?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In this study, the effects of methanol, acetone, and ethyl acetate solvents on total phenolic content and antioxidant activity in Diospyros blancoi seeds extract were examined. Maceration extraction technique was selected in extracting the seeds. Determination of total phenolic content (TPC) and antioxidant activity used Folin-Ciocalteu and 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay, respectively. Radical scavenging activity data were interpreted from the calculations of the half maximal inhibitory concentration (IC50) values. TPC was found to be higher (603.43 ± 0.75 mg gallic acid equivalent/g dry powder) in D. blancoi seeds in the acetone extract compared to the methanol and ethyl acetate extracts. The highest antioxidant activity for D. blancoi seeds extracts was in methanol solvent (IC50: 2.72 μg/mL), with IC50 of ascorbic acid as a positive control was 3.34 μg/mL. These results indicate that TPC contributes to antioxidant activity in D. blancoi seeds.
Introduction
The oxidative stress or oxidative damage is caused by oxygen free radicals and other reactive oxygen species which damage living tissues and cellular components in biological systems (Anthony and Saleh, Citation2013; Nimse and Pal, Citation2015). In biological systems, oxidative stress arises when the ratios of oxidants and endogenous antioxidants are unbalanced. In oxidative stress conditions, cellular constituents of our body are altered, resulting in different disease states (Nimse and Pal, Citation2015). Proteins, lipids, enzymes, and DNA are usually undergoing oxidative damage by covalent binding and lipid peroxidation (Baba and Malik, Citation2015). Antioxidants that can be found in fruits and vegetables, control and reduce the oxidative damage by delaying or inhibiting free radical reactions and cellular damage (Altemimi et al., Citation2017; Nimse and Pal, Citation2015). Hydroxyl groups of phenolic compounds are good electron donors contribute to antioxidant action (Aryal et al., Citation2019). Phenolic compounds exhibit free radical inhibition, metal inactivation or oxygen scavenging, peroxide decomposition, and prevent oxidative disease burden (Babbar et al., Citation2015).
Diospyros blancoi also designated Diospyros discolor, is an endemic Philippine tree cultivated for its commercial fruits (Hung et al., Citation2015; Lee et al., Citation2012). In Indonesia, D. blancoi is locally known as “Bisbul” was brought from the Philippines and only cultivated in the Bogor region (Arrisujaya et al., Citation2019). The bark is traditionally used for the treatment of coughs, fevers, dysentery, and diarrhea. The fruit is used to treat wounds and as a gargle in cases of aphthous stomatitis (Lee et al., Citation2012). D. blancoi plant parts (fruit, bark, leaf, and root) have been widely studied to have parthenocarpic nature (Hung et al., Citation2015), analgesic (Akter et al., Citation2015), anti-asthmatic and anti-inflammatory (Lee et al., Citation2012), antimicrobial (Akter and Sarker, Citation2015), anticancer (Khan et al., Citation2016), and antioxidant activity (Das et al., Citation2010), but there are very few on the antioxidant properties of D. blancoi seeds.
Seeds have been widely studied because of their high content of proteins and oils. Several previous reports contain a high number of contributions dealing with the bioactive compound content and antioxidant capacity of various kinds of seeds. Several seeds represent a potential rich source of phenolic compounds and antioxidant activity like seeds of date (Liu et al., Citation2013), pumpkin (Saavedra et al., Citation2013), quinoa (Carciochi and Dimitrov, Citation2014), black beans, canola, foxtail millet (Chandrasekara et al., Citation2016), litchi, oat (Paliga et al., Citation2017), chia (Pellegrini et al., Citation2018), and fennel (Houhou et al., Citation2018). However, Knowledge of the potential of D. blancoi seeds extract may help increase the contribution to medicinal plant study and their pharmaceutical applications.
D. blancoi seeds is one of agricultural waste products from D. blancoi plants. Several studies have shown that they are abundant in natural bioactive compounds (Akter and Sarker, Citation2015; Das et al., Citation2010; Hung et al., Citation2015; Khan et al., Citation2016; Lee et al., Citation2012). The extraction of bioactive compounds in D. blancoi seed is therefore essential for further analysis and its development. Extract solvent is one of the most important factors influencing plant extracts ‘ chemical composition and biological activity (Do et al., Citation2014; Zhang, Citation2015; Zhu et al., Citation2011). The purpose of this study was then to examine the effect of different polarity extraction solvents (methanol, acetone, and ethyl acetate) on total phenolic content and antioxidant activity of D. blancoi seeds extract using Folin-Ciocalteu and 2,2-diphenyl-1-picrylhydrazyl (DPPH) assays, respectively.
Materials and Methods
Sample Preparation
D. blancoi fruits were bought at a traditional market in Bogor, Indonesia. D. blancoi seeds were cleaned, sundried, ground and mashed into powder using grinder machine and passed through a 100-mesh sieve. The powder was dried in an oven at 40°C for 8 hours and then vacuum packed and stored at room temperature prior to extraction.
Extract of D. Blancoi Seeds
The dried powder (150 g) was diluted in 450 mL of (a) methanol (b) acetone (c) ethyl acetate at room temperature for 4 × 24 hours by maceration technique. The solid-liquid mixtures were filtered separately with Whatman® qualitative filter paper number 1. The final extracts were concentrated with a rotary flash evaporator under reduced pressure at 50°C. All dried extracts were stored at 4°C for further tests.
Determination of Total Phenolic
Total phenolic content (TPC) was measured using spectrophotometric assays with the Folin-Ciocalteu method (Khoddami et al., Citation2013), with minor modifications. The calibration curve was established using gallic acid (10–40 μg/mL). Folin-Ciocalteu reagent was diluted with distilled water at ratio 1:2 v/v. An aliquot of the diluted extract or gallic acid was mixed with 1 mL folin-ciocalteu reagent and mixed thoroughly for 5 minutes. Sodium carbonate (5% w/v, 1 mL) was added to the mixture and allowed to stand for 1 hour at 25°C inside a dark cabin. Absorbance was then measured at 725 nm using UV-VIS spectrophotometer (Optizen POP, Mecasys, Korea). TPC was expressed as milligram gallic acid equivalent (GAE) per gram of dried extract.
2,2-diphenyl-1-picrylhydrazyl (DPPH) Assay
The antioxidant activity of the extract was measured with the DPPH method (Khan et al., Citation2016) with slight modifications. An aliquot of 1 mL the diluted extract in methanol at different concentrations was mixed with 2 mL a solution of DPPH (0.1 mmol/L) in methanol. The mixture was then incubated in the dark at room temperature for 30 minutes. Absorbance was measured at 515 nm using UV-VIS spectrophotometer and ascorbic acid was used as a reference. The percentage of inhibition of free radicals was calculated as follows:
where, A0 is the absorbance of DPPH solution without extract, and A1 is the absorbance of sample or standard with DPPH solution. Antioxidant activity was expressed as the half maximal inhibitory concentration (IC50), which represents the concentrations of the antioxidant or extracts require decreasing 50% of the initial DPPH concentration. IC50 values were calculated from the graph between the percent of inhibition against concentration, and all tests were performed at least in triplicate.
Statistical Analysis
All analyzes were carried out in triplicate and the data were presented as average values along with their standard derivations. Microsoft Excel 2010 (Roselle, IL, USA) was used to analyzing the data. Statistical comparisons were performed using one-way analysis of variance (ANOVA) and p values < .05 were regarded as significant.
Results and Discussion
Total Phenolic Content
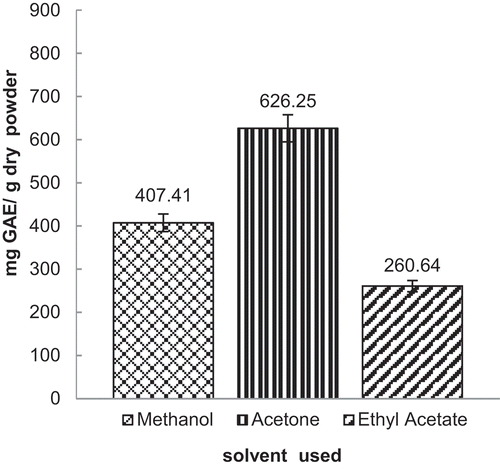
The TPC of extracts from D. blancoi seeds using the three commonly used solvents were shown in . TPC values were obtained from the calibration curve y = 0.0401x + 0.0005 with R2 = 0.9987, where x is the absorbance and y is the concentration of a gallic acid solution expressed as mg GAE/g dry powder extract. The TPC of D. blancoi seeds were in the range of 260.64–603.43 mg GAE/g dry powder. The most effective solvent that produced the highest TPC was acetone, followed by methanol and ethyl acetate. A high TPC in other plant extract has been reported in acetone solvent as well (Chandrasekara et al., Citation2016; Zhang, Citation2015). The different solvents, as the difference in polarity, dispersibility, and penetrability, could selectively extract TPC with different values (Zhang, Citation2015). Solvent polarity is well known to play a key role in the increase of phenolic solubility (Ullah et al., Citation2019).
The present study further compares TPC in other parts of the D. blancoi plant shown in . The TPC of D. blancoi seeds was higher (>260 mg GAE/g dry powder) than the other parts on this plant. The results show that this seed was potentially rich sources of phenolic compounds.
Table 1. Comparison of data on TPC between the seeds and other parts of D. blancoi plant
DPPH Radical Scavenging Activity
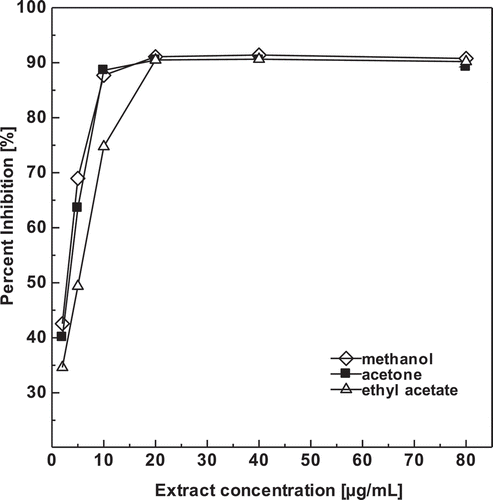
DPPH with an absorbance at 515 nm is a stable free radical that can accept an electron or hydrogen donors to become a stable diamagnetic molecule (Nimse and Pal, Citation2015). It loses this absorption when receiving an electron or a free radical species, which results in discoloration of solution from purple to yellow (Do et al., Citation2014). The solution loses color with increasing concentration antioxidants as electrons taken by DPPH radicals from the antioxidant (Nimse and Pal, Citation2015). DPPH scavenging activities of the D. blancoi seeds extracts in depends on the difference in concentration of was showed in . All extracts produced the highest DPPH radical scavenging activity at concentrations ranging from 2–20 μg/mL (p > .05). However, DPPH radical scavenging activity did not significantly different at concentrations ranging from 20–80 μg/mL (p < .05). The same trend was reported in the study of DPPH radical scavenging activity in other plants (Do et al., Citation2014; Zhu et al., Citation2011)
Antioxidant Activity (IC50)
The IC50 represents the effective concentration of the extract at which DPPH radical scavenging ability up to 50% (Zhang, Citation2015). . shows the IC50 values in the antioxidant activity of D. blancoi seeds extracts, which was obtained by the linear regression equation. Higher antioxidant activity of a compound is indicated by the low IC50 value. It was found that the extract of the three variant solvents possesses DPPH radical activity, which was a high antioxidant category. The IC50 values of all three extracts were in the range of 2.72–5.09 μg/mL.
Table 2. Antioxidant activity (IC50) of D. blancoi seeds extracts in three variant solvents
Phenolic compounds play dynamic roles in delaying or inhibiting free radical reactions and cellular damage (Altemimi et al., Citation2017; Nimse and Pal, Citation2015). In this study, A negative correlation (R = −0.7569) is expected for a good correlation since the analysis was carried out using IC50. The lower the IC50 value, the higher the antioxidant activities. Therefore, the higher the TPC values, it is expected that the IC50 should be inverse (lower) for it to have good correlation. The findings of previous studies on antioxidant activity from other plants can be attributed to various factors such as processes, extract conditions and others (Do et al., Citation2014). However, D. blancoi seeds extracts of all three variant solvents possess high TPC and antioxidant activity. It was also observed that antioxidant activity and TPC of acetone and methanol extracts are higher than the ethyl acetate extract.
Conclusion
The antioxidant activity result indicates that the extracts of all three variant solvents were the source of natural antioxidants with very potent antioxidant categories. Generally, D. blancoi seeds extracts possess high TPC values. Acetone solvent extracted the highest TPC in D. blancoi seeds. The highest antioxidant activity was extracted using methanol solvent, indicated by the smallest IC50 value. Ethyl acetate is a poor solvent extract, with the lowest antioxidant activity and TPC value. In conclusion, the results of this work have shown that a proper extracting solvent is important for obtaining the high antioxidant activity of D. blancoi seeds. The use of D. blancoi seeds as a source of alternative medicinal ingredients after a quantitative investigation of compounds may be established in the future.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Acknowledgments
This work was partially funded by the Penelitian Dosen Pemula scheme, research fund academic year 2018, under contract numbers: 110/SP2H/LT/DRPM/2019, and 2656/L4/PP/2019, from Ministry of Research, Technology and Higher Education of the Republic of Indonesia.
Disclosure Statement
Dian Arrisujaya has received research grants from the Ministry of Research, Technology and Higher Education of the Republic of Indonesia. Devy Susanty declares that she has no conflict of interest. Lisnawati Tri Hastuti declares that she has no conflict of interest.
Additional information
Funding
References
- Akter, S., T. Majumder, R. Karim, Z. Ferdous, and M. Sikder. 2015. Analgesic activities of geodorum densiflorum, diospyros blancoi, baccaurea ramiflora and trichosanthes dioica. J. Pharmacogn. Phytochem. 4(3):209–214.
- Akter, S., and A. Sarker. 2015. Antimicrobial activities of seeds of diospyros blancoi and baccuarea ramiflora. Int. J. Adv. Pharm. Bio. Chem. 4(4):789–793.
- Altemimi, A., N. Lakhssassi, A. Baharlouei, D.G. Watson, and D.A. Lightfoot. 2017. Phytochemicals: extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 6(4):42. doi: 10.3390/plants6040042.
- Anthony, K.P., and M.A. Saleh. 2013. Free radical scavenging and antioxidant activities of silymarin components. Antioxidants 2(4):398–407. doi: 10.3390/antiox2040398.
- Arrisujaya, D., N. Ariesta, and M. Maslahat. 2019. Removal of chromium (VI) from aqueous solutions using diospyros discolor seed activated with nitric acid: isotherm and kinetic studies. Water Sci. Technol. 79(6):1214–1221. doi: 10.2166/wst.2019.125.
- Aryal, S., M.K. Baniya, K. Danekhu, P. Kunwar, R. Gurung, and N. Koirala. 2019. Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from Western Nepal. Plants 8(4):96. doi: 10.3390/plants8040096.
- Baba, S.A., and S.A. Malik. 2015. Determination of total phenolic and flavonoid content, antimicrobial and antioxidant activity of a root extract of arisaema jacquemontii blume. J. Taibah Univ. Sci. 9(4):449–454. doi: 10.1016/j.jtusci.2014.11.001.
- Babbar, N., H.S. Oberoi, and S.K. Sandhu. 2015. Therapeutic and nutraceutical potential of bioactive compounds extracted from fruit residues. Crit. Rev. Food Sci. Nutr. 55(3):319–337. doi: 10.1080/10408398.2011.653734.
- Carciochi, R.A., and K. Dimitrov. 2014. Optimization of antioxidant phenolic compounds extraction from quinoa (Chenopodium Quinoa) seeds. J. Food Sci. Technol. 52(7):4396–4404. doi: 10.1007/s13197-014-1514-4.
- Chandrasekara, A., O.A. Rasek, J.A. John, N. Chandrasekara, and F. Shahidi. 2016. Solvent and extraction conditions control the assayable phenolic content and antioxidant activities of seeds of black beans, canola and millet. JAOCS, J. Am. Oil Chem. Soc. 93(2):275–283. doi: 10.1007/s11746-015-2760-y.
- Das, S.C., K. Hamid, I.J. Bulbul, and S. Sultana. 2010. In vitro antioxidant activity of different parts of the plant diospyros discolor assessment of free radical scavenging activity by. Res. J. Agric. Biol. Sci. 6(4):472–475.
- Do, Q.D., A.E. Angkawijaya, P.L. Tran-Nguyen, L.H. Huynh, F.E. Soetaredjo, S. Ismadji, and Y.H. Ju. 2014. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of limnophila aromatica. J. Food Drug Anal. 22(3):296–302. doi: 10.1016/j.jfda.2013.11.001.
- Houhou, M., K.A. Joutei, C. Rais, and S. Louahlia. 2018. Phenolic compounds, antioxidant and antibacterial activities are affected by sulfur deficiency in foeniculum vulgare seeds. J. Crop Sci. Biotechnol. 21(3):241–247. doi: 10.1007/s12892-018-0077-0.
- Hung, S.F., I.Z. Chen, and S.F. Roan. 2015. Preliminary results of fruit selection and induced parthenocarpy of mabolo (Diospyros Blancoi A. DC.). Genet. Resour. Crop Evol. 62(8):1127–1134. doi: 10.1007/s10722-015-0316-5.
- Khan, M.A., M.M. Rahman, M.N. Sardar, M.S.I. Arman, M.B. Islam, M.J.A. Khandakar, M. Rashid, G. Sadik, and A.H.M.K. Alam. 2016. Comparative investigation of the free radical scavenging potential and anticancer property of diospyros blancoi (Ebenaceae). Asian Pac. J. Trop. Biomed. 6(5):410–417. doi: 10.1016/j.apjtb.2016.03.004.
- Khoddami, A., M.A. Wilkes, and T.H. Roberts. 2013. Techniques for analysis of plant phenolic compounds. Molecules 18(2):2328–2375. doi: 10.3390/molecules18022328.
- Lee, K.Y., J.Y. Jung, M.Y. Lee, D. Jung, E.S. Cho, and H.Y. Son. 2012. Diospyros blancoi attenuates asthmatic effects in a mouse model of airway inflammation. Inflammation 35(2):623–632. doi: 10.1007/s10753-011-9354-0.
- Liu, H., Z. Jiao, J. Liu, C. Zhang, X. Zheng, S. Lai, F. Chen, and H. Yang. 2013. Optimization of supercritical fluid extraction of phenolics from date seeds and characterization of its antioxidant activity. Food Anal. Methods 6(3):781–788. doi: 10.1007/s12161-012-9486-3.
- Nimse, S.B., and D. Pal. 2015. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 5(35):27986. doi: 10.1039/c4ra13315c.
- Paliga, M., Z. Novello, R.M. Dallago, J. Scapinello, J.D. Magro, M.D. Luccio, M.V. Tres, and J. Vladimir Oliveira. 2017. Extraction, chemical characterization and antioxidant activity of litchi chinensis sonn. and avena sativa L. Seeds extracts obtained from pressurized n-butane. J. Food Sci. Technol. 54(3):846–851. doi: 10.1007/s13197-016-2485-4.
- Pellegrini, M., R. Lucas-Gonzalez, E. Sayas-Barberá, J. Fernández-López, J.A. Pérez-Álvarez, and M. Viuda-Martos. 2018. Bioaccessibility of phenolic compounds and antioxidant capacity of chia (Salvia Hispanica L.) seeds. Plant Foods Hum. Nutr. 73(1):47–53. doi: 10.1007/s11130-017-0649-7.
- Saavedra, M.J., A. Aires, C. Dias, J.A. Almeida, M.C.B.M. De Vasconcelos, P. Santos, and E.A. Rosa. 2013. Evaluation of the potential of squash pumpkin by-products (Seeds and Shell) as sources of antioxidant and bioactive compounds. J. Food Sci. Technol. 52(2):1008–1015. doi: 10.1007/s13197-013-1089-5.
- Ullah, S., S.A. Hussain, F. Shaukat, A. Hameed, W. Yang, and Y. Song. 2019. Antioxidant potential and the characterization of arachis hypogaea roots. Biomed. Res. Int. 2019. doi: 10.1155/2019/7073456.
- Zhang, Q. 2015. Effects of extraction solvents on phytochemicals and antioxidant activities of walnut (Juglans Regia L.) green husk extracts. Eur. J. Food Scie. Technol. 3(5):15–21.
- Zhu, K.X., C.X. Lian, X.N. Guo, W. Peng, and H.M. Zhou. 2011. Antioxidant activities and total phenolic contents of various extracts from defatted wheat germ. Food Chem. 126(3):1122–1126. doi: 10.1016/j.foodchem.2010.11.144.