ABSTRACT
The review highlights the significance of mulberry fruits in both chemical and biological sagacity and their role as antioxidant, anticancer, antidiabetic, hepatoprotective, neuroprotective, anti-inflammatory, antiobesity, hypolipidemic, and antibacterial. Besides, having phytochemicals induced biological pathways and nutritional value. Although a number of mulberry fruits species available in nature, the review elucidates the specific role of Morus alba, Morus nigra, Morus rubra, whose functions in living systems are poorly implicit. Many Pharmacological properties of mulberry fruits which are discovered in the recent past for therapeutic purposes also highlighted. Further, ethnopharmacological relevance, medicinal aspects, and bioavailability of mulberry fruits are discussed in detail.
Introduction
Ever since the dawn of earth, human beings are bank on plants for their survival, including their health benefits. The same trend has been continued even now and approximately 80% of the populations around the globe rely on drugs which are obtained from the plant sources (Gryn-Rynko et al., Citation2016). Among plant kingdom horticulture plants, including fruits has special importance. The bioactive compounds such as flavonoids, phenolics, anthocyanins, phenolic acids, and nutritive compounds, namely, sugars, essential oils, carotenoids, vitamins, and minerals present in fruit groups enhancing the overall therapeutics properties (Senica et al., Citation2019; Vijayan et al., Citation2008).
Mulberry is a multi-purpose fruit, belongs to family Moraceae, grows in tropical, subtropical, and temperate conditions (Ramesh et al., Citation2014). More than 150 mulberry species have been found across the world, of which Morus alba (), Morus nigra (), Morus rubra () have been emerged as most potential than the other mulberry species, as they exhibit maximum medicinal properties. The various vegetative parts of mulberry are a subject of the incredible interest in the current scenario due to their amazing therapeutic properties. Mulberry fruits the major concern of the current scenario due to their excellent therapeutic properties and pharmacological relevance. Mulberries are consumed in different forms such as juice, wine, tea, jams jelly and other products for the benefit of human health as they contain high quantity of carbohydrates (Kumaresan et al., Citation2008 ; Singhal et al., Citation2010). The mulberries are known for their medicinal properties, as they useful for the liver and kidney treatment, treat weakness, exhaustion, and anemia. It is also used to extravagance urinary incontinence, tinnitus, dizziness, constipation in the aged, and the anemic, to treat sore throat, depression, and fever. Further, few studies reveal that the mulberry fruit juice can enhance the health by calming the nerves, promoting the metabolism of alcohol, and immunity enrichment. Nutraceuticals such as minerals and vitamins available in the mulberry fruits can treat many chronic diseases. The consumption of mulberry fruit contributes overwhelmingly for the improvement of human health as they are a rich source of nutraceuticals including amino acids, carbohydrates, fats, vitamins, and minerals (). It has also been reported that mulberries contain several bioactive phytochemicals like anthocyanins, phloridzin (), quercetin (), chlorogenic acid (), resveratrol (), rutin () (Gundogdu et al., Citation2011; Shrikanta et al., Citation2015; Tomas et al., Citation2015; Yang et al., Citation2016). The presence of phytochemicals and nutraceuticals in mulberries is responsible for a synergistic and diversified biological activities such as anticancer (Aggarwal et al., Citation2004), antioxidant (Jiang et al., Citation2013), anti-diabetic (Wang et al., Citation2013; Swathi et al., Citation2017), neuroprotective (Rebai et al., Citation2017), hepatoprotective (Deniz et al., Citation2018), anti-inflammatory (Chen et al., Citation2016b), antibacterial (Budiman et al., Citation2017), anti-obesity (Peng et al., Citation2011), and hypolipidemic activities (Yang et al., Citation2010). Availability of required nutrients and phytochemicals from the plant sources is essential. To make those chemicals accessible to different body organs, where they supposed to play their role is more essential. After knowing the natural source for required nutrients and phytochemicals, to make such nutrients accessible to various body parts is more critical through bioavailability, and few studies have revealed the excellent bioavailability in mulberry fruits. The current review is focused predominantly on the chemical composition, nutraceutical value, biological activity of M. alba, M. nigra, and M. rubra with scientifically established recent literature under one roof. Further, we also address the bioavailability of mulberries, which is a neglected part of most of the reviews on mulberries. Fruit weight, moisture, pH and fruit organic acids constituents, nutrition profile, and list of primary and secondary metabolites present in M. alba, M. nigra and M. rubra are deliberated in , and . Mulberries have the unique characteristics to develop as a novel functional food due to their therapeutic properties and also a current topic of interest for many nutritionists. Mulberry fruit juice is one of the exemplary by-products of mulberries, particularly a source for nourishing tonic to treat blood, kidney, and liver-related problems. Juice of mulberry fruits has incredible properties to enhance human health due to the presence of carbohydrates, protein, fat (omega 6 polyunsaturated fatty acids like linoleic acid), free acids, fiber, and minerals. The mulberry juice can sustain up to 3 months under cold storage without reducing its original properties.
Table 1. Nutritional profile of mulberries (value per 100 g.)
Table 2. Physicochemical profile of Morus alba, Morus nigra and Morus rubra.
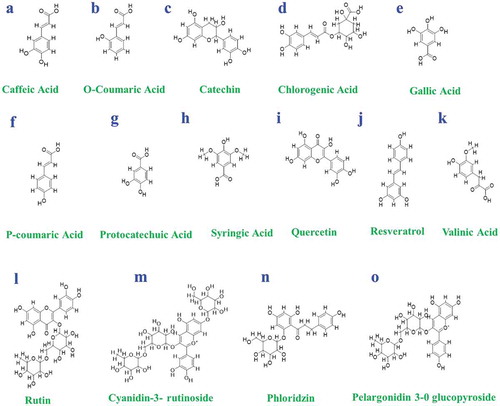
Figure 2. Chemical structures of some biologically active compounds of mulberries: (a) Caffeiec acid, (b) O-Coumaric acid, (c) catechin, (d) chlorogenic acid, (e) gallic acid, (f) p-coumaric acid, (g) protocatechuic acid, (h) syringic acid, (i) quercetin, (j) resveratrol, (k) valinic acid, (l) rutin, (m) cyanidin3-rutinoside, (n) phloridzin, (o) pelargonidin-3-O glucopyroside
Nutraceutical Value of Mulberries
Nutraceuticals are the products derived from food sources with extra health benefits in addition to the essential nutritional value found in foods. A number of studies proved that mulberries are a vital source of nutraceutical products such as amino acids, carbohydrates, fats (), vitamins, minerals (), and phytochemicals ().
Amino Acids
Mulberries contain non-essential amino acids, namely, alanine, arginine, aspartic acid, glutamic acid, glycine, proline, serine, and essential amino acids (EAA) namely isoleucine, leucine, lysine, methionine, cysteine, phenylalanine, tyrosine, threonine, tryptophan, valine, and histidine. The total amino acids ratio (TAA) of mulberries is higher when compared to high-quality protein foods such as milk and fish (Jiang and Nie, Citation2015a). Kusano et al. (Citation2002), using spectroscopic data detected the new structures of amino acids, such as morusimic acid A, morusimic acid B, morusimic acid C, morusimic acid D, morusimic acid in white mulberry.
Carbohydrates
Dimitrova et al. (Citation2015) revealed the presence of carbohydrate (monosaccharide; fructose and glucose) and adequate amount of total phenolic content in mulberry fruits. Their study also suggested that mulberry fruits can be used as dietary food due to the presence of special polysaccharide (inulin). The percentage of the sugar composition of black (M. nigra) and red mulberries (M. rubra) were analyzed, and detected 52% of glucose followed by 48% fructose (Özgen et al., Citation2009). Gundogdu et al. (Citation2018) reported that glucose content in mulberry fruits was recorded about eight times higher than sucrose while fructose content was recorded about five times higher than sucrose.
Fatty Acids
Eighteen types of unsaturated fatty acids including linoleic acid (C16:O) and few saturated fatty acids such as palmitic acid (C18:2n6), myristic acid, stearic acid have been reported in mulberry fruits (refer ) (Jiang and Nie, Citation2015a). Few studies have also revealed that fat content of M. alba is higher than M. nigra (Jelled et al., Citation2017) and the number of total fatty acids content is highest at maturity in Morus alba and stable in M nigra (Ercisli and Orhan, Citation2007b; Sofia et al., Citation2014).
Vitamins and Minerals
Mulberry fruits are rich source of Vitamins such as folates, niacin, pyridoxine, Riboflavin (Lochyñska, Citation2015), Vitamin A, Vitamin C, Vitamin E and Vitamin K, electrolytes being sodium and potassium, minerals like calcium, copper, iron, magnesium, zinc (Ercisli and Orhan, Citation2007a), selenium (Jiang and Nie, Citation2015a), phytonutrients such as carotene-β, carotene, α, lutein-zeaxanthin.
Flavanoids
Flavonoids are responsible for the aroma and color of fruits and play a vital role to enhance the quality and productivity through pollinators. Flavonoids are synthesized by a branched pathway that gives both colored compounds (anthocyanins, one of the essential flavonoid compounds) and colorless compounds (flavonols). Flavanol is a part of the flavonoid group as well (Yuan and Zhao, Citation2017).
Anthocyanins
Anthocyanins are a subclass of flavonoids and contribute coloration of flowers and fruits ranging from red to blue and purple (Yuan and Zhao, Citation2017). Anthocyanins are being exploited in various studies due to the presence of antioxidant activity. Mulberries are the amusing source of anthocyanins. Two major anthocyanins, namely, cyanidin-3-glucoside and cyanidin-3-rutinoside () are present in mulberries (Aramwit et al., Citation2010; Isabelle et al., Citation2008; Liu et al., Citation2004, Citation2008; Qin et al., Citation2010). Jiang and Nie (Citation2015a) reported that mulberry species consist mainly of monomeric anthocyanins. The study also showed that black mulberry (Morus nigra. L.) Species contain the highest contents of total monomeric anthocyanins than white mulberry (Morus alba. L.) and Russian mulberry (Morus alba). Besides, Du et al. (Citation2008) have also reported the presence of anthocyanins in mulberry fruit, namely: cyanidin 3-O-(6″-O-α-rhamnopyranosyl-β-D-glucopyranoside) (C3RG), cyanidin 3-O-(6″-Oa- rhamnopyranosyl-β-D-galactopyranoside) (C3RGa), and cyanidin 7- O-β-D-glucopyranoside (C7 G), C3 G, cyanidin 3-O-β-D-galactopyranoside (C3 Ga).
Flavonols and Flavanols
Flavonols and flavanols are essential dietary sources present in mulberries, which are known to exhibit biological activities against many diseases. Few flavonols identified and isolated from mulberry fruits are: kaempferol hexoside, kaempferol hexosylhexoside, kaempferol rhamnosylhexoside, morin, quercetin (), quercetin glucoronide, quercetin hexoside, and quercetin hexosylhexoside (Natiç et al., Citation2015). Furthermore, Wang et al. (Citation2013) reported the derivatives of quercetin and kaempferol such as quercetin-3-O-β-D-glucopyranoside, quercetin 3-O-(600-O-acetyl)-β-D-glucopyranoside, quercetin 3-O-β-D-rutinoside, quercetin 7-O-β-D-glucopyranoside, quercetin 3,7-di-O-β-D-glucopyranoside, kaempferol 3-O-β-D-glucopyranoside, kaempferol 3-O-β-D-rutinoside, dihydrokaempferol 7-O-ß-D-glucopyranoside. In addition to rutin (), morin, quercetin, mulberry fruits also contain myricetin (Minhas et al., Citation2016; Yang et al., Citation2010). A number of flavanons isolated from mulberry fruits, such as epigallocatechin, epigallocatechin gallate, gallocatechin, gallocatechin gallate, naringin (Natiç et al., Citation2015). Besides, mulberry fruits have reported to contain flavanols such as catechin (), procyanidin B1, procyanidin B2 (Butkhup et al., Citation2013).
Phenolic Acids and Alkaloids
Phenolic compounds which have been reported from mulberry fruits are hydroxycinnamic acid such as chlorogenic acid () (Isabelle et al., Citation2008), caffeic acid () (Gundogdu et al., Citation2011), p-coumaric (), o-coumaric (), and ferulic acid (Lochyñska, Citation2015; Memon et al., Citation2010) and the derivatives of benzoic acid, namely, gallic acid (e) (Bae and Suh, Citation2007), vallinic acid (), syringic acid (), protocatechuic acid () and p-hydroxybenzoic acid (Lochyñska, Citation2015; Memon et al., Citation2010; Yuan and Zhao, Citation2017). These phenolic acids are witnessed through several studies for their therapeutic value. Mulberries are also known for the occurrence of alkaloids which are pharmacologically active, nitrogen-containing essential compounds (Ruby and Rana, Citation2015). Five pyrrole alkaloids (morrole B, C, D, E, F) of white mulberry (M. alba) were observed by NMR Spectroscopy (Kim et al., Citation2002). Similarly five pyrrole alkaloids were reported from mulberry fruits namely, 2-formyl-5-(hydroxymethyl)-1H-pyrrole-1-butanoic acid, 5-(hydroxymethyl)-1H-pyrrole-2 carboxaldehyde, 2-formyl-1H-pyrrole-1-butanoic acid, and 2-formyl-5-(methoxymethyl)-1H-pyrrole-1-butanoic acid. Furthermore, the authors also isolated a new pyrrole alkaloid, morrole A form mulberry fruits (Kim et al., Citation2013).
Another group of researchers identified and isolated five new nortropane alkaloids (i.e., 2α, 3β-dihydroxynortropane, 2α,3β-dihydroxynortropane, 2,3β-6exo-trihydroxynortropane, 2-alpha, 3β,4α-trihydroxynortropane, and 3β,6exo-dihydroxynortropane) (Kusano et al., Citation2002). Some of the tropanes alkaloids are used in the preparation of anticholinergic drugs.
Aromatic and Volatile Compounds
Some active aroma compounds such as benzaldehyde, ethyl butanoate, (E)-2-nonenal, 3-mercaptoethanols, and 1 hexanol were recorded in mulberries (Zhu et al., Citation2018). Few volatile compounds mainly 2,4-nonadienal, methyl hexanate, limonene, octanol, ethyl hexanoate were also reported in black and white mulberry fruits (Sàínchez-Salcedo et al., Citation2015).
Pharmacological Properties of Mulberry Fruits
Anti-Cancer
The active ingredients in mulberries modulate apoptosis and matrix metalloproteinase and thereby block cancer progression (Huang et al., Citation2013). The anti-tumor property of mulberry fruit(s) extract (MFE) was due to progressive inhibition of the nitric oxide (NO) production in lipopolysaccharide (LPS) stimulated RAW 264.7 cells . MFE also suppress the expression of pro-inflammatory mediators and cytokines in LPS stimulated in these cell lines. The dichloromethane present in the MFE efficiently prevents NFkB/p65 and MAPK/Perk signals in LPS stimulated RAW 264.7 cells (Qian et al., Citation2015). One of the studies reports that MFE could suppress acute colitis and inhibits tumorigenesis in mice. MFE (Morus alba) possesses an apoptogenic effect on Ehrlich’s ascites carcinoma-induced mice owing its ability to increase p53 expression and decrease NFkB2 signaling by analyzing antioxidant and polyphenolic contents (Alam et al., Citation2016b). Phenolic contents of ethyl acetate extract of MFE triggered the 70–80% tumor growth inhibition with S-phase cell cycle arrest (Alam et al., Citation2016a).
Mulberries are rich in anthocyanins which improve eyesight by induction of apoptosis in adenocarcinoma cells (Kole, Citation2011). A researcher group studied the effect of MFE on male BALB/c/nude mice and fed them with MFE rich in anthocyanins for 7 weeks and observed that Atypical Glandular Cells (AGS) tumor xenograft growth was inhibited in mice. The anthocyanin of mulberries possesses anti-cancer properties (Huang et al., Citation2011). Anti-cancer activity of anthocyanins was well known as they inhibit the proliferation of cancer cells by modulating signaling pathways.
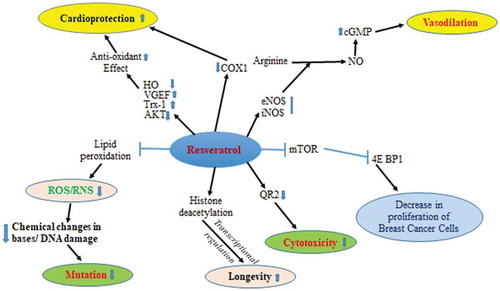
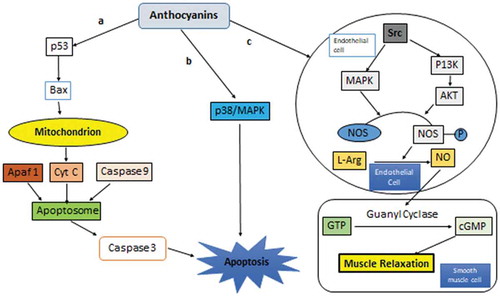
Anthocyanins also induce apoptosis via p53/MAPK signaling pathway (Popa, Citation2014). Anthocyanin’s activates Bax (apoptotic factor) which causes the release the cytochrome C (Cyt C) from mitochondrion. This Cyt C forms apoptosomealong with APAF-1 and Capsase 9. This apoptosome induces apoptosis Caspase 3 (Huang et al., Citation2011). Anthocyanins also induce apoptosis via p38/MAPK signaling pathway (Rehman et al., Citation2017). Further, anthocyanins elevates the NO synthesis from L-arginine by activating the enzyme NOS (nitric oxide synthetase) in endothelial cell (Popa, Citation2014). NO migrates to smooth muscle cells where it activates the guanylcyclase which converts GTP into cGMP. cGMP activates various enzymes which result in the relaxation of smooth muscle cells (Miguel, Citation2011; Speciale et al., Citation2014) ().
Figure 3. Anthocyanins induce apoptosis through p53/MAPK (a), p38/MAPK, (b) signaling pathways. Anthocyanins elevate the NO synthesis from L-arginine by activating the enzyme NOS (nitric oxide synthetase) in endothelial cell (c)
The presence of resveratrol () (3, 5, 4-trihydroxystilbene) in mulberries is also augmenting the anti-carcinogenic, anti-inflammatory, and cardio-protective activities. Further, it has also been reported that resveratrol suppresses the proliferation mechanism of tumor cells of lymphoid, myeloid, breast, prostate, stomach, colon, pancreas, thyroid cancers, ovarian and cervical carcinoma (Aggarwal et al., Citation2004). Resveratrol activates the nitric oxide synthase (NOH). It produces NO (nitric oxide) from the deamination process from arginine which in turn increases the cGMP (secondary messenger) level to cause the vasodilation via various downstream actions (Laupheimer et al., Citation2014). Resveratrol protects from various heart-related diseases either by reducing the oxidant level through reduction of HO and AKT level and increase in Trx-1 and VGEF level or by reducing COX-1 activity (De La Lastra and Villegas, Citation2007). This molecule also suppresses the activity transcription factor 4EBP1 and so decrease the proliferation of cancerous cells especially breast cancer cells (Bishayee, Citation2009; Kim and He, Citation2013) Resveratrol also decreases cytotoxicity by inhibiting the QR2 expression (Herman et al., Citation2018). Resveratrol also reduces ROS (reactive oxygen level) levels by suppressing the lipid peroxidation level. This action also decreases the chances of DNA damage to reduce the mutation (De La Lastra and Villegas, Citation2007; Kim and He, Citation2013; Pandey and Rizvi, Citation2011b) ().
Antioxidant
Mulberries possess exemplary nutritional value and thus attract the substantial number of scientists to analyze the antioxidant properties of mulberry fruits (Hosseini et al., Citation2018). The phenolic composition and antioxidant activity of Morus alba fruit were analyzed and found a significant correlation between antioxidant activity and the total phenolic content and trend was that the antioxidant activity increases with the increase in total phenolic content (Butkhup et al., Citation2013).
Mahmood et al. (Citation2017) evaluated the influence of ripening (un-ripen, semi-ripen, and fully-ripen) and drying (fresh, ambient-dried, and sun-dried) on mulberry fruit of different species, namely, Morus alba, M. nigra, M. macroura, and M. laevigata. Antioxidant activity of fruit extracts was assessed using reducing ability and 1,1-diphenyl-2-picryl hydrazyl scavenging assay. It was observed that as the maturity of fruits progressed, antioxidant activity was observed. Further authors reported that fully ripened mulberry fruit at air drying conditions generally was reported to have higher antioxidant efficacy.
Hong et al. (Citation2004) suggested that mulberry fruits boost the anti-oxidative defense mechanism and lowered down damaging oxidative substances in erythrocytes of streptozotocin-induced rats.
Yigit et al. (Citation2008) investigated the antioxidant activity of black mulberry by preparing its methanol extract, and antioxidant activity was measured using α, α-diphenyl-β-picrylhydrazyl (DPPH) and lipid peroxidation inhibition assays. The results of these studies confirmed the presence of high-level antioxidant activity of methanol extracts of black mulberry fruits. Similarly, the antioxidant activity of the ethanol, ethanol-water (1:1), and water extract of black mulberry (Morus nigra) were reported with higher efficacy (Kostiæ et al., Citation2013).
The antioxidant and anti-fatigue properties were reported from the mulberry fruits anthocyanin using mulberry juice purification (MJP) and mulberry mark purification (MMP). The studies show that anthocyanin of mulberry fruits acts as an antioxidant and assisted in reducing fatigue (Jiang et al., Citation2013).
Similar findings were also reported by Shrikanta et al. (Citation2015) who compared the resveratrol content and antioxidant activity of fruits including Jamun (Syzygium cumini), Jackfruit (Artocarpus heterophyllus), and mulberry (Morus rubra). Further, mulberry fruits exhibited the highest resveratrol content and had the highest antioxidant activity than Jamun and Jackfruit. Resveratrol () reduces reactive oxygen species (ROS) levels by suppressing the lipid peroxidation level (Pandey and Rizvi, Citation2011a). This action also decreased the chances of DNA damage by reducing the mutation () (De La Lastra and Villegas, Citation2007).
Anti-Inflammatory
Cyanidin-3-glucoside (C3 G) is a known anthocyanin found in mulberry fruits, which is responsible to alter different hematological parameters in animal systems such as inhibiting inflammatory response serum triglycerides, and high-density lipoprotein cholesterol (Kim and Park, Citation2006). It has been reported that C3 G possesses anti-inflammatory and free radical scavenging activity which can prevent endothelial distinction (Kang et al., Citation2006). The anti-inflammatory response of flavonoids of black mulberries against pro-inflammatory cytokines in mice serum was also reported by the researchers (Chen et al., 2016).
Anti-Obesity and Hypolipidemic
Obesity is becoming a serious concern in today’s world as it increases the risk of cardiovascular disease, diabetes, and cancer. Several novel studies are conducted on mulberry fruits, where they have exhibited the best potent anti-obesity characteristics. Yang et al. (Citation2010) revealed the reduction in serum level, liver triglyceride, total cholesterol serum, low-density lipoprotein cholesterol, and atherogenic index when they administered mulberry fruit powder in a fat-induced rats. In addition to this, there was a significant increase in serum high density- lipoprote in cholesterol. Similarly, Sirikanchanarod et al. (Citation2016) reported in their study, when hypercholesterolemic individuals were allowed to consume 45 g freeze-dried mulberry fruits for 6 weeks, a significant reduction in lower cholesterol level (LDL) was observed in the independent group. These studies showed a clear indication that mulberry fruits are potential therapeutic agents to reduce hyperlipidemia.
Lim et al. (Citation2013) observed blending effect of mulberry fruits and leaf extracts on the high fat-induced obese mouse and found that the combination of mulberry fruits and leaves extracts reduced plasma triglyceride, liver lipid peroxidation level, adipocyte size, and improvement hepatic steatosis. Peng et al. (Citation2011) investigated the antilipidemic effect of mulberry fruit water extract on male hamster rat model and reported that mulberry fruit water extract reduced the body weight, lowered the serum triglyceride and free fatty acid in a male hamster rat model.
The effect of mulberry fruit extract (MFE) against development on atherosclerosis in rabbits fed with high cholesterol diet was studied and found that MFE possesses the anti-hyperlipidemic activity and reduced the risk of atherosclerosis (Chen et al., Citation2005). Another study by Liu et al. (Citation2008) reported that mulberry water extract (MWES) and mulberry anthocyanin-rich extracts (MACS) inhibited the relative electrophoretic mobility (REM), ApoB fragmentation and thiobuturic acid reactive substances (TBARS) in LDL oxidation mediated by Cu2+. Authors also observed a reduction in oxLDL induced macrophage death. Further, LDL oxidation inhibition also reported. These studies suggested that mulberry extract possesses an anti-atherosclerosis effect.
Anti-Diabetic
Diabetes is a hyperglycemic condition due to a defect in insulin secretion (Kalofoutis et al., Citation2007). Taking view of diabetes, several studies have been conducted to evaluate the anti-diabetic potential of mulberries and gave potential anti-diabetic effects of white mulberry (Morus alba) extract. It was found that Mulberry fruit extract (MFE) hampered alpha-glucosidase activity, suppressed fasting glucose, and glycosylated serum protein in Streptozotocin-induced diabetic mice (Wang et al., Citation2013).
Jiao et al. (Citation2017) reported when diabetic rats were treated with two fractions of MFP (Mulberry fruit polysaccharide) MFP50 and MFP90 for 7 weeks, reduction in fasting glucose, and serum insulin was observed. Similar findings were also reported by Guo et al. (Citation2013) who investigated the anti-diabetic effect of Ramulus mori polysaccharide derived from white mulberry (Morus alba) on diabetic rats and the results indicate the reduction in fasting glucose of diabetic rat model.
The anti-diabetic effect of mulberry anthocyanins was examined in the rats. Results of the study showed a reduction in fasting blood glucose, cholesterol, leptin serum insulin, and triglyceride levels including enhancement in adiponectin level. This indicates the possession of anti-hyperglycemic and anti-hyperlipidemic effects of mulberry fruits (Yan et al., Citation2016).
In order to find out the hypoglycemic effect of white mulberry fruit extract, the chemically induced diabetic mice model was used. Reduction in fasting glycemia, glycated serum protein, total cholesterol, triglycerides, postprandial blood glucose were observed. In addition, increase in insulin sensitivity, AMPK and GLUT4 expression levels were reported in the mice model (Choi et al., Citation2016).
Chen et al. (Citation2015) isolated the Mulberry fruit polysaccharides (MFP). Four eluents of water (MFP-1), 0.1 M NaCl (MFP-2), 0.2 M NaCl (MFP-3), and 0.3 M NaCl (MFP-4) were fractionated and their antioxidant and hypoglycemic activities were evaluated. The results revealed that MFP-3 and MFP-4 displayed better antioxidant activity inhibitory effects on α-amylase and α-glucosidase than MFP-1 and MFP-2.
Neuroprotective
Essential fatty acids are long-chain polyunsaturated fatty acids derived from α-linolenic (Omega 3 fatty acid) and linoleic acids (omega 6 fatty acid), which are necessary for the formation of the healthy cell membrane, and boost neural function through the production of hormones particularly eicosanoids, thromboxane’s, leukotrienes, and prostaglandins (Feng et al., Citation2015). Mulberries contain essential fatty acids, which cannot be synthesized by the human body. It has also been reported that fruits, leaves, and bark of white mulberry enhanced the brain activity, reduced low-density lipoprotein cholesterol levels and protects against cholesterol neurotoxicity (El-Sayyad, Citation2015).
Neuroprotective activity of mulberry fruit extracts has been reported through in-vivo and in-vitro models of neural disorders (Rebai et al., Citation2017). The study was performed to know the effect of mulberry fruits extracts on Parkinson’s disease. These agents, having antioxidant and anti-inflammatory properties, provide a safe and effective means of ameliorating chronic diseases.
It has been reported that anthocyanin’s in mulberry fruit extract (MFE) protected dopaminergic neurons against neurotoxicity (Kim et al., Citation2010). Further, Yang et al. (Citation2016) investigated the neuroprotective activity of mulberry fruits in different ripening stages using a cellular model of Alzheimer and the neuroprotective activity of mulberry fruits was reported by authors. Authors also observed that ripened fruits contain neuroprotective activity due to the presence of a high amount of total phenolic compounds.
Mulberry fruit extract (MFE) improved the memory and enhanced the densities of neurons and cholinergic activity in rat model suffering from memory impairment and hippocampal damage in vascular dementia. This study confirmed the neuroprotective action of mulberry fruits against memory impairment (Kaewkaen et al., Citation2012).
Hepatoprotective
Deniz et al. (Citation2018) studied the hepatoprotective effect of black mulberry (Morus nigra) extracts on carbon tetrachloride (CCL4) induced hepatic injury. Carbon tetrachloride dissolved in soyabean oil (1 ml/kg/twice a week) was injected in rats. In post hepatic injury, rats were administrated with black mulberry extracts in the blood of rats, serum activities of liver enzymes aspartate aminotransferase (AST), alanine aminotransferase (ALT) and gamma-glutamyltransferase (GGT) were recorded. The outcome of their finding revealed that black mulberry extract inhibited the oxidation generated by carbon tetrachloride and it enhanced the activities of antioxidant enzymes superoxide dismutase (SOD) and glutathione peroxide (GPX) including an increase in (AST) and (GGT) expression.
An investigation confirmed that MFE can block the synthesis and increase the oxidation of fatty acid and confirmed its hepato-protective potential (Chang et al., Citation2013). Further, evaluation of the hepatoprotective effect of anthocyanins present in mulberry fruits was done on carbon tetrachloride – induced liver fibroin in rats. Oral feeding of mulberry fruit to rats shows a decrease in the levels of liver enzymes, hydroxyproline, hyaluronidase, ALT (Alanine aminotransferase), AST (aspartate aminotransferase) and collagen type 3 (Li et al., Citation2016). Further, Hu et al. (Citation2019) isolated the three different mulberry fruit polysaccharide fractions (MFP-I, MFP-II, and MFP-III) through stepwise precipitation with 30%, 60%, and 90% ethanol, respectively, and evaluated their protective efficacy against palmitic acid (PA)-induced hepatocyte lipotoxicity. It was observed among all the MFPs, MFP-II significantly reduced PA-induced hepatic lipotoxicity at 0.1 and 0.2 mg/mL while other two (MFP-I and MFP-III) displayed weak protection. The study revealed that MFPII amplified the nuclear factor erythroid-2-related factor 2 (Nrf2) phosphorylation and its nuclear translocation, consequently activating the Nrf2/ARE signaling pathway and also augmented heme oxygenase 1, NAD(P)H:quinone oxidoreductase 1, and γ-glutamate-cysteine ligase gene expressions and enhanced the activities of catalase and glutathione peroxidase activities which provided protection to hepatocytes against PA-induced oxidative stress and lipotoxicity.
Anti-Bacterial
A research was conducted to examine the antibacterial property of black mulberry M. nigra fruit extract for acne, which is mainly caused by bacteria S. epidermidis and Placacnes places. The author reported the presence of antibacterial activity black M. nigra fruit extract against these acne-causing bacteria (Budiman et al., Citation2017).
In a study carried out to investigate the antibacterial activity of Morin isolated from Morus alba, Yang and Lee found that Morin showed antibacterial activity against Streptococcus mutants (Yang and Lee, Citation2012).
The antibacterial of activity of total flavonoid of black mulberry (Morus nigra) and non black mulberry (Morus mongolica and Morus alba ‘Zhenzhubaiwas) was studies, and it was revealed that total flavonoid of black mulberry showed remarkably higher antibacterial activities against Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus than the total flavonoid of non black mulberry (Chen et al., Citation2018).
Bioavailability of Mulberries
Mulberry fruits are mainly used in the form of ripened fruit, wine, tea, etc. These are all known for their delicious taste and health improving properties due to the presence of numerous phytochemicals and phenolic compounds which are responsible for biological activities against many serious diseases. Nutrients and phytochemicals of mulberry fruits are easily bio-accessible and play a vital role in enhancing health due to an account of their biological activities.
Anthocyanins from black mulberry (9%) have shown high bio-accessibility than raspberry (5.3%), sourberry (2.8%), and ruddy mulberry (0.34%) (Liang et al., Citation2012). Absorption of anthocyanins took place very rapidly after consuming them and noticed in plasma membrane 15–50 minutes postprandial and these were excreted entirely within 6–8 hours (Hassimotto et al., Citation2008). It demonstrated that the metabolism of mulberry anthocyanins take place through methylation, glucuronidation, and sulfo conjugation pathways after its absorption (Boutin et al., Citation1993). In the course of these pathways, UDP-glucuronosyl conjugates with glucuronic acid and sulphotransferase conjugate with sulfate then catalyzed in the liver, small intestine, and kidney. Further, the effects of antioxidant of black mulberry juice by LQToF-KMS to identify anthocyanins, two major anthocyanins (cyanidin-3-glucoside, cyanidin-3-rutinoside (fig.1.13)), phenolic acids such as caffeoylquinic acid and 3 flavanols (quercetin-3 glucoside, quercetin-malonyl-glucoside, and rutin) was tested. The authors analyzed the bioavailability of mulberry antioxidants which expressed that anthocyanins for fruit matrix were reported to be higher for the juice matrix of black mulberry. Intestinal microflora also metabolized anthocyanins. Cleavage of C-ring takes place and phenolic acids produced were absorbed quickly (Bravo, Citation1998). Glucosides such as quercetin were also metabolized with the help of gut microflora of ilium. Anthocyanins of mulberry were metabolized to phenolic acid such as chlorogenic acid, ferulic acid, protocatechuic acid by intestinal microflora (Liang et al., Citation2012).
While evaluating the digestibility of mulberry fruit polysaccharides (MFPs) and its effect on lipid digestion in the simulated saliva, gastric and intestinal model, Chen et al. (2016) reported that salivary amylase posed no effect on MFPs whereas gastric juice showed higher effects on the digestion of MFPs that intestinal juice which can evidently be seen from the decrease in molecular weight (Mw) from 128.7 ± 6.1, 13.6 ± 1.4 and 2.9 ± 0.1 to 84.3 ± 4.7, 5.2 ± 0.1 and 1.2 ± 0.1 KDa, respectively. Further authors reported that amount of reducing ends were found to be increased from 0.051 ± 0.003 to 0.451 ± 0.011 mM and during the entire digestion period, no monosaccharide was released from the polysaccharide suggesting that gastrointestinal digestion cleaved the glycosidic bonds and produced no free monosaccharide. In addition to this, it was found observed that MFP might decrease the rate and degree of lipid digestion in a concentration-dependent manner.
Conclusion
Though the considerable amount of research and review articles have published on the health benefits of mulberry fruits, still their interests are not much visible and percolated among the users significantly. Hence, the current review predominantly describes the role of antioxidants, phenols, polyphenols, and other phytochemicals, which are responsible for anti-cancer, anti-inflammatory, anti-obesity, hypolipidemic, antidiabetic, neuroprotective, and hepatoprotective activities with the bioavailability of mulberries, which is lacking part of the earlier publications. Further, there is an urgent need to develop high fruit yielding mulberry varieties through molecular techniques, and conducting further research on human beings to ensure such nutraceuticals bioavailability in humans will help indeed in understanding various diseases and treatment selections. In a nutshell, multi-dimensional use of mulberries would be useful for the Natural Product Research where scientist looking for new sources of functional foods. Fruits of mulberry have become a more prominent part when compared to root, stem barks, leaf and latex of its plant of mulberry, due to their edible nature and wide usages as a functional food. However, further intensive research on mulberries will undoubtedly reveal more biological activities from the untouched areas like antiviral properties, which is the need for a current scenario.
Acknowledgments
The authors are greatly indebted to Dr. R. K. Sinha, Assistant Editor, Indian silk, CSB, Bangalore for providing the Photographs of different mulberry species for the current review. This work was supported by DST-SERB, New Delhi.
Disclosure Statement
No potential conflict of interest was reported by authors.
Additional information
Funding
References
- Aggarwal, B.B., A. Bhardwaj, R.S. Aggarwal, N.P. Seeram, S. Shishodia, and Y. Takada. 2004. Role of resveratrol in prevention and therapy of cancer: Preclinical and clinical studies. Anticancer Res. 24(5A):2783–2840.
- Alam, A.K., A.S. Hossain, M.A. Khan, S.R. Kabir, M.A. Reza, M.M. Rahman, M.S. Islam, M.A.A. Rahman, M. Rashid, M.G. Sadik, et al. 2016a. The antioxidative fraction of white mulberry induces apoptosis through regulation of p53 and NF+¦B in EAC cells. PLoS ONE 11:e0167536. doi: 10.1371/journal.pone.0167536.
- Alam, A.K., A.S. Hossain, M.A. Khan, S.R. Kabir, M.A. Reza, M.M. Rahman, M.S. Islam, M.A.A. Rahman, M. Rashid, M.G. Sadik, et al. 2016b. The antioxidative fraction of white mulberry induces apoptosis through regulation of p53 and NF+¦B in EAC cells. PLoS ONE. 11:e0167536. doi: 10.1371/journal.pone.0167536.
- Aljane, F., and N. Sdiri. 2016. Morphological, phytochemical and antioxidant characteristics of white (Morusalba L.), red (Morusrubra L.) and black (Morusnigra L.) mulberry fruits grown in arid regions of Tunisia. J. New Sci. 35(1):1940–1947.
- Aramwit, P., N. Bang, and T. Srichana. 2010. The properties and stability of anthocyanins in mulberry fruits. Food Res. Int. 43(4):1093–1097. doi: 10.1016/j.foodres.2010.01.022.
- Bae, S.H., and H.J. Suh. 2007. Antioxidant activities of five different mulberry cultivars in Korea. LWT-Food Sci. Technol. 40:955–962. doi: 10.1016/j.lwt.2006.06.007.
- Bishayee, A. 2009. Cancer prevention and treatment with resveratrol: From rodent studies to clinical trials. Cancer Prev. Res. (Phila) 2(5):409–418. doi: 10.1158/1940-6207.CAPR-08-0160.
- Boutin, J.A., F. Meunier, P.H. Lambert, P. Hennig, D. Berti, B. Serkiz, J.P. Volland. 1993. In vivo and in vitro glucuronidation of the flavonoid diosmetin in rats. Drug Metab. Dispos. 21(6):1157–1166.
- Bravo, L. 1998. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 56(11):317–333. doi: 10.1111/j.1753-4887.1998.tb01670.x.
- Budiman, A., D.L. Aulifa, A.S.W. Kusuma, and A. Sulastri. 2017. Antibacterial and antioxidant activity of black mulberry (Morus nigra) extracts for acne treatment. Pharmacog. J. 9(5):611–614. doi: 10.5530/pj.2017.5.97.
- Butkhup, L., W. Samappito, and S. Samappito. 2013. Phenolic composition and antioxidant activity of white mulberry (Morus alba L.) fruits. Int. J. Food Sci. Technol. 48:934–940. doi: 10.1111/ijfs.12044.
- Chang, J.J., M.J. Hsu, H.P. Huang, D.J. Chung, Y.C. Chang, and C.J. Wang. 2013. Mulberry anthocyanins inhibit oleic acid-induced lipid accumulation by reduction of lipogenesis and promotion of hepatic lipid clearance. J. Agric. Food Chem. 61:6069–6076. doi: 10.1021/jf401171k.
- Chen, C., B. Zhang, X. Fu, L.-J. You, A.M. Abbasi, and R.H. Liu. 2016a. The digestibility of mulberry fruit polysaccharides and its impact on lipolysis under simulated saliva, gastric and intestinal conditions. Food Hydrocoll. 58:171–178. doi: 10.1016/j.foodhyd.2016.02.033.
- Chen, C., L.-J. You, A.M. Abbasi, X. Fu, R.H. Liua, and C. Li. 2015. Characterization of polysaccharide fractions in mulberry fruit and assessment of their antioxidant and hypoglycemic activities in vitro. Food Func. doi: 10.1039/c5fo01114k.
- Chen, C.C., L.K. Liu, J.D. Hsu, H.P. Huang, M.Y. Yang, and C.J. Wang. 2005. Mulberry extract inhibits the development of atherosclerosis in cholesterol-fed rabbits. Food Chem. 91:601–607. doi: 10.1016/j.foodchem.2004.06.039.
- Chen, H., J. Pu, D. Liu, W. Yu, Y. Shao, G. Yang, Z. Xiang, N. He. 2016b. Anti-inflammatory and antinociceptive properties of flavonoids from the fruits of black mulberry (Morus nigra L.). PLoS ONE. 11:e0153080. doi: 10.1371/journal.pone.0153080.
- Chen, H., W. Yu, G. Chen, S. Meng, Z. Xiang, and N. He. 2018. Antinociceptive and antibacterial properties of anthocyanins and flavonols from fruits of black and non-black mulberries. Molecules 23(1):4. doi: 10.3390/molecules23010004.
- Choi, K.H., H.A. Lee, M.H. Park, and J.S. Han. 2016. Mulberry (Morus alba L.) fruit extract containing anthocyanins improves glycemic control and insulin sensitivity via activation of AMP-activated protein kinase in diabetic C57BL/Ksj-db/db mice. J. Med. Food. 19:737–745. doi: 10.1089/jmf.2016.3665.
- De La Lastra, C.A., and I. Villegas. 2007. Resveratrol as an antioxidant and pro-oxidant agent: Mechanisms and clinical implications. Biochem. Soc. Trans. 5(Pt 5):1156–1160. doi: 10.1042/BST0351156.
- Deniz, G.Y., E. Laloglu, K. Koc, H. Nadaroglu, and F. Geyikoglu. 2018. The effect of black mulberry (Morus nigra) extracts on carbon tetrachloride-induced liver damage. Arch. Bio. Sci. 70:371–378. doi: 10.2298/ABS171009055D.
- Dimitrova, M., N.T. Petkova, P.P. Denev, and I.N. Aleksieva. 2015. Carbohydrate composition and antioxidant activity of certain Morus species. Int. J. Pharmacogn. Phytochem. Res. 7:621–627.
- Du, Q.Z., J.F. Zheng, and Y. Xu. 2008. Composition of anthocyanins in mulberry and their antioxidant activity. J. Food Composit. Anal. 21(5):390–395. doi: 10.1016/j.jfca.2008.02.007.
- El-Sayyad, H.I. 2015. Cholesterol overload impairing cerebellar function: The promise of natural products. Nutrition 31(5):621–630. doi: 10.1016/j.nut.2014.10.017.
- Ercisli, S., and E. Orhan. 2007a. Chemical composition of white (Morusalba), red (Morusrubra) and black (Morus nigra) mulberry fruits. Food Chem. 103:1380–1384. doi: 10.1016/j.foodchem.2006.10.054.
- Ercisli, S., and E. Orhan. 2007b. Chemical composition of white (Morus alba), red (Morus rubra) and black (Morus nigra) mulberry fruits. Food Chem. 103(4):1380–1384. doi: 10.1016/j.foodchem.2006.10.054.
- Feng, Y., M. Liu, Y. Ouyang, X. Zhao, Y. Ju, and Y. Fang. 2015. Comparative study of aromatic compounds in fruit wines from raspberry, strawberry, and mulberry in central Shaanxi area. Food Nutr. Res. 59:29290. doi: 10.3402/fnr.v59.29290.
- Gryn-Rynko, A., G. Bazylak, and D. Olszewska-Slonina. 2016. New potential phytotherapeutics obtained from white mulberry (Morus alba L.) leaves. Biomed. Pharmacother. 84:628–636. doi: 10.1016/j.biopha.2016.09.081.
- Gundogdu, M., F. Muradoglu, R.G. Sensoy, and H. Yilmaz. 2011. Determination of fruit chemical properties of Morus nigra L., Morus alba L. and Morus rubra L. by HPLC. Sci Horti. 132(5):37–41. doi: 10.1016/j.scienta.2011.09.035.
- Gundogdu, M., M. Tunçtürk, S. Berk, N. Şekeroğlu, and S. Gezici. 2018. Antioxidant capacity and bioactive contents of mulberry species from Eastern Anatolia region of Turkey. Ind. J. Pharmac. Edu. Res. 52(4):S96–S101. doi: 10.5530/ijper.52.4s.82.
- Guo, C., R. Li, N. Zheng, L. Xu, T. Liang, and Q. He. 2013. Anti-diabetic effect of ramulus mori polysaccharides, isolated from Morus alba L., on STZ-diabetic mice through blocking inflammatory response and attenuating oxidative stress. Int. Immunopharmacol. 16(1):93–99. doi: 10.1016/j.intimp.2013.03.029.
- Hassimotto, N.M., M.I. Genovese, and F.M. Lajolo. 2008. Absorption and metabolism of cyanidin-3-glucoside and cyanidin-3-rutinoside extracted from wild mulberry (Morus nigra L.) in rats. Nutr. Res. 28(3):198–207. doi: 10.1016/j.nutres.2007.12.012.
- Herman, F., S. Westfall, J. Brathwaite, and G.M. Pasinetti. 2018. Suppression of presymptomatic oxidative stress and inflammation in neurodegeneration by grape-derived polyphenols. Front. Pharmacol. 28;9:867. doi: 10.3389/fphar.2018.00867.
- Hong, J.H., J.M. Ahn, S.W. Choi, and S.J. Rhee. 2004. The effects of mulberry fruit on the antioxidative defense systems and oxidative stress in the erythrocytes of streptozotocin-induced diabetic rats. Nut. Sci. 7:127–132.
- Hosseini, A.S., M. Akramian, A. Khadivi, and H. Salehi-Arjmand. 2018. Phenotypic and chemical variation of black mulberry (Morus nigra) genotypes. Indust. Crops Prod. 117:260–271. doi: 10.1016/j.indcrop.2018.03.007.
- Hu, D., T. Bao, Y. Lu, H. Su, H. Ke, and W. Chen. 2019. Polysaccharide from mulberry Fruit (Morus alba L.) protects against palmitic-acid-induced hepatocyte lipotoxicity by activating the Nrf2/ARE signaling pathway. J. Agri. Food Chem. doi: 10.1021/acs.jafc.9b03335.
- Huang, H.P., T.T. Ou, and C.J. Wang. 2013. Mulberry (SangShén ZÎ) and its bioactive compounds, the chemoprevention effects and molecular mechanisms in vitro and in vivo. J. Tradit. Complement Med. 3(1):7–15. doi: 10.4103/2225-4110.106535.
- Huang, H.P., Y.C. Chang, C.H. Wu, C.N. Hung, and C.J. Wang. 2011. Anthocyanin-rich Mulberry extract inhibits the gastric cancer cell growth in vitro and xenograft mice by inducing signals of p38/p53 and c-jun. Food Chem. 129(4):1703–1709. doi: 10.1016/j.foodchem.2011.06.035.
- Isabelle, M., B.L. Lee, C.N. Ong, X. Liu, and D. Huang. 2008. Peroxyl radical scavenging capacity, polyphenolics, and lipophilic antioxidant profiles of mulberry fruits cultivated in southern China. J. Agric. Food Chem. 56(20):9410–9416. doi: 10.1021/jf801527a.
- Jelled, A., R.B. Hassine, A. Thouri, G. Flamini, H. Chahdoura, A.E. Arem, J. Ben Lamine, A. kacem, Z. Haouas, H. Ben Cheikh, et al. 2017. Immature mulberry fruits richness of promising constituents in contrast with mature ones: A comparative study among three Tunisian species. Indus. Crops Prod. 95:434–443. doi: 10.1016/j.indcrop.2016.10.053.
- Jiang, D.Q., Y. Guo, D.H. Xu, Y.S. Huang, K. Yuan, and Z.Q. Lv. 2013. Antioxidant and anti-fatigue effects of anthocyanins of mulberry juice purification (MJP) and mulberry marc purification (MMP) from different varieties mulberry fruit in China. Food Chem. Toxicol. 59:1–7. doi: 10.1016/j.fct.2013.05.023.
- Jiang, Y., and W.J. Nie. 2015a. Chemical properties in fruits of mulberry species from the Xinjiang province of China. Food Chem. 174:460–466. doi: 10.1016/j.foodchem.2014.11.083.
- Jiang, Y., and W.J. Nie. 2015b. Chemical properties in fruits of mulberry species from the Xinjiang province of China. Food Chem. 174:460–466. doi: 10.1016/j.foodchem.2014.11.083.
- Jiao, Y., X. Wang, X. Jiang, F. Kong, S. Wang, and C. Yan. 2017. Antidiabetic effects of Morus alba fruit polysaccharides on high-fat diet-and streptozotocin-induced type 2 diabetes in rats. J. Ethnopharmacol. 199:119–127. doi: 10.1016/j.jep.2017.02.003.
- Kaewkaen, P., T. Tong-un, J. Wattanathorn, S. Muchimapura, W. Kaewrueng, and S. Wongcharoenwanakit. 2012. Mulberry fruit extract protects against memory impairment and hippocampal damage in an animal model of vascular dementia. Evid. Based Complement Altern. Med. doi: 10.1155/2012/263520.
- Kalofoutis, C., C. Piperi, A. Kalofoutis, F. Harris, D. Phoenix, and J. Singh. 2007. Type II diabetes mellitus and cardiovascular risk factors: Current therapeutic approaches. Exp. Clin. Cardiol. 12(1):17–28.
- Kang, T.H., J.Y. Hur, H.B. Kim, J.H. Ryu, and S.Y. Kim. 2006. Neuroprotective effects of the cyanidin-3-O-b-d-glucopyranoside isolated from mulberry fruit against cerebral ischemia. Neurosci. Let. 391(3):122–126. doi: 10.1016/j.neulet.2005.08.053.
- Kim, A-J., and Park, S. 2006. Mulberry Extract Supplements Ameliorate the Inflammation-Related Hematological Parameters in Carrageenan-Induced Arthritic Rats. J Med Food. 9 (3): 431–435. https://doi.org/10.1089/jmf.2006.9.431 doi: 10.1089/jmf.2006.9.431.
- Kim, H.G., M.S. Ju, J.S. Shim, M.C. Kim, S.H. Lee, Y. Huh, S.Y. Kim, M.S. Oh. 2010. Mulberry fruit protects dopaminergic neurons in toxin-induced Parkinson’s disease models. Br. J. Nutr. 104(1):8–16. doi:10.1017/S0007114510000218.
- Kim, I., and Y.Y. He. 2013. Targeting the AMP-activated protein kinase for cancer prevention and therapy. Front Oncol. 3:175. doi: 10.3389/fonc.2013.00175.
- Kim, S.B., B.Y. Chang, Y.H. Jo, S.H. Lee, S.B. Han, B.Y. Hwang, S.Y. Kim, and M.K. Lee. 2002. Macrophage activating activity of pyrrole alkaloids from Morus alba fruits. J. Ethnopharmacol. 145:393–396. doi: 10.1016/j.jep.2012.11.007.
- Kim, S.B., B.Y. Chang, Y.H. Jo, S.H. Lee, S.B. Han, B.Y. Hwang, S.Y. Kim, M.K. Lee. 2013. Macrophage activating activity of pyrrole alkaloids from Morus alba fruits. J. Ethnopharmacol. 145(1):393–396. doi:10.1016/j.jep.2012.11.007.
- Kole, C. 2011. Wild crop relatives: Genomic and breeding resources vegetables. Springer. ISBN 978-3-642-20449-4.
- Kostiæ, D.A., D.S. Dimitrijeviæ, S.S. Mitiæ, M.N. Mitiæ, G.S. Stojanoviæ, and A.V. Živanoviæ. 2013. Phenolic content and antioxidant activities of fruit extracts of Morus nigra L. (Moraceae) from Southeast Serbia. Tropic J. Pharmaceut. Res. 12(1):105–110.
- Kumaresan, P., A. Tikader, and C.K. Kamble. 2008. Mulberry fruit and its medicinal values. Indian Silk. 46:10–13.
- Kusano, G., S. Orihara, D. Tsukamoto, M. Shibano, M. Coskun, A. Guvenc, and C.S. Erdurak. 2002. Five new nortropane alkaloids and six new amino acids from the fruit of Morusalba LINNE. growing in Turkey. Chem. Pharm. Bull. 50(2):185–192. doi: 10.1248/cpb.50.185.
- Laupheimer, M.W., M. Perry, S. Benton, P. Malliaras, and N. Maffulli. 2014. Resveratrol exerts no effect on inflammatory response and delayed onset muscle soreness after a marathon in male athletes.: A randomised, double-blind, placebo-controlled pilot feasibility study. Transl. Med. UniSa. 10:38–42.
- Li, Y., Z. Yang, S. Jia, and K. Yuan. 2016. Protective effect and mechanism of action of mulberry marc anthocyanins on carbon tetrachloride-induced liver fibrosis in rats. J. Funct. Foods. 24:595–601. doi: 10.1016/j.jff.2016.05.001.
- Liang, L., X. Wu, T. Zhao, J. Zhao, F. Li, Y. Zou, G. Mao, L. Yang. 2012. In vitro bioaccessibility and antioxidant activity of anthocyanins from mulberry (Morus atropurpurea Roxb.) following simulated gastro-intestinal digestion. Food Res. Int. 46(1):76–82. doi:10.1016/j.foodres.2011.11.024.
- Lim, H.H., S.O. Lee, S.Y. Kim, S.J. Yang, and Y. Lim. 2013. Anti-inflammatory and antiobesity effects of mulberry leaf and fruit extract on high fat diet-induced obesity. Exp. Bio. Medic. 238(10):1160–1169. doi: 10.1177/1535370213498982.
- Liu, L.K., H.J. Lee, Y.W. Shih, C.C. Chyau, and C.J. Wang. 2008. Mulberry anthocyanin extracts inhibit LDL oxidation and macrophage-derived foam cell formation induced by oxidative LDL. J. Food Sci. 73(6):H113–H121. doi: 10.1111/j.1750-3841.2008.00801.x.
- Liu, X., G. Xiao, W. Chen, Y. Xu, and J. Wu. 2004. Quantification and purification of mulberry anthocyanins with macroporous resins. Biomed. Res. Int. 326–331. doi: 10.1155/S1110724304403052.
- Lochyñska, M. 2015. Energy and nutritional properties of the white mulberry (Morus alba L.). J. Agri. Sci. Technol. 5:709–716. doi: 10.17265/2161-6256/2015.09.001.
- Mahmood, T., F. Anwar, N. Afzal, R. Kausar, S. Ilyas, and M. Shoaib. 2017. Influence of ripening stages and drying methods on polyphenolic content and antioxidant activities of mulberry fruits. J. Food Meas. Charact. 11(4):2171–2179. doi: 10.1007/s11694-017-9602-6.
- Memon, A.A., N. Memon, D.L. Luthria, M.I. Bhanger, and A.A. Pitafi. 2010. Phenolic acids profiling and antioxidant potential of mulberry (Morus laevigata W., Morus nigra L., Morus alba L.) leaves and fruits grown in Pakistan. Pol. J. Food Nutr. Sci. 60(1):25–32.
- Miguel, M.G. 2011. Anthocyanins: Antioxidant and/or anti-inflammatory activities. J. App. Pharm. Sci. 1(6):7–15.
- Minhas, M.A., A. Begum, S. Hamid, M. Babar, R. Ilyas, S. Ali, F Latif, S Andleeb. 2016. Evaluation of antibiotic and antioxidant activity of Morus nigra (Black Mulberry) extracts against soil borne, food borne and clinical human pathogens. Pak. J. Zoo 48:1381–1388.
- Natiç, M.M., D.C. Dabiç, A. Papetti, M.M.F. Akšíç, V. Ognjanov, M. Ljubojeviç, Ž.L. Tešić. 2015. Analysis and characterisation of phytochemicals in mulberry (((Morus alba L.))) fruits grown in Vojvodina, North Serbia. Food Chem. 171:128–136. doi: 10.1016/j.foodchem.2014.08.101.
- Özgen, M., S. Serçe, and C. Kaya. 2009. Phytochemical and antioxidant properties of anthocyanin-rich Morus nigra and Morus rubra fruits. Sci. Hortic. 119(3):275–279. doi: 10.1016/j.scienta.2008.08.007.
- Pandey, K.B., and S.I. Rizvi. 2011a. Anti-oxidative action of resveratrol: Implications for human health. Arab. J. Chem. 4:293–298. doi: 10.1016/j.arabjc.2010.06.049.
- Pandey, K.B., and S.I. Rizvi. 2011b. Anti-oxidative action of resveratrol: Implications for human health. Arab. J. Chem. 4:293–298. doi: 10.1016/j.arabjc.2010.06.049.
- Peng, C.H., L.K. Liu, C.M. Chuang, C.C. Chyau, C.N. Huang, and C.J. Wang. 2011. Mulberry water extracts possess an anti-obesity effect and ability to inhibit hepatic lipogenesis and promote lipolysis. J. Agri. Food Chem. 59(6):2663–2671. doi: 10.1021/jf1043508.
- Popa, A. 2014. Better lower and upper bounds for the minimum rainbow subgraph problem. Theor. Comput. Sci. 543:1–8. doi: 10.1016/j.tcs.2014.05.008.
- Qian, Z., Z. Wu, L. Huang, H. Qiu, L. Wang, L. Li, L. Yao, K. Kang, J. Qu, Y. Wu, et al. 2015. Mulberry fruit prevents LPS-induced NF-kB/pERK/MAPK signals in macrophages and suppresses acute colitis and colorectal tumorigenesis in mice. Sci. Rep. 5:17348. doi: 10.1038/srep17348.
- Qin, C., Y. Li, W. Niu, Y. Ding, R. Zhang, and X. Shang. 2010. Analysis and characterization of anthocyanins in mulberry fruit. Czech J. Food Sci. 28(2):117–126. doi: 10.17221/228/2008-CJFS.
- Ramesh, H.L., V. Sivaram, and V.N.Y. Murthy. 2014. Antioxidant and medicinal properties of Mulberry (Morus SP.): A review. World J. Pharmaceut. Res. 3(6):320–343.
- Rebai, O., M. Belkhir, S. Fattouch, and M. Amri. 2017. Phytochemicals from mulberry extract (Morus sp.): Antioxidant and neuroprotective potentials. J. App. Pharmaceut. Sci. 7(1):217–222. doi: 10.7324/JAPS.2017.70133.
- Rehman, S.U., S.A. Shah, T. Ali, J.I. Chung, and M.O. Kim. 2017. Anthocyanins reversed D-galactose-induced oxidative stress and neuroinflammation mediated cognitive impairment in adult rats. Mol. Neurobiol. 54:255–271. doi: 10.1007/s12035-015-9604-5.
- Ruby, T., and C.S. Rana. 2015. Plant secondary metabolites: A review. Int. J. Eng. Res. Gen. Sci. 3(5):661–670.
- Sánchez-Salcedo, E.M., P. Mena, C. García-Viguera, J.J. Martínez, and F. Hernández. 2015. Phytochemical evaluation of white (Morus alba L.) and black (Morus nigra L.) mulberry fruits, a starting point for the assessment of their beneficial properties.J. Funct. Foods. 12: 399–408. doi:10.1016/j.jff.2014.12.010.
- Senica, M., F. Stampar, and M. Mikulic-Petkovsek. 2019. Different extraction processes affect the metabolites in blue honeysuckle (Lonicera caerulea L. subsp. edulis) food products. Turk. J. Agric. For. 43:576–585. doi: 10.3906/tar-1907-48.
- Shrikanta, A., A. Kumar, and V. Govindaswamy. 2015. Resveratrol content and antioxidant properties of underutilized fruits. J. Food Sci. Technol. 52(1):383–390. doi: 10.1007/s13197-013-0993-z.
- Singhal, B.K., A.K. Mohammad, D. Anil, M.B. Farooq, and B.B. Bharat. 2010. Approaches to industrial exploitation of mulberry (Mulberry sp.) fruits. J. Fruit Ornamen Plant Res. 18(1):83–99.
- Sirikanchanarod, A., A. Bumrungpert, W. Kaewruang, T. Senawong, and P. Pavadhgul. 2016. The effect of mulberry fruits consumption on lipid profiles in hypercholesterolemic subjects: A randomized controlled trial. J. Pharmac. Nutri. Sci. 6:7–14. doi: 10.6000/1927-5951.2016.06.01.2.
- Sofia, P.G., V. Riana-Bianca, C. Corina, I. Gogoasa, G. Corina, and P. Cerasela. 2014. Chemical characterization of white (Morus alba), and black (Morus nigra) mulberry fruits. J. Horti. Forest Biotech. 18(3):133–135.
- Speciale, A., F. Virgili, A. Saija, and F. Cimino. 2014. Anthocyanins in vascular diseases, p. 923–941. In: R.R. Watson, V.R. Preedy, and S. Zibadi (eds.). Polyphenols in human health and disease. Elsevier.
- Swathi, P., G. Manjusha, M. Vivekanand, A. Ramkishan, and B. Bhavani. 2017. Effect of Morus alba against Hyperglycemic and Hyperlipidemic activities in streptozotocin-induced Diabetic Nephropathy. Biosci. Biotechnol. Res. Asia. 14:1441–1447. doi: 10.13005/bbra/2589.
- Tomas, M., G. Toydemir, D. Boyacioglu, R. Hall, J. Beekwilder, and E. Capanoglu. 2015. The effects of juice processing on black mulberry antioxidants. Food Chem. 186:277–284. doi: 10.1016/j.foodchem.2014.11.151.
- Vijayan, K., S.P. Chakraborti, S. Ercisli, and P.D. Ghosh. 2008. NaCI induced morpho-biochemical and anatomical changes in mulberry (Morus spp.). Plant Growth Regul. 56(1):61–69. doi: 10.1007/s10725-008-9284-5.
- Wang, Y., L. Xiang, C. Wang, C. Tang, and X. He. 2013. Antidiabetic and antioxidant effects and phytochemicals of mulberry fruit (Morus alba L.) polyphenol enhanced extract. PLoS ONE 8(7):e71144. doi: 10.1371/journal.pone.0071144.
- Yan, F., G. Dai, and X. Zheng. 2016. Mulberry anthocyanin extract ameliorates insulin resistance by regulating PI3K/AKT pathway in HepG2 cells and db/db mice. J. Nutri. Biochem. 36:68–80. doi: 10.1016/j.jnutbio.2016.07.004.
- Yang, J., X. Liu, X. Zhang, Q. Jin, and J. Li. 2016. Phenolic profiles, antioxidant activities, and neuroprotective properties of mulberry (MorusatropurpureaRoxb.) fruit extracts from different ripening stages. J. Food Sci. 81:C2439–C2446. doi: 10.1111/1750-3841.13426.
- Yang, J.Y., and H.S. Lee. 2012. Evaluation of antioxidant and antibacterial activities of morin isolated from mulberry fruits (Morus alba L.). J. Korean Soc. App. Bio. Chem. 55:485–489.
- Yang, X., L. Yang, and H. Zheng. 2010. Hypolipidemic and antioxidant effects of mulberry (Morus alba L.) fruit in hyperlipidaemia rats. Food Chem. Toxicol. 48(8–9):2374–2379. doi: 10.1016/j.fct.2010.05.074.
- Yigit, D., A. Mavi, and M. Aktas. 2008. Antioxidant activities of black mulberry (Morus nigra). Erzincan Univ. J. Sci. Technol. 1:223–232.
- Yuan, Q., and L. Zhao. 2017. The mulberry (Morus alba L.) fruit: A review of characteristic components and health benefits. J. Agri. Food Chem. 65(48):10383–10394. doi: 10.1021/acs.jafc.7b03614.
- Zhu, J., L. Wang, Z. Xiao, and Y. Niu. 2018. Characterization of the key aroma compounds in mulberry fruits by application of gas chromatography GÇôolfactometry (GC-O), odor activity value (OAV), gas chromatography-mass spectrometry (GC-MS) and flame photometric detection (FPD). Food Chem. 245:775–785. doi: 10.1016/j.foodchem.2017.11.112.