 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
1-methylcyclopropene (1-MCP) extends shelf-life of banana fruit, however, it induces uneven ripening. The strong effect 1-MCP has on ethylene receptors probably condition the fruit to ripen unevenly. Combined application of 1-MCP and ethephon has been reported to alleviate this negative effect. However, limited work reported the effect of the treatment at cold storage. Hence, the present study determined whether the combined application of 1-MCP and ethylene prolongs banana green-life during cold storage and induces uniform ripening during shelf-life. The study comprised four treatments: control (untreated), gaseous 1-MCP at 400 nL L−1 and combination of 400 nL L−1 1-MCP with liquid ethephon at 50 or 100 µL L−1 concentrations. The treatments were arranged in a completely randomized design (CRD) with 4 replicates. The treated fruit were cold stored at 14°C for 30 d and thereafter 23°C to simulate shelf-life for 9 d. The results showed that 400 nL L−1 1-MCP and combined with 50 and 100 µL L−1 ethephon prolonged green-life and delayed ripening during cold storage compared to the control. In addition, there was more softening, yellow color development, and accumulation of soluble sugars in fruit treated with 1-MCP+ethephon compared to 1-MCP during shelf-life. The combined application of 1-MCP and ethylene is an effective strategy that prolongs banana green-life during cold storage and induces uniform ripening during shelf-life. This strategy has potential to be used when banana fruit are exported to distant markets, since it also prolongs green-life for a long time.
Introduction
Banana fruit (Musa acuminate, AAA group) is an important source of food that have a wide range of health benefits in humans, which include micro and macro nutrients (Anyasi et al., Citation2013; Pereira and Maraschin, Citation2015; Singh et al., Citation2016). In South Africa, banana fruit are sold both at domestic and export markets (DAFF, Citation2016). However, export markets have been concentrated in African continent. This is despite the fact that South Africa has potential to export banana fruit in lucrative markets such as the Middle East and Russian Federation. However, the limited technologies in banana industry to prolong storage and green-life thwart these markets to be exploited.
Bananas are highly perishable climacteric fruit that loss green-life rapidly at ambient temperature and are susceptible to chilling injury at temperatures below 13°C (Facundo et al., Citation2015; Jiang et al., Citation2004). Therefore, fruit are kept at 13–15°C to prolong green-life (Facundo et al., Citation2015; Huang et al., Citation2013; Jedermann et al., Citation2014). These temperatures can effectively prolong banana green-life for up to 2–3 weeks in a storage (Huang et al., Citation2013). Bananas for long distant export markets are harvested at pre-climacteric mature green stage and should remain green until reaching their destination. Thereafter, ripening is initiated artificially using propylene or ethylene at 18–24°C. However, long distant involved in export markets could result in fruit ripening in transit and subsequently spoilage, which may affect fruit acceptability by both retailers and customers. Thus, various postharvest strategies that have potential in prolonging banana green-life have been investigated.
One of the technologies that have been widely investigated is ethylene antagonist 1-methylcyclopropene (1-MCP) (Watkins, Citation2006). This technology has been effective in extending shelf-life of apricot (Egea et al., Citation2010), bananas (Zhu et al., Citation2015), broccoli (Gómez-Lobato et al., Citation2012), mango (Vázquez-Celestino et al., Citation2016), pears (Cheng et al., Citation2012; Yu and Wang, Citation2017). Upon application of 1-MCP onto the fruits, the interaction of both exogenous and endogenous ethylene with ethylene receptors is inhibited, thus ethylene-depended processes such as ripening (Sisler and Serek, Citation1997; Sisler et al., Citation2006, Citation2009; Watkins, Citation2006). Although 1-MCP has been effective in delaying ripening in climacteric fruits, the application onto banana fruit led to uneven ripening (Botondi et al., Citation2014; Harris et al., Citation2000; Zhu et al., Citation2015). It is unclear what causes 1-MCP to induce uneven ripening in banana fruit. However, higher 1-MCP concentrations (0.7–1 µL L−1) detrimentally affected banana fruit ripening. With 300–500 nL L−1 1-MCP being optimum. For example, 400 nL L−1 1-MCP extended banana shelf-life at ambient temperature compared to 600 nL L−1, which made the fruit not to ripen properly (Zhu et al., Citation2015). In another banana study, 300 nL L−1 1-MCP effectively delayed fruit ripening at 14 °C (Botondi et al., Citation2014). Furthermore, Harris et al. (Citation2000) and Pongprasert and Srilaong (Citation2014) reported that 500 nL L−1 1-MCP delayed banana ripening. 1-MCP delays ripening in climacteric fruits such as banana by binding ethylene receptors (Sisler and Serek, Citation1997; Sisler et al., Citation2006). Therefore, this probably suggest that 1-MCP effect on receptors is strong and can be detrimental to the treated fruit, especially if higher concentrations are used.
Unfortunately, the uneven ripening phenomenon induced by 1-MCP makes the fruit less suitable for export or fetches less market price. This phenomenon also appears to be a major factor that hinders the utilization of 1-MCP technology in banana industry in various countries (Blankenship and Dole, Citation2003; Watkins, Citation2006) including South Africa. The commercial success of 1-MCP technology is based on delaying fruit ripening than preventing it (Lurie, Citation2007). Therefore, research on strategies that mitigate 1-MCP negative effect in fruit with the aim of prolonging banana green-life with subsequent uniform ripening is warranted.
Previous studies have shown that the application of 1-MCP in combination with ethylene has a potential to prolong banana green-life with subsequent uniform ripening (Botondi et al., Citation2014; Zhu et al., Citation2015). Zhu et al. (Citation2015) found that the application of liquid ethephon at 50 µL L−1 for 1 min followed by gaseous 1-MCP at 400 nL L−1 for 16 h prolonged banana shelf-life, without affecting normal ripening. In another banana study, Botondi et al. (Citation2014) observed that the simultaneous application of gaseous 300 nL L−1 1-MCP alone and combined with 1200 or 2400 nL L−1 ethylene for 16 h at 14°C prolonged fruit shelf-life, with subsequent normal ripening compared to 300 nL L−1 1-MCP alone or combined with 4800 nL L−1 ethylene. These studies demonstrated that the combined application of 1-MCP and ethylene allows for a normal or proper ripening after ripening initiation compared to 1-MCP alone. However, the treatment is not yet applied commercially in various banana-producing countries.
Furthermore, little is known about the combined effect of 1-MCP and ethylene on banana fruit green-life during cold storage or after long-term cold storage and long-distant transportation, which is crucial for banana export markets. Botondi et al. (Citation2014) evaluated the effect of the combined application of 1-MCP and ethylene after 20 d cold storage at 14°C. Whereas Zhu et al. (Citation2015) evaluated the treatments for 14 d at 23°C, before artificially initiating ripening. Thus, investigating the combined effect of 1-MCP and ethylene treatment under long-term cold storage could indicate the storage potential of the treatment for use in the marketing and transportation of banana fruit into distant markets. The objective of the present work was, therefore, to determine whether the combined application of 1-MCP and ethylene prolongs banana green-life during cold storage and subsequently allows a proper ripening during shelf-life.
Materials and Methods
Plant Materials
Green banana (cv. Williams) fruit with reduced but visible angularity (three-quarter full) was obtained from Burgershall Research Station of the Agricultural Research Council (ARC)-Institute for Tropical and Subtropical Crops, Mpumalanga Province, South Africa (25°6′0” S, 31°4′60”. After harvest, fruit were transported in a well-ventilated vehicle to the Postharvest Laboratory at the University of KwaZulu Natal, Pietermaritzburg, arriving within 8h. Upon arrival, fruit were equilibrated at 14°C for 16 h. To avoid fruit with uneven maturity, only hands located in the middle of the bunch from both inner and outer whorl rows were used.
Postharvest Treatments, Storage and Sampling
In a preliminary study, fruit were fumigated with 400 or 500 nL L−1 1-MCP for 16 h at 14°C then immersed in 0, 50 or 100 µL L−1 ethephon for 5 min. Thereafter stored at 14 °C for 30 d. Fruit treated with 500 nL L−1 1-MCP alone, 500 nL L−1 1-MCP+50 or 500 1-MCP+100 µL L−1 ethephon did not ripen properly. Therefore, 400 nL L−1 1-MCP alone, 400 nL L−1 1-MCP+50 µL L−1 ethephon and 400 nL L−1 1-MCP +100 µL L−1 ethephon were used in this present study.
Fruit were randomly divided into four groups and packed into cardboard boxes and replicated four times. Each group received one of the four treatments: control (untreated), 400 nL L−1 1-MCP alone, 400 nL L−1 1-MCP combined with 50 or 100 µL L−1 ethephon. Fruit were immersed in ethephon treatments for 5 min. Thereafter 1-MCP concentrations exposure for 16 h (in 100-L sealed plastic drum) at 14 °C as described by Botondi et al. (Citation2014). Both control and treated fruit were then stored at 14 °C for 30 d. Reagents used were Ethephon (480 SL, Villa) and 1-MCP (SmartFreshTM, 0.14% AgroFresh Inc., Rohm and Hass, Philadelphia, PA). The 1-MCP powder was calculated from percent active ingredient according to the manufacturer’s instruction. The calculated 1-MCP powder was diluted with deionized water in a glass vial. Physico-chemical parameters were measured at 0 d before storage, and at 10 d intervals during storage until 30 d. Thirty days after cold storage, fruit from all the groups were immersed in 800 µL L−1 ethephon for 5 min to initiate ripening as described by Zhu et al. (Citation2015). Thereafter, stored at 23°C to simulate shelf-life conditions, where physico-chemical parameters were measured at 3 d intervals for up to 9 d.
Determination of Peel Color Using Color Chart
A total of 7 fruit per replicate was used to evaluate peel color based on the Dole® color chart (Dole Food Company, Inc., CA, USA). Dole color chart was used to rate peel color based on scale 1 to 7, where 1 = all green, 2 = light green, 3 = half green and yellow, 4 = more yellow than green, 5 = yellow with green tips, 6 = full yellow and 7 = yellow flecked with brown color. Thereafter, the color index was calculated using EquationEq. 1:
Determination of Peel Color Using Colorimeter
Fruit peel color was determined by measuring the middle part of the fruit around the equatorial region at three points using a portable colorimeter (Chroma Meter, Konica Minolta Sensing Inc., Japan) expressing CIELAB color space; L*, a* and b*. The colorimeter was calibrated using calibration white tile before taking the readings in each treatment and during each sampling day. Furthermore, the color parameters Chroma and hue angle were calculated using EquationEqs. 2 and Equation3
, respectively.
Determination of Chlorophylls and Total Carotenoids
Freeze-dried peel tissue of banana fruit was ground into a fine powder using mortar and pestle. Thereafter, chlorophylls (Chls) and total carotenoids were extracted by immersing 0.2 g powder of peel tissue in 80% (v/v) ice-cold acetone. After extraction in the dark for 30 min, the extract was centrifuged for 20 min using GenVac® (SP Scientific, Genevac LTD., Suffolk, UK) at room temperature. The absorbance of the supernatant was read on UV-1800 Spectrophotometer (Shimadzu Scientific Instruments INC., Columbia, USA) at the wavelengths required for Chlorophyll a and b and total carotenoids (Lichtenthaler and Buschmann, Citation2001). Chlorophyll a (Chla), chlorophyll b (Chlb), total chlorophyll (Chla+b) and total carotenoids (Cx+c) were calculated according to equations enumerated by Lichtenthaler and Buschmann (Citation2001). The results were expressed as µg/g on dry mass basis.
Determination of Firmness and Mass Loss
Fruit firmness was measured at 10 and 3 d intervals during storage and shelf-life, respectively, using hand-held firmness tester (Bareiss, Germany). Fruit firmness was expressed as the mean of two readings taken at the equatorial region of the fruit on opposite sides and the results were expressed as Newton (N). Fruit mass was measured using a digital weighing balance (RADWAG Wagi Electronic Inc., Poland) and mass loss was calculated as a cumulative percentage (%) loss in mass based on mass weight before storage and at the sampling time during storage. The mass loss was calculated using EquationEq. 4 and expressed as %.
Determination of Pulp Non-structural Carbohydrates
Non-structural carbohydrates were extracted and quantified using the methodology described by Ncama et al. (Citation2018), with modification. Briefly, non-structural carbohydrates were extracted by immersing 0.5 g of freeze-dried banana pulp powder in 10 mL of 80% (v/v) ethanol. The samples were placed in water bath at 80 °C for 1 h, with occasional agitation to prevent layering, and left overnight to cool at 4 °C. Thereafter, the supernatant was evaporated in Genvac evaporator (Genvac ® EZ 2.3; IPSWICH; England) to remove the alcohol. The evaporated samples were diluted with 2 mL ultra-pure water before filtered through a 0.45 µm syringe nylon filter and transferred into glass vial for high performance liquid chromatography (HPLC) analysis. Concentrations of glucose, fructose and sucrose were quantified using the HLPC binary pump (Shimadzu Scientific Instruments INC., Columbia, USA), equipped with refractive index detector. The extracts were injected into a Rezex RCM monosaccharide CA+ (8%) column of 7.8 mm diameter x 300 mm length (Phenomenex, Torrance, CA, USA). Ultra-pure water was used as a mobile phase and analysis was performed at a flow rate of 0.6 µL/min and column temperature of 85 °C (thermo-stated column compartment; G1316A, Shimadzu Scientific Instruments INC., Columbia, USA). The presence and concentration of three individual sugars were calculated by comparing the peak area of samples against those of known fructose (0.5–2.5 mg/mL; Y = 297093X – 7852.9; R2 = 0.99), glucose (0.5–2.5 mg/mL; Y = 249543X – 11,170; R2 = 0.99) and sucrose (0.5–2.5 mg/mL; Y = 261,145 X – 15,260; R2 = 0.99) standard concentration curves and expressed in mg/mL on a dry weight basis.
Statistical Analysis
The experiment was arranged in a completely randomized design (CRD) with four replicates for each treatment. Experimental data were statistically analyzed using analysis of variance (ANOVA) to determine whether the combined application of 1-MCP and ethylene treatments significantly (p ≤ 0.05) prolonged banana green-life during storage and maintained good quality at shelf-life. Duncan Multiple Range (DMR) test was used to test significant differences among treatments means. All the analyses were performed using GenStat statistical software (GenStat®, 18th edition, VSN International, UK).
Results
Banana Ripening during Cold Storage and Shelf-life
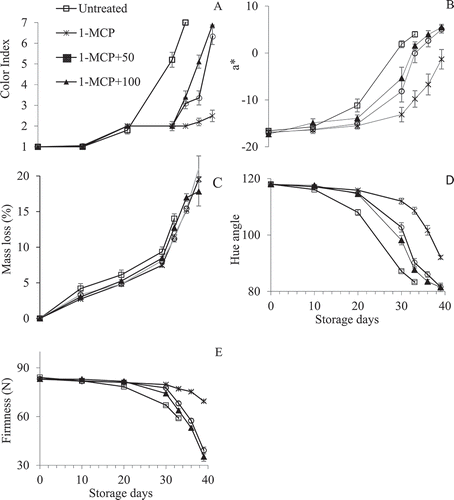
Banana ripening was significantly (p < .001) delayed by 400 nL L−1 1-MCP alone or combined with 50 or 100 µL L−1 ethephon during cold storage, at 14°C. In control fruit (untreated), peel color significantly turned yellow within 20d at 14°C compared to 1-MCP treatments. During shelf-life, at 23°C, fruit treated with 1-MCP+50 µL L−1 ethephon and 1-MCP+100 µL L−1 ethephon significantly turned yellow within 6 d and 9 d, respectively, compared to 1-MCP alone, which remained green. In fact, fruit treated with 1-MCP combined with 50 or 100 µL L−1 ethephon were fully yellow at 9 d compared to the 1-MCP alone, which remained green. Overall, untreated fruits were fully yellow on 30 d at cold storage, whereas those treated with 400 nL L−1 1-MCP plus 50 or 100 µL L−1 ethephon were fully yellow at 9 d, shelf-life. Fruit treated with 1-MCP alone remained green throughout shelf-life period (,,)), indicating that the fruit did not ripen.
Figure 1. Combined effect of ethephon and 1-MCP on color index (A), a* (B), mass loss (C), hue angle (D) and firmness (E) stored at 14 and 23 °C for 30 d and 9 d, respectively. Fruit were green from 0–30 d and treated with ethephon at 30 d to initiate ripening, which lasted for 30–39 d. Each value is a mean of four replicates (n= 4) ± standard error. 1-MCP represents 400 nL L−1 1-MCP; 1-MCP+50, 1-MCP plus 50 µL L−1 ethephon; 1-MCP+100, 1-MCP plus 100 µL L−1 ethephon
Color, Chlorophyll and Carotenoids Change
The degreening of banana peel was observed in control fruit at 20 d, as observed by lower a* (). and hue (h) () values, compared with 1-MCP and 1-MCP+ethephon-treated fruit. The application of 1-MCP treatments significantly (p < .001) delayed the peel degreening during cold storage. However, the h values for fruit treated with 1-MCP plus 50 or 100 µL L−1 ethephon fruit started to decrease significantly (p < .001) (degreening) on 3 d shelf-life from 90.23 to 81.54 and 87.68 to 81.54, respectively. In 1-MCP-treated fruit, h value was 92.06 at 9 d (). The a* values increased from negative to positive, in untreated and 1-MCP+50 and 100 µL L−1 ethephon fruit, indicating that the fruit was losing green color. During shelf-life, a* value of fruit treated with 1-MCP+50 and 100 µL L−1 ethephon increased from −8.18 to 5.05 and −14. 89 to 5.49, respectively, compared to those in 1-MCP-treated, which increased from −13.14 to −1.33 (). The lightness value reached its peak (58.95) at 30 d in control fruit compared to 1-MCP and 1-MCP+50 µL L−1 ethephon and 1-MCP+100 µL L−1 ethephon, which reached its peak at 9 (50.50), 6 (56.76) and 3 (59.03) d shelf-life, respectively (). The effect of 1-MCP treatments on the concentrations of chlorophyll and carotenoids during cold storage and shelf-life was significant (p < .001). 1-MCP alone and combined with 50 or 100 µL L−1 ethephon significantly reduced the concentration of total chlorophyll and carotenoids throughout the cold and shelf-life storage compared to the control. However, in 1-MCP-treated fruit the concentrations of total chlorophyll and carotenoids were significantly higher, 307.7 and 145.03 µg/g, respectively, compared to those in 1-MCP+50 µL L−1 ethephon (69.60 and 75.82 µg/g, for total chlorophyll and carotenoids, respectively) and 1-MCP+100 µL L−1 ethephon (135 and 77.39 µg/g, for total chlorophyll and carotenoids, respectively) fruit. Irrespective of the treatments, both carotenoids and chlorophyll content significantly decreased during cold storage and shelf-life ().
Table 1. Combined effect of ethephon and 1-MCP on color parameters of banana stored at 14 and 23 °C for 30 and 9d, respectively
Firmness Change
The effect of 1-MCP alone and combined with 50 or 100 µL L−1 ethephon on banana firmness during cold storage and shelf-life is shown in . As shown in , the firmness of control fruit was significantly (p < .001) lower compared to fruit treated with 1-MCP and 1-MCP+ethephon at 20 d. The firmness of fruit treated with 1-MCP+50 µL L−1 ethephon or 1-MCP+100 µL L−1 ethephon started to decrease significantly at 30 d through shelf-life, from 77.67 to 39.14 N and 74.20 to 35.28 N, respectively, compared to 1-MCP treated (79.70 to 69.45 N). The firmness of 1-MCP-treated fruit remained higher during shelf-life.
Mass Loss
As shown in , control fruit significantly (p < .001) lost 10% mass at 20 d cold storage compared to those treated with 1-MCP alone or combined with 50 or 100 µL L−1 ethephon, which lost 5%. On 30 d, mass loss in 1-MCP-treated fruit was significantly lower (7.5%) compared to untreated (9.3%), 1-MCP+50 µL L−1 ethephon (8.1%) and 1-MCP+100 µL L−1 ethephon (8.5%) fruit. During shelf-life, fruit significantly lost 5.08, 8.11 and 9.79% mass in 1-MCP alone, 1-MCP+50 µL L−1 and 1-MCP+100 µL L−1 ethephon treatments, respectively.
Glucose, Fructose and Sucrose Concentrations
During harvest, on 0 d, only the concentration of sucrose was detected in banana fruit and continued to increase during cold storage and shelf-life compared to the reducing sugars (glucose and fructose). The reducing sugars were only detected at 30 d and increased significantly (p < .001) during shelf-life (). The application of 1-MCP alone and combined with 50 or 100 µL L−1 ethephon significantly (p < .001) delayed the increase of sugars compared to the control. Fruit treated with 1-MCP alone had a significantly lower sucrose concentration (0.81 mg/mL) at 30 d and throughout the shelf-life compared to those treated with 1-MCP+50 µL L−1 ethephon (3.05 mg/mL) and 1-MCP+100 µL L−1 ethephon (4.61 mg/mL). During shelf-life, 1-MCP+50 µL L−1 ethephon significantly decreased sucrose concentration from 4.61 to 3.82 mg/mL in banana fruit compared to 1-MCP+50 µL L−1 ethephon (4.79 to 4.23 mg/mL). In control fruit, the sucrose concentration was 4.93 mg/mL. Furthermore, the application of 1-MCP+100 µL L−1 ethephon increased glucose and fructose (0.63 to 0.85 and 0.51 to 0.67 mg/mL, respectively) concentrations, whereas 1-MCP+50 µL L−1 ethephon increased the concentrations from 0.42 to 0.80 and 0.34 to 0.62 mg/mL for glucose and fructose, respectively ().
Table 2. Combined effect of ethephon and 1-MCP on sugar parameters of banana stored at 14 and 23 °C for 30 and 9d, respectively
Discussion
The objective of the present work was to determine whether the combined application of 1-MCP and ethylene prolongs banana green-life during cold storage and subsequently allow a proper ripening during shelf-life. In the present study, fruit treated with 400 nL L−1 1-MCP alone and combined with 50 or 100 µL L−1 ethephon remained green for up to 30 d without a sign of ripening compared to the control. The control fruit started ripening at 20 d during storage, as can be seen by the decline of firmness and hue values (,)). In accordance with our results, the application of 1-MCP alone and combined with ethylene delayed banana green-life for 14 d at 14°C (Botondi et al., Citation2014). This is probably a result of synergistic effect of lower temperature and 1-MCP on fruit ripening, since both 1-MCP and lower temperature reduce fruit ripening. However, they may differ in their mode of action and the efficacy to reduce ripening. Lower temperatures (13–15°C) can prolong banana green-life for up to 14–20 days at (Facundo et al., Citation2015); also, as seen in the present study (,,)). Although there are limited reports on the efficacy of 1-MCP at cold storage, the present study showed that 400 nL L−1 1-MCP alone and combined with 50 or 100 µL L−1 ethephon prolong banana green-life during cold storage. These results are important for banana transportation to distant export markets. Furthermore, fruit treated with 1-MCP+50 µL L−1 ethephon and 1-MCP+100 µL L−1 ethephon in the present study had a proper ripening compared to 1-MCP alone during shelf-life. This indicates that, although both 1-MCP alone and 1-MCP+ethephon prolong banana green-life, their effects change and differ at shelf-life, after ripening initiation.
The delay in color change was related to the delay in chlorophyll degradation (). During shelf-life, there was a decrease in the concentration of chlorophyll and a visible yellow color development on 400 nL L−1 1-MCP plus 50 or 100 µL L−1 ethephon-treated fruit. However, on 1-MCP-treated fruit there was no visible yellow color, despite the continuous decrease of chlorophyll content (). In agreement with our findings, Zhu et al. (Citation2015) and Botondi et al. (Citation2014) also found that 1-MCP-treated banana fruit were green compared to the control and 1-MCP+ethphon- treated fruit at shelf-life, although they did not measure the concentration of chlorophylls. The effect of 1-MCP on chlorophyll catabolism in a peel of banana fruit is seldom reported in the literature. However, in pear (Cheng et al., Citation2012; Zhao et al., Citation2018) and broccoli (Gómez-Lobato et al., Citation2012), 1-MCP maintained higher chlorophyll content by downregulating the expression and activities of chlorophyll degradation pathway genes and enzymes such as chlorophyllase and pheophytin pheophorbide hydrolase. We did not measure the activities of these enzymes in our study. However, based on the generally accepted chlorophyll degradation pathway (Barry, Citation2009), it is likely that 1-MCP mode of action in chlorophyll catabolism is similar in all the fruits. The combined application of 1-MCP+ethephon probably inhibited these enzymes transiently, hence its effect subsided at shelf-life after ripening initiation compared to 1-MCP alone.
These findings indicated that the application of 1-MCP in combination with ethylene is an effective strategy in prolonging green-life and shelf-life as observed in other banana studies (Botondi et al., Citation2014; Zhu et al., Citation2015). Therefore, the strategy could be deployed when exporting banana fruit to distant markets. However, care should be taken not to apply the treatment onto the fruit of different maturities. It has been reported that there is a variation in maturity of banana fruit within a bunch which contributed to the reduction of 1-MCP efficacy and compromised fruit quality (Harris et al., Citation2000; Moradinezhad et al., Citation2008).
During a normal ripening of banana fruit, there is a degradation of chlorophylls and the unmasking of carotenoids (Yang et al., Citation2009). In the present study, irrespective of 1-MCP treatments, both the concentration of total chlorophyll and carotenoids decreased. In agreement with our findings, Barreto et al. (Citation2011) observed that ethylene and 1-MCP reduced total carotenoid content in papaya fruit during ripening. Similarly, as yellowing of celery increased, total carotenoids decreased in both ethylene and 1-MCP-treated produce (Massolo et al., Citation2019). It was speculated that ethylene and 1-MCP probably inhibited carotenoids synthesis (Barreto et al., Citation2011). It is unclear whether the concentration of carotenoids increased with decreasing chlorophylls in order to confer a yellow color. The decreasing chlorophyll and carotenoid concentrations in ripening banana observed in this present study may indicate that peel yellowing is closely correlated with chlorophyll degradation rather than carotenoids synthesis and accumulation. Since this is speculation, more research is needed in order to understand mechanisms underlying color change in banana fruit. Moreover, for an unknown reason, total carotenoid was increasing in 1-MCP-treated fruit during storage compared to the control and 1-MCP+ethephon-treated, however, it decreased during shelf-life. In apricot (Prunus armeniaca L.), Egea et al. (Citation2010) found that 1-MCP increased the total carotenoid content. These authors speculated that 1-MCP may have increased carotenoids as a response to oxidative stress conferred by lower temperatures, 2°C. We did not find any evidence to suggest that fruits were under any oxidative stress. Therefore, it is unclear why total carotenoids increased during storage. However, these findings support the fact that carotenoids are more than just color pigments.
During banana ripening there is a degradation of the cell wall, which leads to fruit softening (Zhu et al., Citation2015). In the present study, 1-MCP treatments inhibited fruit softening during cold storage, indicating that the treatments were effective in reducing fruit ripening. Immediately after cold storage removal and ripening initiation, softening occurred in fruit treated with 1-MCP+ethephon compared to 1-MCP alone. Similar results were obtained in banana fruit (Botondi et al., Citation2014; Zhu et al., Citation2015). It was found that 1-MCP delays fruit softening by inhibiting the activities of cell wall softening related enzymes such as pectin lyase, pectin methylesterase, cellulase, and polygalacturonase (Zhu et al., Citation2015). These authors further found that in 1-MCP+ethephon treated fruit, the activities of these enzymes increased rapidly, concomitant with a decrease in firmness compared to 1-MCP alone. Our results show that there is a close relationship between softening and mass loss as reported in earlier study (Vázquez-Celestino et al., Citation2016). For instance, the higher mass loss, the lower firmness, leading to increased fruit softening. 1-MCP treatments delayed both mass loss and softening compared to the control (,)).
The fruit sweetness is influenced by the composition and amount of accumulated sugars (Itai and Tanahashi, Citation2008). In the present study, banana fruit had higher sucrose concentration at harvest, which continued to increase during cold storage and shelf-life. This indicates that sucrose is a major soluble sugar in green banana fruit at harvest, which is important to sustain higher respiration rate (Bekele et al., Citation2015). However, glucose and fructose started to accumulate at shelf-life (). In a ripening fruit, sucrose is converted to glucose and fructose (Yu et al., Citation2015). 1-MCP alone and combined with 50 µL L−1 ethephon significantly reduced sucrose content compared to 1-MCP+100 µL L−1 ethephon and control during cold storage. This is probably due to the lower respiration rate and thus delay in fruit ripening as can be seen in , with a color index being significantly higher in control and 1-MCP+100 µL L−1 ethephon-treated fruit. There are limited reports on the role of combined 1-MCP with ethephon on soluble sugars in fruit. Our results suggest that sucrose metabolism in 1-MCP+ethephon-treated fruit is ethylene concentration depended. With higher ethylene concentration favoring rapid ripening after storage and higher soluble sugar accumulation.
It is surmised that applying 1-MCP and ethylene simultaneously allows for rapid and uniform fruit ripening due to one part of the receptor occupied by 1-MCP and the other by ethylene (Yu and Wang, Citation2017). However, the affinity of 1-MCP is believed to be 10 times more than that of ethylene (Sisler et al., Citation2009). This probably indicates that ethylene has to be applied first before 1-MCP, as done in this present study and previously by Zhu et al. (Citation2015) compared to simultaneous application. Zhu et al. (Citation2015) hypothesized that ethephon activates certain ethylene receptors and alleviates the effect of 1-MCP alone. It has been argued that 1-MCP inhibits ethylene response rather than ethylene production. Therefore, 1-MCP treatment may allow for ethylene production at system 1. For climacteric fruit to ripen, ethylene production must be in a system 2 (Barry and Giovannoni, Citation2007). Therefore, the transition from system 1 to 2 in 1-MCP treated fruit may depends on the quantities of ethylene receptors. We surmise that in 1-MCP+ethylene treated fruit there are more ethylene receptors than 1-MCP-treated. The more ethylene receptors in 1-MCP+ethylene fruit probably allow the fruit to respond better to endogenous ethylene; hence a proper ripening was observed after ripening initiation in the present study (,,)).
It is cumbersome to quantify ethylene receptors in fruit. Hence limited reports quantifying receptors in the literature. In banana (Zhu et al., Citation2015) and pear (Chiriboga et al., Citation2013), the activities of ethylene enzymes were higher in 1-MCP+ethylene treatment fruit compared to 1-MCP alone. These studies reported higher ethylene production and respiration rate with subsequent ripening recovery in 1-MCP+ethylene-treated fruit compared to those treated with 1-MCP alone. We did not measure respiration rate, ethylene production or ethylene-related enzymes in the present study. However, in climacteric fruit such as banana, it has been shown that firmness and green color decrease with increasing respiration rate and ethylene production during ripening (Barry and Giovannoni, Citation2007). Therefore, the drastic changes of these variables at shelf-life, probably indicate that the fruit ethylene production was high (at system 2) in 1-MCP+ethephon fruit compared to 1-MCP alone. The 1-MCP-treated fruit were green and firm. Perhaps indicating that the fruit ethylene production was lower at system 1.
Conclusion
In the present study, banana fruit immersed in 50 or 100 µL L−1 ethephon and subsequently treated with 400 nL L−1 1-MCP had extended green-life during cold storage and a normal ripening during shelf-life, after ripening initiation. Fruit treated with 1-MCP alone were green and firm during shelf-life. The application of 400 nL L−1 1-MCP combined with 50 or 100 µL L−1 ethephon reduced firmness and moisture losses, color change and accumulation of soluble sugars during storage. The application of 400 nL L−1 1-MCP+50 µL L−1 ethephon and 400 nL L−1 1-MCP+100 µL L−1 has the potential for use in marketing and transportation of banana fruit into distant markets. However, a careful fruit grading for uniformity in maturity will be important for treatment application. Therefore, the concentration of 1-MCP plus ethephon may be different according to fruit maturity.
Acknowledgments
This work is based on a research supported by Agricultural Research Council through the program ‘Professional and Graduates Development.
ReferencesReferences
- Anyasi, T.A., A.I. Jideani, and G.R. Mchau. 2013. Functional properties and postharvest utilization of commercial and noncommercial banana cultivars. Comp.r Rev. Food Sci. Food Saf. 12(5):509–522. doi: 10.1111/1541-4337.12025.
- Barreto, G.P., J.P. Fabi, V.V. De Rosso, B.R. Cordenunsi, F.M. Lajolo, J.R. Do Nascimento, and A.Z. Mercadante. 2011. Influence of ethylene on carotenoid biosynthesis during papaya postharvesting ripening. J. Food Compos. Anal. 24(4–5):620–624. doi: 10.1016/j.jfca.2011.02.006.
- Barry, C.S. 2009. The stay-green revolution: Recent progress in deciphering the mechanisms of chlorophyll degradation in higher plants. Plant Sci. 176(3):325–333. doi: 10.1016/j.plantsci.2008.12.013.
- Barry, C.S., and J.J. Giovannoni. 2007. Ethylene and fruit ripening. J. Plant Growth Regul. 26(2):143. doi: 10.1007/s00344-007-9002-y.
- Bekele, E.A., W.F. Beshir, M.L. Hertog, B.M. Nicolai, and A.H. Geeraerd. 2015. Metabolic profiling reveals ethylene mediated metabolic changes and a coordinated adaptive mechanism of ‘Jonagold’apple to low oxygen stress. Physiol. Plant. 155(3):232–247. doi: 10.1111/ppl.12351.
- Blankenship, S.M., and J.M. Dole. 2003. 1-Methylcyclopropene: A review. Postharvest Biol. Technol. 28(1):1–25. doi: 10.1016/S0925-5214(02)00246-6.
- Botondi, R., F. De Sanctis, S. Bartoloni, and F. Mencarelli. 2014. Simultaneous application of ethylene and 1‐MCP affects banana ripening features during storage. J. Sci. Food Agric. 94(11):2170–2178. doi: 10.1002/jsfa.6599.
- Cheng, Y., Y. Dong, H. Yan, W. Ge, C. Shen, J. Guan, L. Liu, and Y. Zhang. 2012. Effects of 1-MCP on chlorophyll degradation pathway-associated genes expression and chloroplast ultrastructure during the peel yellowing of Chinese pear fruits in storage. Food Chem. 135(2):415–422. doi: 10.1016/j.foodchem.2012.05.017.
- Chiriboga, M.-A., M. Saladié, J.G. Bordonaba, I. Recasens, J. Garcia-Mas, and C. Larrigaudière. 2013. Effect of cold storage and 1-MCP treatment on ethylene perception, signalling and synthesis: Influence on the development of the evergreen behaviour in ‘Conference’pears. Postharvest Biol. Technol. 86:212–220. doi: 10.1016/j.postharvbio.2013.07.003.
- DAFF. 2016. A profile of the South African banana market value chain. DAFF, Preroria, South Africa. https://www.daff.gov.za
- Egea, I., F.B. Flores, M.C. Martínez‐Madrid, F. Romojaro, and P. Sánchez‐Bel. 2010. 1‐Methylcyclopropene affects the antioxidant system of apricots (Prunus armeniaca L. cv. Búlida) during storage at low temperature. J. Sci. Food Agric. 90(4):549–555. doi: 10.1002/jsfa.3842.
- Facundo, H.V.D.V., P.D. Gurak, A.Z. Mercadante, F.M. Lajolo, and B.R. Cordenunsi. 2015. Storage at low temperature differentially affects the colour and carotenoid composition of two cultivars of banana. Food Chem. 170:102–109. doi: 10.1016/j.foodchem.2014.08.069.
- Gómez-Lobato, M.E., J.H. Hasperué, P.M. Civello, A.R. Chaves, and G.A. Martínez. 2012. Effect of 1-MCP on the expression of chlorophyll degrading genes during senescence of broccoli (Brassica oleracea L. Sci. Hort. 144:208–211. doi: 10.1016/j.scienta.2012.07.017.
- Harris, D., J. Seberry, R. Wills, and L. Spohr. 2000. Effect of fruit maturity on efficiency of 1-methylcyclopropene to delay the ripening of bananas. Postharvest Biol. Technol. 20(3):303–308. doi: 10.1016/S0925-5214(00)00150-2.
- Huang, H., G. Jing, L. Guo, D. Zhang, B. Yang, X. Duan, M. Ashraf, and Y. Jiang. 2013. Effect of oxalic acid on ripening attributes of banana fruit during storage. Postharvest Biol. Technol. 84:22–27. doi: 10.1016/j.postharvbio.2013.04.002.
- Itai, A., and T. Tanahashi. 2008. Inhibition of sucrose loss during cold storage in Japanese pear (Pyrus pyrifolia Nakai) by 1-MCP. Postharvest Biol. Technol. 48(3):355–363. doi: 10.1016/j.postharvbio.2007.10.015.
- Jedermann, R., U. Praeger, M. Geyer, and W. Lang. 2014. Remote quality monitoring in the banana chain. Philos. T R Soc. A 372(2017):20130303. doi: 10.1098/rsta.2013.0303.
- Jiang, Y., D.C. Joyce, W. Jiang, and W. Lu. 2004. Effects of chilling temperatures on ethylene binding by banana fruit. Plant Growth Regul. 43(2):109–115. doi: 10.1023/B:GROW.0000040112.19837.5f.
- Lichtenthaler, H.K., and C. Buschmann. 2001. Chlorophylls and carotenoids: Measurement and characterization by UV‐VIS spectroscopy. Curr. Protoc. Food Anal. Chem. M 1(1):F4. 3.1–F4. 3.8. doi: 10.1002/0471142913.faf0403s01.
- Lurie, S. 2007. 1-MCP in post-harvest: Physiological mechanisms of action and applications. Fresh Produce 1(1):4–15. doi: 10.1002/0471142913.faf0403s01.
- Massolo, J.F., L.G. Forte, A. Concellón, S.Z. Viña, and A.R. Vicente. 2019. Effects of ethylene and 1-MCP on quality maintenance of fresh cut celery. Postharvest Biol. Technol. 148:176–183. doi: 10.1016/j.postharvbio.2018.11.007.
- Moradinezhad, F., M. Sedgley, A. Klieber, and A. Able. 2008. Variability of responses to 1‐methylcyclopropene by banana: Influence of time of year at harvest and fruit position in the bunch. Ann. Appl. Biol. 152(2):223–234. doi: 10.1111/j.1744-7348.2007.00206.x.
- Ncama, K., S.Z. Tesfay, O.A. Fawole, U.L. Opara, and L.S. Magwaza. 2018. Non-destructive prediction of ‘Marsh’grapefruit susceptibility to postharvest rind pitting disorder using reflectance Vis/NIR spectroscopy. Sci. Hort. 231:265–271. doi: 10.1016/j.scienta.2017.12.028.
- Pereira, A., and M. Maraschin. 2015. Banana (Musa spp) from peel to pulp: Ethnopharmacology, source of bioactive compounds and its relevance for human health. J. Ethnopharmacol. 160:149–163. doi: 10.1016/j.jep.2014.11.008.
- Pongprasert, N., and V. Srilaong. 2014. A novel technique using 1-MCP microbubbles for delaying postharvest ripening of banana fruit. Postharvest Biol. Technol. 95:42–45. doi: 10.1016/j.postharvbio.2014.04.003.
- Singh, B., J.P. Singh, A. Kaur, and N. Singh. 2016. Bioactive compounds in banana and their associated health benefits–A review. Food Chem. 206:1–11. doi: 10.1016/j.postharvbio.2014.04.003.
- Sisler, E.C., R. Goren, A. Apelbaum, and M. Serek. 2009. The effect of dialkylamine compounds and related derivatives of 1-methylcyclopropene in counteracting ethylene responses in banana fruit. Postharvest Biol. Technol. 51(1):43–48. doi: 10.1016/j.postharvbio.2008.06.009.
- Sisler, E.C., V.P. Grichko, and M. Serek. 2006. Interaction of ethylene and other compounds with the ethylene receptor: Agonists and antagonists, p. 1–34. In: Ethyl plant. Springer Verlag, Berlin, Heidelberg. doi: 10.1007/978-3-540-32846-9_1.
- Sisler, E.C., and M. Serek. 1997. Inhibitors of ethylene responses in plants at the receptor level: Recent developments. Physiol Plant. 100(3):577–582. https://doi.org/10.1111/j.1399-3054.1997.tb03063.x
- Vázquez-Celestino, D., H. Ramos-Sotelo, D.M. Rivera-Pastrana, M.E. Vázquez-Barrios, and E.M. Mercado-Silva. 2016. Effects of waxing, microperforated polyethylene bag, 1-methylcyclopropene and nitric oxide on firmness and shrivel and weight loss of ‘Manila’mango fruit during ripening. Postharvest Biol. Technol. 111:398–405. doi: 10.1016/j.postharvbio.2015.09.030.
- Watkins, C.B. 2006. The use of 1-methylcyclopropene (1-MCP) on fruits and vegetables. Biotechnol. Adv. 24(4):389–409. doi: 10.1016/j.biotechadv.2006.01.005.
- Yang, X., X. Pang, L. Xu, R. Fang, X. Huang, P. Guan, W. Lu, and Z. Zhang. 2009. Accumulation of soluble sugars in peel at high temperature leads to stay-green ripe banana fruit. J. Exp. Bot. 60(14):4051–4062. doi: 10.1093/jxb/erp238.
- Yu, F., Z. Ni, X. Shao, L. Yu, H. Liu, F. Xu, and H. Wang. 2015. Differences in Sucrose Metabolism in Peach Fruit Stored at Chilling Stress versus Nonchilling Stress Temperatures. Hort Sci. 50(10):1542–1548. doi: 10.21273/HORTSCI.50.10.1542.
- Yu, J., and Y. Wang. 2017. The combination of ethoxyquin, 1-methylcyclopropene and ethylene treatments controls superficial scald of ‘d’Anjou’pears with recovery of ripening capacity after long-term controlled atmosphere storage. Postharvest Biol. Technol. 127:53–59. doi: 10.1016/j.postharvbio.2017.01.012.
- Zhao, J., X. Xie, W. Dai, L. Zhang, Y. Wang, and C. Fang. 2018. Effects of precooling time and 1-MCP treatment on ‘Bartlett’fruit quality during the cold storage. Sci. Hortic. 240:387–396. doi: 10.1016/j.scienta.2018.06.049.
- Zhu, X., L. Shen, D. Fu, Z. Si, B. Wu, W. Chen, and X. Li. 2015. Effects of the combination treatment of 1-MCP and ethylene on the ripening of harvested banana fruit. Postharvest Biol. Technol. 107:23–32. doi: 10.1016/j.postharvbio.2015.04.010.
