanderi, H.M. Patil, V. Patel, and M. Desai. 2018. Response of soaking time and chemicals on germination and growth of tamarind (Tamarindus indica L.). Plant Archives 18:51–56.
- Patil, A.S., S.R. Patil, and A.S. Kale. 2015. Influence of growth Regulators on germination and growth of endangered medicinal plant Nothapodytes nimmoniana j.graham under shade net conditions. Global J. Res. Med. Plants Indigen. Med. 4(1):01–09.
- Pradip, K. and Singh H.S. 2010. Tree wealth of the non-forest areas of Gujarat. A report tree census in non-forest areas-2009. Forest department, Gujarat state. p 47
- Ramzan, A., I.A. Hafiz, T. Ahmad, and N.A. Abbasi. 2010. Effect of priming with potassium nitrate and dehusking on seed germination of gladiolus (Gladiolus alatus). Pak. J. Bot. 42:247–258.
- Schuch, U.K., E. Davison, and J. Kelly. 2001. Seed Propagation of Cordia boissieri and Cordia parvifolia. Turfgrass and Ornamental Research Report. http://ag.arizona.edu/pubs/crops/az1246
- Shabaq, M.N.A. 2013. The role of the different concentrations of GA3 on seed germination and seedling growth of loquat (Eriobotrya japonica L.). IOSR J. Agric. Vet. Sci. 4(5):03–06. doi: 10.9790/2380-0450306.
- Shanmugavalli, M. Renganayaki, P.R, and Menaka, C. 2007. Seed dormancy and germination improvement treatments in fodder sorghum. International Crops Research Institute for the Semi-Arid Tropics 3: 1-3.
- Sheoran, O.P., D.S. Tonk, L.S. Kaushik, R.C. Hasija, and R.S. Pannu. 1998. Statistical Software Package for Agricultural Research Workers, p. 139–143. In: D.S. Hooda and R.C. Hasija (eds.). Recent advances in information theory, statistics & computer applications. Department of Mathematics Statistics, CCS HAU, Hisar.
- Singh, R. 2018. Activities of research wing of Haryana forest department. Retrieved from http://haryanaforest.gov.in/Portals/0/Research_Activities_Final%20as%20on%2031-3-2018.pdf
- Sirova, J., M. Sedlarova, J. Piterkova, L. Luhova, and M. Petrivalsky. 2011. The role of nitric oxide in the germination of plant seeds and pollen. Plant Sci. 181:560–572. doi:10.1016/j.plantsci.2011.03.014.
- Song, J.Q., and H. Fujiyama. 1996. Ameliorative effect of potassium on rice and tomato subjected to sodium salinization. Soil Sci. Plant Nutr. 42:493–501. doi:10.1080/00380768.1996.10416318.
- Tuan, P.A., R. Kumar, P.K. Rehal, P.K. Toora, and B.T. Ayele. 2018. Molecular mechanisms underlying abscisic acid/gibberellin balance in the control of seed dormancy and germination in cereals. Front. Plant Sci. 9:668. doi:10.3389/fpls.2018.00668.
- Varner, J.E. 1965. Enzymes, p. 14–20. In: J. Bonner and J.E. Vamer (eds.). Plant Biochemistry. Academic Press, London and New York.
- Waman, A.A., P. Bohra, and A. Norman. 2017. Chemical pre-treatments improve seed germination and seedling growth in Semecarpus kurzii: An ethnomedicinally important plant. J. For. Res. 29:1283. doi:10.1007/s11676-017-0562-9.
- Yücel, E., and G. Yilmaz. 2009. Effects of different alkaline metal salts (Na Cl, KNO3), acid concentrations (H2SO4) and growth regulator (GA3) on the germination of Salvia cyanescens Boiss. & Bal. seeds. Gazi Unive. J. Sci. 22:123–127.
- Zhang, A., D.F. Huang, and Z. Hou. 2002. Effect of potassium nutrient on development and photosynthesis of melon plant. J. Shanghai Agricultural Coll. 20(1):13–17.