?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The present study deals with the extraction and characterization of Jackfruit seed polyphenol (JSP) and its application in the stabilization of peanut oil. The optimized extract was obtained at condition of 95% ethanol for 30 min by using conventional solvent extraction method. The extract exhibited a total phenolic content of 120.5 µg GAE/mL, flavonoid content of 0.43 mg CE/ml, condensed tannin content of 0.34 mg CE/ml and DPPH scavenging EC50 value of 14.22 µg/ml. The characterization of extract by LC-ESI-Q-TOF-MS/MS revealed the presence of phenolic acid, flavonoids, and stilbenes. The JSP extract in the presence of synthetic antioxidants such as butylated hydroxytoluene (BHT) and α-Tocopherol-acetate (vitamin-E acetate) were tested for the stabilization of cold-pressed peanut oil. Furthermore, natural antioxidant mediated stabilization of cold-pressed peanut oil was also investigated using α-Tocopherol (vitamin E) and curcumin. The JSP extract and curcumin significantly retarded lipid oxidation in peanut oil under Schaal oven test conditions of 60°C for a week, as compared to BHT, α-Tocopherol, and α-Tocopherol acetate. Also, the JSP extract and curcumin showed better antioxidant effects on the oxidative stability of oil at frying temperature of 150–180°C for 2 hr and thus can prove potential in ensuring the retardation of oil oxidation process. Hence, JSP extract and curcumin can have industrial application of serving as a natural preservative in oil.
Introduction
Polyphenols are secondary metabolites produced by plants. They contain one or two hydroxyl group attached to the phenyl ring. Several studies support the health benefits of polyphenols rich diets which include fruits, vegetables, whole grains, nuts, and legumes. There are more than 8000 compounds, which are isolated from various plant sources. Polyphenols are divided into four subclasses – namely flavonoids, phenolic acids, stilbenes, and lignans. These compounds show excellent antioxidant, antimicrobial, anti-mutagenic, anti-bacterial, and anti-inflammatory properties (Gharras, Citation2009). Polyphenols also interfere with the lipid oxidation process by donating hydrogen atoms to unstable highly reactive free radicals and convert them to phenoxy radical intermediate which is relatively more stable. They also act as efficient metal chelators (Hussain et al., Citation2016). Many studies have revealed protective and beneficial effects of polyphenols against various diseases. Polyphenols are known to reduce the risk of non-communicable disease like cardiovascular diseases, inflammatory diseases, cancer, and diabetes. Anticancer effects of polyphenols are exhibited by reducing the number and growth rate of tumors. Several polyphenols like catechins, quercetin, isoflavones, flavanones, lignans, ellagic acid, resveratrol, and red wine polyphenols are found to display anti-cancer effects in various studies (Pandey and Rizvi, Citation2009). Many reports have also explained that the consumption of polyphenol limits the incidence of coronary heart diseases and lower the risk of myocardial infarction. They can inhibit LDL oxidation which results in atherosclerosis. Resveratrol present in red wine is established in prevention of platelet aggregation and vasoconstrictor. The polyphenols such as catechins, epicatechin, epicatechin gallate, epigallocatechin, tannic acid, isoflavones, chlorogenic acid, glycyrrhizin, and saponins are known to reduce the risk of diabetes by inhibiting the intestinal glycosidase and glucose transporter (Pandey and Rizvi, Citation2009).
Though jackfruit has many health benefits, it is still an underutilized fruit in India as compared to other fruits. The waste products of jackfruit are more than any other fruits. A recent report revealed that annually 2000 and 35 Crore of jackfruit goes to waste in Karnataka and Kerela state, respectively (Ranganathan, Citation2018). The waste part of this fruit includes mainly its peel or rind and perianths along with seeds. Despite enormous health benefit and Jackfruit seed/seed flour-based food preparations, they are still non-optimally utilized and have very limited scientific literature.
Heating and frying of oil is a common process of preparing foods. The oxidation of oil takes place when it is exposed to a high temperature during frying in the presence of the substrate, water, and air, and hence increases the free fatty acids (Alireza et al., Citation2010). The generation of monomers, polymers, and volatile compounds leads to the decomposition of oil (Falade and Oboh, Citation2015). Natural antioxidants are the polyphenols obtained from plant and are known for their safe and nontoxic nature. Hence, natural antioxidants are gaining more demand in current markets nowadays owing to its health benefits attributes (Anbudhasan et al., Citation2014).
Therefore, the aim of this current investigation was to optimize the extraction conditions for jackfruit seeds polyphenols followed by LC-MS/MS characterization of the phenolic profile. And also to understand the effect of jackfruit seed polyphenols in enhancing the stability of peanut oil during Schaal oven test and at frying temperature.
Materials and Method
Chemicals and Reagents
The Folin–Ciocalteu’s reagent, gallic acid, catechin, and 2,2-diphenyl-1-picrylhydrazyl (DPPH) were purchased from SRL Company, Mumbai. Ethanol was procured from SD Fine Ltd, Mumbai. All other chemicals and solvents were obtained from Himedia laboratories Pvt Ltd and were of high analytical grade.
Preparation of Jackfruit Seeds Powder (JSP)
The fresh jackfruit seeds were purchased from the local market. The seeds were manually cleaned with fresh water and grounded into small pieces. The seeds were then dried in a tray drier at 55°C for 24 h. The seed powder was then prepared using a mixer grinder and was further passed through a 60-mesh sieve. The powder thus obtained was then stored in an airtight bag, at room temperature away from light.
Extraction Optimization
The optimization of extraction procedure of jackfruit seed powder was carried out by using different combinations of three parameters namely solid to solvent ratio (20–100 mg of powder/ml of solvent), extraction time (5–30 min), and concentration of ethanol (40%, 60%, 80%, and 95%). These parameters were used to optimize the extraction of phenolic compounds from the sample by conventional solvent extraction method. In each optimization, two factors were kept constant and the third factor was considered as variable. During the extraction procedure, the samples were kept covered, in order to prevent evaporation of the solvent. At the end of each extraction, the samples were filtered using Whatman’s filter paper and the filtrate was used to determine total phenolic count of the extract at different parameters.
Phytochemical Analysis
Total Phenolics Content (TPC)
TPC content was measured by the method followed by Belwal et al. (Citation2016) with some modifications. The 0.5 ml of extract was mixed with 2.5 ml of 10% Folin–Ciocalteu reagent solution (v/v) and was kept for 8 min. 2 ml of 7.5% (w/v) sodium bicarbonate was added to this solution. The mixture was then kept in dark for 1 hr at room temperature. The absorbance was measured at 765 nm using UV-Vis spectrophotometer (Hitachi U-2001, Japan). The standard curve of gallic acid (100 µg/ml) was prepared in methanol and results were expressed in mg gallic acid/100 g dry weight of the sample.
Total Flavonoid Content
The total flavonoid content of jackfruit seed extract powder (JSE powder) was determined by the aluminum chloride colorimetric method as described by Wang et al. (Citation2018). The 250 µl of sample extract was mixed with 75 µl of 5% sodium nitrite. The mixture was kept at 27°C for 5 min and then, 125 µl of 2% aluminum chloride with 125 µl of 1 M sodium hydroxide was added. The mixture was incubated for 10 min followed by measurement of absorbance at 510 nm. The standard curve was made by using catechin (0.125–1.25 mg/ml) and total flavonoid content was calculated as µg catechin equivalent (CE)/ml of extract.
Condensed Tannin Content
The condensed tannin content or proanthocyanidins were determined using vanillin-HCl assay (Wang et al., Citation2018). The 200 µl of the sample was reacted with 3 ml of the mixture of 30% sulfuric acid and 4% vanillin prepared in methanol (1:1 v/v). The mixture was kept at room temperature for 20 min and absorbance was measured at 510 nm using UV-Vis spectrophotometer. Catechin (0.125–2 mg/ml) was used to plot a standard curve. The condensed tannin content was calculated as mg catechin equivalent (CE)/ml extract.
Antioxidant Capacity Assay
The antioxidant activity was determined by the method described in a previous study (Altemimi et al., Citation2016). 1 ml of samples extract was mixed with 3 ml of DPPH solution and was kept for 30 mins in a dark place. The absorbance was then measured at 517 nm using spectrophotometer. Blank was prepared using 1 ml of methanol mixed with 3 ml of DPPH. Percent inhibition was calculated using the formula given below:
(where Ab = absorbance of control and As = absorbance of sample)
Bioactive Profiling of Optimized JSP Extract by LC-Q-TOF-MS/MS
The characterization of the optimized JSP extract by LC-Q-TOF-MS/MS was done by the method described previously by Kadam et al. (Citation2018). The column used was an Agilent 6200 series Liquid Chromatography system utilizing a Zorbax Eclipse C18 (5 µm, 150 mm × 2.1 mm). The solvent system used was water 95% (Solvent A): acetonitrile 5% (Solvent B) with the following gradient of 2–20 min (A: 95%, B: 5%), 20–25 min (A: 5%, B: 95%), 26–30 min (A: 95%, B: 5%). The column temperature was 25°C with the flow rate of 0.3 ml/min. The total run time was of 30 min with 5 µl injection volume. An Agilent G6550A ultra high definition Accurate-Mass Quadrupole Time of Flight Mass Spectrophotometer using Agilent Mass Hunter Software version B.05.01 (B5125) was employed. Agilent personal Compound Database Library (PCDL) version B.05.01 build 92 was used to create the custom database. The mass spectrophotometer was operated in 2 GHz and the full scan covered the range from m/z 100 to 1000 m/z in the automatic mode. Sample ionization was achieved by Electrospray Ionization (ESI) interface in both positive and negative ion mode. In the negative ion mode, the gas and vaporizer temperatures were set to 250°C, with a gas flow rate of 13 L/min. The nebulizer was set to 35 psig with a corona current of 20 mA, fragmentor 175 V, skimmer 65 V, Octopole RF 750 V, and a capillary voltage of 5500 V.
Oxidative Stability Experiments
Frying Temperature Test
The frying oil test was performed according to the method of Sundaresan (Citation2015). The 4 mg of JSP extract and each antioxidant (BHT, vitamin-E acetate, curcumin, and vitamin E) were added into 20 gm of fresh cold-pressed peanut oil. All the samples of different oil mixtures were heated until the oil temperature reaches between 150°C and 180°C, on a hot plate for 2 hrs. The temperature was maintained by using a temperature sensor and manual stirring. The control sample without any added antioxidant was heated at the same temperature mentioned above. The experiments were performed in six parts and all the samples were cooled down to determine its peroxide value, p-anisidine value, and totox value.
Schaal Oven Test
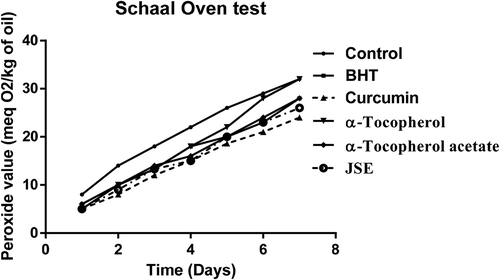
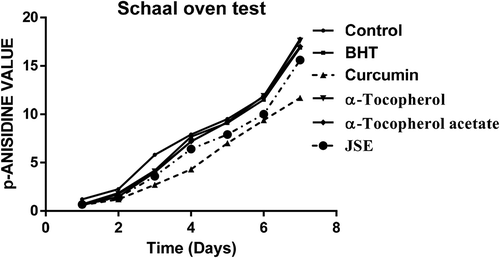
The Schaal oven test was performed according to the method of Poyato et al. (Citation2013). 10 mg of each antioxidant (BHT, vitamin-E acetate, curcumin, and vitamin E) and 10 mg of JSP extract was added into 50 gm of fresh cold-pressed peanut oil separately. The mixtures of all oil samples were stored in an oven at the temperature of 60°C for 7 days. All the oil samples were withdrawn periodically to check its peroxide value, p-anisidine value, and totox value at an interval of 24 h.
Oxidative Analysis
Peroxide Value
The peroxide value was determined according to the method described by Wang et al. (Citation2018). The 2.5 gm of oil samples was allowed to react with 15 ml of acetic acid-chloroform solution (3:2 v/v). The 0.25 ml of saturated potassium iodine solution was added and stirred on a magnetic stirrer for 1 min. Then, 15 ml of distilled water with 0.5 ml of 1% starch solution was added and titrated against the solution of 0.1 N sodium thiosulfate. The endpoint was purple to colorless. The peroxide value was calculated as the milli equivalent of oxygen per kg of oil or fat.
Peroxide value (meq O2/kg oil) = [(S-B) × N × 1000]/weight of sample in gram (where S = titration value of sample, B = titration of blank and N = equivalent concentration of sodium thiosulfate)
P-Anisidine Value
The p-anisidine value was determined according to the method described previously by Wang et al. (Citation2018). For anisidine value, 1 gm of oil sample was added in 25 ml of volumetric flask and the volume was made up to 25 ml with isooctane. The absorbance (Ab) of the solution was measured at 350 nm against blank as a pure isooctane. 5 ml of the oil solution with the isooctane from the above was reacted with 1 ml of p-anisidine reagent (0.25 g/100 ml glacial acetic acid) and absorbance (As) was measured against blank as the isooctane with the p-anisidine reagent at 350 nm.
Totox Value
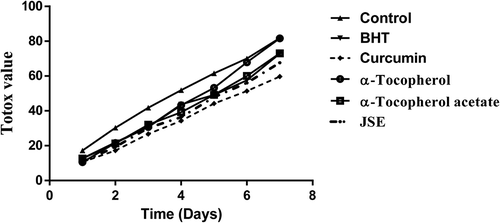
The totox value was calculated from the peroxide value (POV) and p-anisidine value (p-AV) of the above samples by the following equation (Sun-Waterhouse et al., Citation2011). The totox value was used to calculate overall oxidation status of oil.
Statistical Analysis
Data represents mean ± error bar. Statistical analysis was performed using the software “GraphPad Prism”- version 6.01. The significance of difference among the groups was assessed using a one-way analysis of variance (ANOVA) test followed by Tukey’s honest significant difference (HSD) post hoc test of difference between all group means.
Results and Discussion
Optimization of Extraction of JSP
For the extraction of JSP extract, ethanol was used, which serve as a common solvent for extraction of natural phenolic compounds. The mixture of water and alcohol is more efficient solvents for extracting phenolic compounds because of its minimal toxicity, high extraction yield, and environment-friendly characteristic than other pure organic solvents (Daniel et al., Citation2008).
To optimize solid to solvent ratio, the two factors, i.e., extraction time of 20 min and concentration of ethanol (95%) were kept constant. The solvent volume should be sufficiently high enough to dissolve all required compounds more effectively and to obtain a better yield of extraction (Prakash Maran et al., Citation2013). The high solvent to solute ratio increases the hydration and fragmentation process which enhances the mass transfer of the phytochemicals from the plant matrix to the solvent (Toma et al., Citation2001).
The JSP extract obtained possess TPC of 39.02, 83.07, 105.7, 115.45, and 120.1 μg/ml of extract using solute: solvent ratio of 0.5:25, 1:25, 1.5:25, 2:25, and 2.5:25 g of JSP/ml of ethanol, respectively (). The results indicate that solid:solvent ratio of 2.5:25 gram/ml had the highest TPC content and hence it is used as the optimized value of solid: solvent ratio.
Figure 1. Optimization condition for extraction of jackfruit seeds powder (JSP) extract. (a) TPC of solid: solvent ratio, (b) TPC of solutions with the different time period and (c) TPC of solutions with different concentration of ethanol
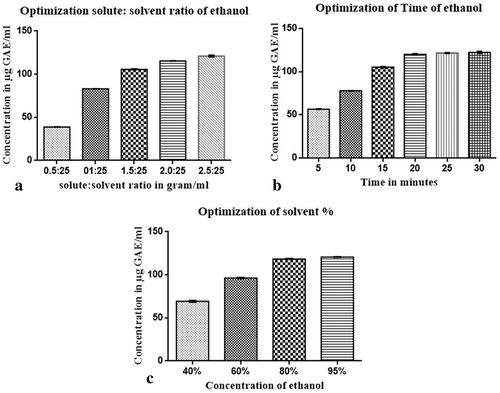
For the optimization of time the optimized solid:solvent ratio, i.e., 2.5:25 gm/ml and concentration of ethanol (95%) were kept constant. It has been observed that with the increase in the extraction time, the output of polyphenols from the plant matrix increases. The TPC of solution mixture were 56.1, 77.6, 104.3, 119.2, 121.1, and 121.9 μg/ml of extract of different time period of 5, 10, 15, 20, 25 and 30 minutes, respectively (), which is due to the fact that, as the time increases the cell disruption by solvent causes an increase in extraction of polyphenols.
For optimization of ethanol concentration the optimized solid: solvent ratio, i.e., 2.5:25 gm/ml and optimized time of 30 min were taken as a constant. It is found that 95% of ethanol had higher TPC than other concentration of ethanol. The TPC obtained were 68.42, 95.54, 117.98, and 122.17 μg/ml of extract of 40%, 60%, 80%, and 95% of ethanol, respectively (). Various studies have reported that the water and organic solvent mixture has great effects on polyphenols extraction. Both ethanol-water mixtures were suitable for the extraction of phenolic compounds because of different polarity of both solvents and were also safe for incorporation in food products (Waszkowiak and Gliszczyńska-Swigło, Citation2016). Binary-solvent system was more efficient according to the principle of “like dissolve like”, in which solvent extract only those compounds which have similar polarity like that of solvent (Chew et al., Citation2011). Thus, the final optimized condition for JSP extract was considered to be Solid: solvent ratio as 2.5:25 g/ml with the time period of 30 minutes in 95% ethanol concentration.
Phytochemical Analysis of Optimized JSP Extract
Phytochemical is naturally present in the plants and shows biologically significance by playing an essential role in the plants to defend themselves against various pathogenic microbes by showing the antimicrobial activity mediated through inhibition or killing mechanisms. These phytochemicals are efficient and reported to reduce the risk of cancer, possibly due to dietary fibers, polyphenolic antioxidants, and anti-inflammatory effects (Chang and Lin, Citation2011). In the present study, total phenolic content was found to be 122.17 ± 0.41 μg GAE/ml where GAE means gallic acid equivalent.
Flavonoids are the largest group of plant phenolics, and they are known to have high antioxidant activity along with many health benefits. The chemical structure of flavonoids has 15-carbon skeleton with two phenyl rings (A and B) linked to one heterocyclic rings (C) (Kumar and Pandey, Citation2013). The flavonoid content of optimized JSP extract obtained from the conventional extraction method for 30 mins in 95% ethanol was revealed to be 0.47 ± 0.02 mg CE/ml, where CE is the catechin equivalent.
The condensed tannin or proanthocyanidins are flavonoid-based phenolic compounds present in plants. The vanillin is an aromatic aldehyde compound. The vanillin assay involves the formation of red adduct when vanillin reacts with a meta-substituted ring of flavanols. The condensed tannin content of optimized JSP extract obtained from the conventional extraction method for 30 mins in 95% ethanol was found to be 0.34 ± 0.08 mg CE/ml.
The DPPH scavenging ability assay is commonly used to indicate the antioxidant activity of plant extract. The E50 signifies the concentration at which antioxidants used to scavenge 50% of initial radical. The smaller value of EC50 the better antioxidant ability it has. The EC50 value of optimized JSP extract obtained from the conventional extraction method for 30 mins in 95% ethanol was unveil to be 14.22 ± 0.9 µg/ml.
LC-Q-TOF-MS/MS Analysis
The LC-Q-TOF-MS/MS was performed to identify different phytochemical compounds of the JSP extract which was prepared in 95% ethanolic solvent. It is a highly sensitive analytical technique for separating bioactive compounds as compared to conventional LC method. It is a rapid method of metabolic analysis with proper peak separation.
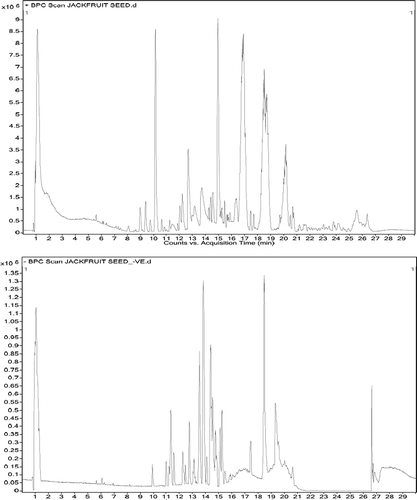
The chromatogram of LC-Q-TOF-MS/MS was relatively complex containing peaks of flavonoid, phenolic acid, stilbene, and other compounds (). Total 34 peaks were identified with the compounds of different classes of polyphenols in which, 14 phenolic acids, 17 flavonoids, and 3 stilbenes were identified (). The retention time of individual compounds was referred to analyze the MS/MS spectra. Peaks were identified in reference to the retention times, fragmentation patterns for an individual component.
Table 1. List of detected compounds from JSP extracts
MS spectral interpretation allowed the identification of 14 phenolic acid and their derivatives (peak 1, 2, 3, 5, 9, 15, 17, 19, 20, 23, 25, 31, 32 and 34) in which peak 1, 2, 3, 9, 15, 17,20, 23, 25, 31, and 34 were identified to be hydroxycinnamic acid such as, diferulic acid, benzyl (E)-Ferulate, trans-3-(Trifluoromethyl) cinnamic acid octadecyl ester, trans-3-Tri-fluoromethyl cinnamic acid 4-cyanophenyl ester, trans-cinnamic acid benzyl dimethylsilyl ester, trans-cinnamamide, N,N,-didecyl-3-trifluoromethyl, coumaroylquinic acid, 1-caffeoylquinic acid TMS, caffeic acid 3 TBDMS derivative, dihydroferulic acid 4-O-glucuronide, hydrocinnamic acid benzyl-dimethylsilyl ester according to pseudo molecular [M–H]¯ ion m/z at 386.36, 284.3, 468.63, 317.26, 296.43, 495.7, 337, 761.35, 522.94, 372.33, and 298.45, respectively.
The peak 1, 2 and 31 were identified as the ferulic acid and its derivatives which has many biological applications. It exhibits antioxidant activity by scavenging free radicals and possess anti-inflammatory properties. It also shows anti-diabetic effects by reducing the toxicity of streptozotocin in the pancreas and stabilizing free radicals (Kumar and Pruthi, Citation2014). It prominently displays anticancer activity against lung and colon cancer and is also reported to suppress the metastatic breast cancer cell line, i.e., MDA-MB (Zhang et al., Citation2016). It shows promising antitumor activity against osteosarcoma cells by inhibiting its proliferation and inducing apoptosis via inhibiting the P13 K/Akt pathway (Wang et al., Citation2016). A recent report established anti-wrinkle and whitening activity of ferulic acid and its derivatives when used as a functional food (Park et al., Citation2018).
The peak 20 was identified as p-Coumaric Acid derivative which was shown to possess anti-inflammatory activity against rheumatic arthritis on rat model (Zhu et al., Citation2018). It also harbors the antimicrobial activity (Naga et al., Citation2014) and protects vital organs against oxidative damages induced by Cisplatin in animal model by exhibiting high antioxidant activity (Ekinci Akdemir et al., Citation2017).
The peak 23 and 25 were identified as caffic acid derivatives, the intake of which results in increased plasma insulin with decreased urine output and blood sugar and thus found to be potential as anti-diabetic agent (Chao et al., Citation2010). It is also known for its antioxidant activity against hormesis by reducing reactive oxygen species (Li et al., Citation2015).
The peak 3, 9, 15, 17, and 34 were identified as cinnamic acid and its derivatives. These classes of the compound act as a strong reducing agent hence displays an excellent antioxidant activity. The amides derivatives have shown promising effect of radical scavenging in free radical-mediated diseases (Sova, Citation2012). They are potential antiviral agent and are well known for suppression of acute inflammation and inhibition of viral multiplication (Leonardo et al., Citation2001). Its anti-tumor activity is recently documented and was found to inhibit the growth of K562 cells of leukemia (Zhang et al., Citation2014). They have demonstrated anti-diabetic effects in various studies on the rat model in which it stimulates insulin secretion and improves glucose tolerance (Adisakwattana, Citation2017). They have also shown antibacterial activity against harmful pathogenic bacteria (Sova, Citation2012).
The peak 5, 19 and 32 are identified as hydroxybenzoic acids and their derivatives. Vanillic acid exerts anti-mutagenic activity by protecting DNA from damage (Almeida et al., Citation2016). Gallic acid act against the reactive oxygen species showing promising antioxidant effect. It harbors anti-tumor effect against lung cancer, leukemia breast cancer, and melanoma in in-vitro studies (Locatelli et al., Citation2013). The peak 4, 6 7 8 10 11 12 13 14 16 18 21 22 24 26 27 28 29 30, and 33 were identified as flavonoids and there derivatives, such as 5-methoxy flavones, Phloretin-2ʹ-O-xylosyl-glucoside, Sappanone A dimethyl ether, Cudraflavone A, Myricetin, Resorcinol 2 TBDMS Derivative, Licoflavone C, Rotenone, 4-methoxy-4ʹ-octoxy-trans-stilbene, e-Viniferin, (-)-Epigallocatechin 3-O-gallate, Flavanone 5-hydroxy-3ʹ,4ʹ,6,7,8-pentamethoxy, 3-O-glucosyl rhamnosyl galactoside, 4-Methoxy-5,7-dihydroxy isoflavone, Artocarpetin A, Dihydrodaidzein (keto), Quercetin-3-Rutinoside, Flavone,3,5,7-trihydroxy, bis-TMS, Apigenin-6-glucoside and 7-methoxy flavones with m/z ratio of 240.16, 568.52, 312.09, 418.44, 318.23, 338.63, 418.44, 394.41, 338.48, 454.24, 458.37, 390.38, 756.65, 284.26, 368.38, 256.25, 610.52, 414.59, 432.39, and 268.26, respectively.
The peak 4, 8, 12, 26, 29, 30 and 33 were identified as flavones and their derivatives. They are reported to exhibit high antioxidant, anti-inflammatory, and anticancer activity (Jiang et al., Citation2016). They harbor antimicrobial activity against both Gram positive and Gram negative bacteria (Naik et al., Citation2017). Apigenin has shown anticancer activity against colon, thyroid skin, and breast cancer in in-vitro studies (Yan et al., Citation2017).
The peak 10, 18, 22 and 28 were identified as flavonols and their derivatives. Myricetin acts against diabetes by executing insulin-mimetic effect and by exerting anti-inflammatory along with anti-oxidant activity (Li and Ding, Citation2012). In the murine model, it has shown to inhibit an UVB ray which leads to wrinkle and thus are known to exhibit anti-aging effects. It has shown anti-cancer activity against brain, breast, cervix, colon, leukemia, prostate, and uterus cancer cell lines (Semwal et al., Citation2016). The (-)-Epigallocatechin 3-O-gallate has shown anti-diabetic effects in rat with nephrectomy by suppressing hypoglycemia, lipid peroxidation, and proteinuria, and also work against renal damage and glucose toxicity (Yamabe et al., Citation2006). It has displayed anti-proliferative effects against human colorectal cancer (HCT-116) cell line by inducing apoptosis (Du et al., Citation2012). The EGCG and quercetin have shown chemoprotective effects (Jagtap et al., Citation2009). Quercetin demonstrates anticancer effects in colon cancer by inhibiting p21-Ras expression. It is evidently reported to present antioxidant effects by scavenging reactive oxygen species and also acts against allergic causing substances such as hay fever and hives, by stabilizing cell membrane (Lakhanpal and Rai, Citation2007). Kaempferol harbors antioxidant property by ameliorating free radicals and has shown admirable anticancer effect by inducing apoptosis followed by reduced inflammation, angiogenesis, and metastasis (Chen and Chen, Citation2013).
The peak 7, 13, 24 and 27 were identified as isoflavones and their derivatives. They are known for its anti-inflammatory effects owing to its antioxidant activity (Yu et al., Citation2016). The sappanone A has shown an anti-cancer effect against mouse B16 melanoma cells by inhibiting melanogenesis and suppressing tyrosinase activity (Chang et al., Citation2012).
The peak 6 was identified as chalcone class of flavanoid. The phloretin is found potential to scavenge hydroxyl radicals and reactive oxygen species indicating its antioxidant effects. It also inhibits cytokines, chemokines, and adhesion molecules of inflammation-promoting agents hence signifying anti-inflammatory effects (Behzad et al., Citation2017).
The peak 11, 14 and 16 were identified as stilbene and its derivatives. Resveratrol and their derivatives have reported to harbor many health benefits. They are involved in cardioprotective effects due to their high antioxidant activity and radical scavenging property. They also exert anticancerous effects by inhibition of tumor growth, cell proliferation, and inducing apoptosis (Ko et al., Citation2017).
The Effect of JSP Extract on Oxidative Stability of Groundnut Oil
Frying Temperature Test
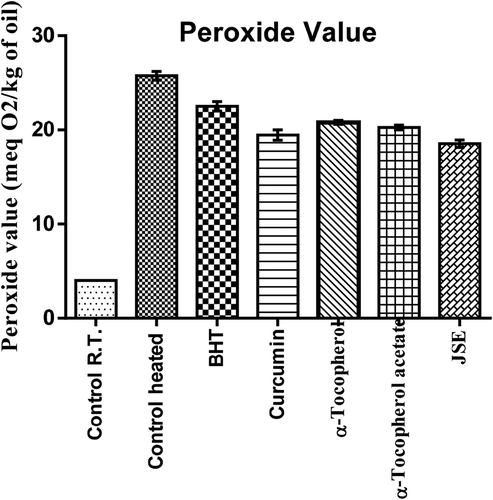
The groundnut oil was heated at the temperature of 150–180°C for 2 hrs to check the effect of JSP extract along with other antioxidants. The peroxide value indicates primary oxidation products in oil and it was found that peroxide value greatly increases after frying. The groundnut oil with the 200 ppm of tocopherol and BHT had minimal effects at the frying temperature. The curcumin and JSE was found to have lowest peroxide value. The JSE extract showed better performance than α-Tocopherol, synthetic antioxidant BHT, and α-Tocopherol-acetate (). In the present research investigation, it was revealed that oil with curcumin and JSP extract was most thermally stable at frying temperature and had low peroxide value than the other antioxidants and control (without any added antioxidant).
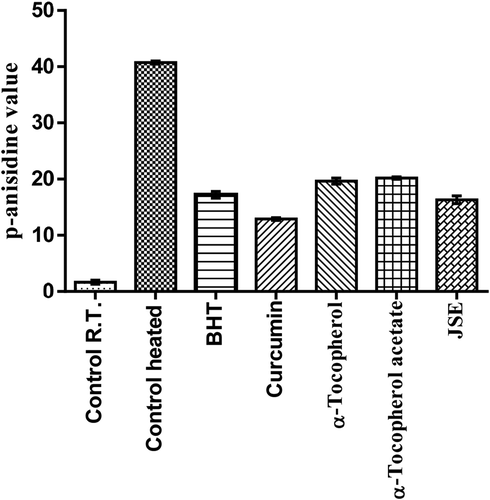
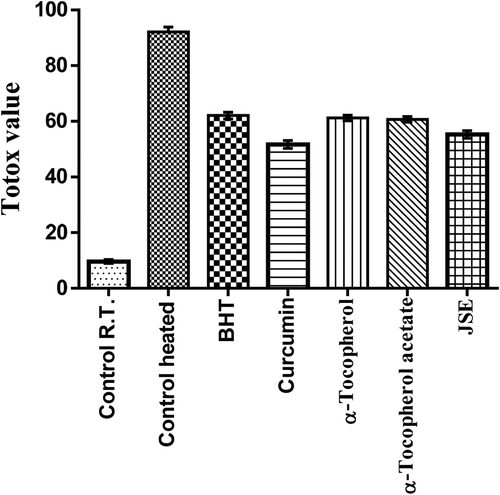
The thermal oxidation at frying temperature involves the formation of both primary and secondary products of oxidation. These secondary oxidation products are released due to the low peroxide’s stability at high temperature. These include the formation of aldehydes, ketones, and dienes (Halvorsen and Blomhoff, Citation2011). To detect these secondary oxidation products, the p-anisidine value (p-AV) was determined which provides more information on oil degradation at frying temperature. The p-AV of control shows more degradation in the 2 h than the oil with added antioxidants. The oil with curcumin and JSP extract had significant resistance toward formation of secondary oxidation products. The synthetic antioxidants exerted minimal effects on the stability of oil at frying temperature. The p-AV was highest in control followed by α-tocopherol-acetate, α-tocopherol, BHT, JSP extract, and curcumin (). The control which was reserved at room temperature had the lowest p-AV. The totox value gives an overall oxidative status of oil which unfolded the fact that the JSP extract and curcumin harbor superior oil stabilizing property than synthetic antioxidants (). Overall data obtained revealed that JSP extract had better antioxidant activity and significantly retarded oil oxidation at frying temperature.
Schaal Oven Test
Storage life or oxidative stability, until development of rancidity, is an important factor in the processing and marketing of fats, oils and fat-containing foods. Methods for determining oxidative stability are therefore essential, particularly where antioxidant is being investigated for effectiveness in retarding rancidity in these products. One widely used method involves storage tests in which oil, fat or food products are stored under controlled conditions and evaluated periodically for organoleptic (odor and flavor) changes. Storage test conducted under normal conditions of product use provide the most realistic determination of stability. A simplest accelerated method utilizes oven storage. The Schaal Oven Test at 145°F, is often used for evaluating fats, baked good and oils. Oven test at lower temperature are used for such products as essential oil and cheese spreads where elevated temperatures tend to distill volatile ingredients, break emulsions, or otherwise destroy original physical characteristics of the product. If supplementation of an antioxidant delays or completely prevents the changes in the physical and chemical composition of the food product then the antioxidant is contemplated to be effective (Geahart et al., Citation1957; Stuckey et al., Citation1958).
The groundnut oil with curcumin and JSE after Schaal oven test showed low peroxide value followed by α-Tocopherol-acetate, BHT and α-Tocopherol, and control (). Also, the synthetic antioxidants BHT and α-Tocopherol-acetate were not able to reduce the oxidation of oil incubated at the temperature of 60°C for 7 days. The addition of curcumin and JSP extract in oil effectively reduces the oxidation. α-Tocopherol was not very effective and had the same p-AV value as the control without antioxidant (). The totox value was also calculated to determine the overall oxidative status of groundnut oil in a Schaal oven test conditions at the 60°C. The totox value was highest in control without antioxidant followed by α-tocopherol, BHT, α-Tocopherol-acetate, JSP extract, and curcumin (). These results showed the ineffectiveness of synthetic antioxidants such as α-Tocopherol-acetate, and BHT in preventing oxidation process. The ineffectiveness of BHT supplementation was also studied in vegetable oils during intermitted heating by Tsaknis et al. (Citation2002). In present investigation, curcumin was found to be very effective at storage condition of schaal oven test for a week. Other research work is also published on the same lines expressing the effectiveness of curcumin at 0.03% concentration in 10 times fried oil by reducing the formation of trans fatty acids of oil (Andi and Eva, Citation2014). The color retention was seen higher in oil with curcumin, JSP extract, and α-tocopherol after frying of oil for 2 hr. The synthetic antioxidants, BHT, and α-tocopherol-acetate were completely turbid and lost their color (). Also, on determination of peroxide and p-anisidine levels, the results showed that the supplementation of 200 ppm of JSP extract significantly increases the oxidative stability of peanut oil. Thus, it can be used as a natural antioxidant to increase the stability of the oil at industrial scale.
Conclusion
A conventional extraction method for 30 mins at room temperature with a 95% ethanol solution presented the optimal extraction condition of jackfruit seed powder extract. The optimized JSP extract exhibited significantly higher content of total phenolic, flavonoid, and tannins. Various polyphenolic compounds such as phenolic acids, flavonoids, and stilbenes were identified from optimized JSP extract with the help of liquid chromatography coupled with Q-TOF-MS. It clearly indicates that the underutilized jackfruit seeds have many helpful phytochemical constituents which might prove beneficial in mitigating various oxidative stress associated diseases. The Schaal oven test of peanut oil with JSP extract at 60°C for 7 days showed significant effect in imparting stability and preventing oil oxidation than the BHT, α-Tocopherol, and α-Tocopherol-acetate. In presence of Curcumin or JSP extract, peanut oil at frying temperature of 150–180°C for 2 hrs showed better antioxidant ability than the oil with the addition of BHT, α-Tocopherol, and α-Tocopherol-acetate. Thus, JSP extract and curcumin can be used as a natural antioxidant in food products to retard their lipid oxidation and to improve their shelf life.
References
- Adisakwattana, S. 2017. Cinnamic acid and its derivatives: Mechanisms for prevention and management of diabetes and its complications. Nutrients 9(2):1. doi: 10.3390/nu9020163.
- Alireza, S., C.P. Tan, M. Hamed, and Y.B. Che Man. 2010. Effect of frying process on fatty acid composition and iodine value of selected vegetable oils and their blends. Int. Food Res. J. 17: 295–302.
- Almeida, I.V., F.M. Cavalcante, and V.E. Vicentini. 2016. Different responses of vanillic acid, a phenolic compound, in HTC cells: Cytotoxicity, antiproliferative activity, and protection from DNA-induced damage. Genet. Mol. Res. 15(4). doi: 10.4238/gmr15049388.
- Altemimi, A., D.G. Watson, R. Choudhary, M.R. Dasari, D.A. Lightfoot, and W.-X. Lin. 2016. Ultrasound assisted extraction of phenolic compounds from peaches and pumpkins. PLoS One 11(2):e0148758. doi: 10.1371/journal.pone.0148758.
- Anbudhasan, P., A. Surendraraj, S. Karkuzhali, and P. Sathishkumaran. 2014. Natural antioxidants and its benefits. Int. J. Food Nutr. Sci. 3(6):225–232.
- Andi, A., and J. Eva. 2014. Turmeric extract as an antioxidant in repeatedly used cooking oil. Int. J. Sci. Technol. Res. 3(12):347–350.
- Behzad, S., A. Sureda, D. Barreca, S.F. Nabavi, L. Rastrelli, and S.M. Nabavi. 2017. Health effects of phloretin: From chemistry to medicine. Phytochemistry Reviews 16(3):527–533. doi: 10.1007/s11101-017-9500-x.
- Belwal, T., P. Dhyani, I.D. Bhatt, R.S. Rawal, and V. Pande. 2016. Optimization extraction conditions for improving phenolic content and antioxidant activity in Berberis asiatica fruits using response surface methodology (RSM). Food Chem. 207:115–124. doi: 10.1016/j.foodchem.2016.03.081.
- Chang, C.L., and C.S. Lin. 2011. Phytochemical composition, antioxidant activity, and neuroprotective effect of terminalia chebula retzius extracts. Evid. Based Compl. Alternat. Med. 2012:125247. doi: 10.1155/2012/125247.
- Chang, T.-S., S.-Y. Chao, and H.-Y. Ding. 2012. Melanogenesis inhibition by homoisoflavavone sappanone A from Caesalpinia sappan. Int. J. Mol. Sci. 13(8):10359–10367. doi: 10.3390/ijms130810359.
- Chao, C.-Y., M.-C. Mong, K.-C. Chan, and M.-C. Yin. 2010. Anti-glycative and anti-inflammatory effects of caffeic acid and ellagic acid in kidney of diabetic mice. Mol. Nutr. Food Res. 54(3):388–395. doi: 10.1002/mnfr.200900087.
- Chen, A.Y., and Y.C. Chen. 2013. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chemistry 138(4):2099–2107. doi: 10.1016/j.foodchem.2012.11.139.
- Chew, K.K., M.Z. Khoo, S.Y. Ng, Y.Y. Thoo, W.M. Wan Aida, and C.W. Ho. 2011. Effect of ethanol concentration, extraction time and extraction temperature on the recovery of phenolic compounds and antioxidant capacity of Orthosiphon stamineus extracts. Int. Food Res. J. 18(4):1427–1435.
- Daniel, F., S. Jorge, R. Monica, S. Marivel, J. Maria, P. Manuel, C. Noelia, and J.N. Maria. 2008. Polyphenols from plant materials: Extraction and antioxidant power. EJEAF Che 7(8):3210–3216.
- Du, G.-J., Z. Zhang, X.-D. Wen, C. Yu, T. Calway, C.-S. Yuan, and C.-Z. Wang. 2012. Epigallocatechin gallate (EGCG) is the most effective cancer chemopreventive polyphenol in green tea. Nutrients 4(11):1679–1691. doi: 10.3390/nu4111679.
- Ekinci Akdemir, F.N., M. Albayrak, M. Calik, Y. Bayir, and İ. Gulçin. 2017. The Protective effects of p-coumaric acid on acute liver and kidney damages induced by Cisplatin. Biomedicines 5(4):18. doi: 10.3390/biomedicines5020018.
- Falade, A.O., and G. Oboh. 2015. Thermal oxidation induces lipid peroxidation and changes in the physicochemical properties and β-carotene content of arachis oil. Int. J. Food Sci. 2015:1–7. doi: 10.1155/2015/806524.
- Geahart, W.M., B.N. Stuckey, and J.J. Austin. 1957. Comparison of methods for testing the stability of fats and oils, and of foods containing them. JAOCS 34(9):427–430.
- Gharras, H.E. 2009. Polyphenols: Food sources, properties and applications - A review. Int. J. Food Sci. Technol. 44(12):2512–2518. doi: 10.1111/j.1365-2621.2009.02077.x.
- Halvorsen, B.L., and R. Blomhoff. 2011. Determination of lipid oxidation products in vegetable oils and marine omega-3 supplements. Food Nutr. Res. 55:10.3402. doi: 10.3402/fnr.v55i0.5792.
- Hussain, T., B. Tan, Y. Yin, F. Blachier, M.C. Tossou, and N. Rahu. 2016. Oxidative stress and inflammation: What polyphenols can do for us? Oxid. Med. Cell. Longev. 7432797. doi: 10.1155/2016/7432797.
- Jagtap, S., K. Meganathan, V. Wagh, J. Winkler, J. Hescheler, and A. Sachinidis. 2009. Chemoprotective mechanism of the natural compounds, epigallocatechin- 3-O-gallate, quercetin and curcumin against cancer and cardiovascular diseases. Curr. Med. Chem. 16(12):1451–1462. doi: 10.2174/092986709787909578.
- Jiang, N., Doseff, A. I., & Grotewold, E. 2016. Flavones: From Biosynthesis to Health Benefits. Plants (Basel, Switzerland), 5(2), 27. https://doi.org/10.3390/plants5020027
- Kadam, D., S. Palamthodi, and S.S. Lele. 2018. LC–ESI-Q-TOF–MS/MS profiling and antioxidant activity of phenolics from L. Sativum seedcake. Journal of Food Science and Technology 55(3):1154–1163. doi: 10.1007/s13197-017-3031-8.
- Ko, J. H., Sethi, G., Um, J. Y., Shanmugam, M. K., Arfuso, F., Kumar, A. P., Bishayee, A., & Ahn, K. S. 2017. The Role of Resveratrol in Cancer Therapy. International journal of molecular sciences, 18(12), 2589. https://doi.org/10.3390/ijms18122589
- Kumar, N., and V. Pruthi. 2014. Potential applications of ferulic acid from natural sources. Biotechnology Reports. 4:86–93. doi: 10.1016/j.btre.2014.09.002.
- Kumar S, Pandey, S.K. 2013. Chemistry and biological activities of flavonoids: An overview. Sci. World J.2013 1–16.
- Lakhanpal P, Rai, D.K. 2007. Quercetin: A versatile flavonoid. Internet J. Med. Update. 2(2).22-37.
- Leonardo, S., F. Craig, and G. Silvia. 2001. Hydroxycinnamic acids as natural antioxidants. Sci. Technol. 83:1–5.
- Li, Y., L.J. Chen, F. Jiang, Y. Yang, X.X. Wang, Z. Zhang, Z. Li, and L. Li. 2015. Caffeic acid improves cell viability and protects against DNA damage: Involvement of reactive oxygen species and extracellular signal-regulated kinase. Braz. J. Med.Biol. Res. 48(6):502–508. doi: 10.1590/1414-431X20143729.
- Li, Y., and Y. Ding. 2012. Minireview: Therapeutic potential of myricetin in diabetes mellitus. Food Sci. Hum. Wellness 1(1):19–25. doi: 10.1016/j.fshw.2012.08.002.
- Locatelli, C., F. Filippin Monteiro, A. Centa, and T. Creczynski-Pasa. 2013. Antioxidant, antitumoral and anti-inflammatory activities of gallic acid. In: Handbook on gallic acid: Natural occurrences, antioxidant properties and health implications.Nova pulishing Editors: Michelle A. Thompson, Parker B. Collins, pp.1-23
- Naga, V.K., M.D. Nadeem, M. Pardha Saradhi, B. Mahendran, and S. Bharathi. 2014. Cumulative activity of the p-coumaric acid and syringaldehyde for antimicrobial activity of different microbial strains. Eur. J. Exp. Biol. 4(6):40–43.
- Naik, K., S. Thangavel, A. Alam, and S. Kumar. 2017. Flavone analogues as antimicrobial agents. Recent Pat. Inflamm. Allergy Drug Discov. 11(1):53–63. doi: 10.2174/1872213X11666170119094702.
- Pandey, K.B., and S.I. Rizvi. 2009. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2(5):270–278. doi: 10.4161/oxim.2.5.9498.
- Poyato, C., I. Navarro-Blasco, I. Calvo, Y. Cavero, I. Astiasarán, and D. Ansorena. 2013. Oxidative stability of O/W and W/O/W emulsions: Effect of lipid composition and antioxidant polarity. Food Res. Int. 51(1):132–140. doi: 10.1016/j.foodres.2012.11.032.
- Park, H., Cho, J., Hong, S. et al. Whitening and anti-wrinkle activities of ferulic acid isolated from Tetragonia tetragonioides in B16F10 melanoma and CCD-986sk fibroblast cells. J Nat Med 72, 127–135 (2018). https://doi.org/10.1007/s11418-017-1120-7
- Prakash Maran J, Sivakumar V, Thirugnanasambandham K, Sridhar R (2013) Optimization of microwave assisted extraction of pectin from orange peel. Carbohydr Polym 97:703–709
- Ranganathan, A.A. 2018. Jackfruit taxonomy and waste utilization. Vegetos 31(1):67–73.
- Semwal, D.K., R.B. Semwal, S. Combrinck, and A. Viljoen. 2016. Myricetin: A dietary molecule with diverse biological activities. Nutrients 8(2):90. doi: 10.3390/nu8020090.
- Sova, M. 2012. Antioxidant and antimicrobial activities of cinnamic acid derivatives. Mini-Rev. Med. Chem. 749–767. doi: 10.2174/138955712801264792.
- Stuckey, B.N., E.R. Sherwin, and F.D. Hannah Jr. 1958. Improved techniques for testing fats and oils by the oxygen bomb method. Journal of the American Oil Chemists Society 35(11):581–584. doi: 10.1007/BF02633811.
- Sundaresan, C.R. 2015. Influence of natural and synthetic antioxidants on the degradation of Soybean oil at frying temperature. J. Food Sci. Technol. 52(8):5370–5375.
- Sun-Waterhouse, D., J. Zhou, G.M. Miskelly, R. Wibisono, and S.S. Wadhwa. 2011. Stability of encapsulated olive oil in the presence of caffeic acid. Food Chem. 126(3):1049–1056. doi: 10.1016/j.foodchem.2010.11.124.
- Toma, M., M. Vinatoru, L. Paniwnyk, and T.J. Mason. 2001. Investigation of the effects of ultrasound on vegetal tissues during solvent extraction. Ultrason. Sonochem. 8(2):137–142. doi: 10.1016/s1350-4177(00)00033-x.
- Tsaknis, J., S. Lalas, and E. Protopapa. 2002. Effectiveness of the antioxidants BHA and BHT in selected vegetable oils during intermittent heating. Grasas Aceites. 53(2): 199–205.
- Wang, T., X. Gong, R. Jiang, H. Li, W. Du, and Kuang. 2016. Ferulic acid inhibits proliferation and promotes apoptosis via blockage of PI3K/Akt pathway in osteosarcoma cell. Am. J. Transl. Res. 8(2) :968–980.
- Wang, Y.-Z., S.-G. Fu, S.-Y. Wang, D.-J. Yang, Y.-H.S. Wu, and Y.-C. Chen. 2018. Effects of a natural antioxidant, polyphenol-rich rosemary (Rosmarinus officinalis L.) extract, on lipid stability of plant-derived omega-3 fatty-acid rich oil. LWT - Food Sci. Technol. 89:210–216. doi: 10.1016/j.lwt.2017.10.055.
- Waszkowiak, K., and A. Gliszczyńska-Swigło. 2016. Binary ethanol–water solvents affect phenolic profile and antioxidant capacity of flaxseed extracts. Eur. Food Res. Technol. 242(5):777–786. doi: 10.1007/s00217-015-2585-9.
- Yamabe, N., T. Yokozawa, T. Oya, and M. Kim. 2006. Therapeutic potential of (-)-epigallocatechin 3-O-gallate on renal damage in diabetic nephropathy model rats. J. Pharmacol. Exp. Ther. 319(1):228–236. doi: 10.1124/jpet.106.107029.
- Yan, X., M. Qi, P. Li, Y. Zhan, and H. Shao. 2017. Apigenin in cancer therapy: Anti-cancer effects and mechanisms of action. Cell & Bioscience 7(1):50. doi: 10.1186/s13578-017-0179-x.
- Yu, J., Bi, X., Yu, B., & Chen, D. 2016. Isoflavones: Anti-Inflammatory Benefit and Possible Caveats. Nutrients, 8(6), 361. https://doi.org/10.3390/nu8060361
- Zhang, J., A. Xiao, T. Wang, X. Liang, J. Gao, P. Li, and T. Shi. 2014. Effect and mechanism of action of cinnamic acid on the proliferation and apoptosis of leukaemia cells. Biomed. Res. 25(3):405–408.
- Zhang, X., D. Lin, R. Jiang, H. Li, J. Wan, and H. Li. 2016. Ferulic acid exerts antitumor activity and inhibits metastasis in breast cancer cells by regulating epithelial to mesenchymal transition. Oncol. Rep. 36(1):271–278. doi: 10.3892/or.2016.4804.
- Zhu, H., Q.H. Liang, X.G. Xiong, Y. Wang, Z.H. Zhang, M.J. Sun, X. Lu, and D. Wu. 2018. Anti-inflammatory effects of p-coumaric acid, a natural compound of Oldenlandia diffusa, on arthritis model rats. Evid. Based Compl. Alternat. Med. 5198594. doi: 10.1155/2018/5198594.