ABSTRACT
In this study, phylogenetic analysis of some Turkish Prunus armeniaca L. genotypes was conducted based on RAPD-PCR, ISSR-PCR, and chloroplast DNA (trnL-F) sequence analyses. Prunus armeniaca genotypes were obtained from Malatya province, and brought to the laboratory for genomic DNA isolation. Eleven RAPD and fifteen ISSR primers were used to determine molecular characterization of Prunus armeniaca genotypes. For the amplification of the trnL-F region, trne and trnf primers were used in Polymerase Chain Reaction (PCR). A total of 46 and 95 bands were obtained with RAPD and ISSR analysis, respectively. The trnL-F sequences varied from 398 to 403 nucleotides. Average nucleotide composition of trnL-F sequences was 31.0% T, 14.4% C, 37.4% A and 17.2% G. The genetic distance between the five apricot genotypes is 0.00. A maximum likelihood tree was generated to determine genetic relationships among Prunus armeniaca genotypes. Analysis of Prunus armeniaca genotypes based on RAPD, ISSR, and trnL-F sequences revealed that the rate of polymorphism obtained using RAPD and ISSR were higher than those obtained through the trnL-F sequences.
Introduction
The Rosaceae family is a large family of woody trees, shrubs, climbers and herbaceous plants, and members of the family are spread all over the world (Hürkul and Köroğlu, Citation2019). The Rosaceae family, consisting of more than 100 genera and 3000 species, is the third most economically important family of plants in temperate regions (Zarei et al., Citation2017). The vast majority of the economically important fruits of the temperate regions are produced by members of this family, including Malus, Pyrus, Prunus, Rubus, and Fragaria species (Potter et al., Citation2002). The genus Prunus contains 400 species of trees and shrubs, covering some of the economically important temperate stone fruit species (Abbasi et al., Citation2019; Batnini et al., Citation2019). Prunus armeniaca L. (apricot), an economically important fruit species, is grown worldwide with a large number of varieties, mostly in temperate regions, the Mediterranean Basin, North America, and Asia (Hagen et al., Citation2004; Gürcan et al., Citation2019; Zhang et al., Citation2019a). Apricot is among the most commonly cultivated stone fruits in the world (Yılmaz et al., Citation2012). Although Turkey is not the place of origin of apricots, the country has an important role in apricot production in the world. Turkey is the leader in apricot production in the world with 846.606 tons (in 2019) which meets the 18–23% of the world production (TUIK, Citation2020). The most important apricot growing region in Turkey is Eastern Anatolia (Özbek, Citation1978). Malatya is located in this region, and it is Turkey’s most important apricot production center (Asma et al., Citation2007). Malatya has favorable conditions for apricot cultivation due to the cold-hot climate in the winter and the calcareous soils. The majority of apricot production in Malatya consists of genotypes suitable for drying. These genotypes consists of 63% (‘Hacıhaliloğlu,’ 32% ‘Kabaaşı,’ and 5% are other apricot genotypes ‘Soğancı,’ ‘Hasanbey,’ ‘Çataloğlu,’ and ‘Zerdali’) (Asma, Citation2011). In 2019, fresh apricot production in Malatya was 391,801 tons (TUIK, Citation2020). Apricot is also vital for human health. It is rich in potassium, phytochemicals, vitamin A and carotene, which makes it a crucial nutrient in human diet. Dietary fiber is one of the most essential compounds of dried apricots. Apricot involved functional nutrients that support the defense mechanisms against free radical damage, delay aging and prevent diseases, which in sum, can be recommended for a healthy life (Ozdemir and Gur, Citation2018; Zhang et al., Citation2019a). In addition, the endocarp of this fruit is usually highly lignified, hence it provides nutrients to matured seeds and prevents damage due to insects and diseases. Furthermore, endocarp plays an important role in sustaining the development of the emerging seeds (Zhang et al., Citation2019b).
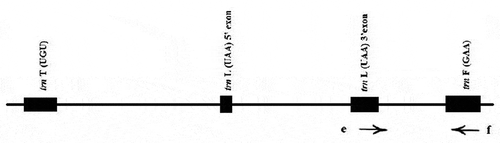
Morphological, biochemical, and DNA-based markers are used to determine the genetic diversity of plants (Jafari et al., Citation2019). But today, DNA-based markers are widely used thanks to their advantages compared to traditional morphochemical markers (Ipek et al., Citation2019). Genetic markers have wide-ranging potential applications in identifying varieties and revealing genetic diversity between germplasms (Mekapogu et al., Citation2020). RAPD and ISSR, among various markers, are mostly preferred due to their sensitivity, simplicity, and cost-effectiveness (Kala et al., Citation2017). RAPD markers offer many advantages such as higher polymorphism frequency, fast results, simplicity, low amount DNA requirement, and no prior knowledge of DNA sequence (Ruzi-Chután et al., Citation2019). ISSR is one of the simplest and most widely used PCR-based marker techniques that amplifies DNA segments between two identical microsatellite repeat regions (Marimuthu Somasundaram et al., Citation2019). However, among molecular markers, ISSR markers have some advantages comparing to low reproducibility of RAPD markers, high cost of the AFLP markers and the limitations of the complex SSR markers (Ramzan et al., Citation2018). The chloroplast is an important organelle for green plants where photosynthesis and carbon fixation take place (Zhou et al., Citation2019). The chloroplast genome is a circular multiple copy DNA molecule (Liang et al., Citation2019) containing four segments as follows: a large single-copy (LSC) segment, a small single-copy (SSC) segment, and two inverted repeats (IRs) segment (Deng et al., Citation2020). Chloroplast DNA (cpDNA) sequences are widely used as a tool in plant phylogenetics and genome development studies (Hao et al., Citation2010). Non-coding sequences of the chloroplast genome are the primary data source for molecular systematic and population genetic studies of plants (Shaw et al., Citation2007). The trnL(UAA)-F(GAA) intergenic spacer () which encodes transfer RNAs for leucine and phenylalanine, is commonly used for taxonomic and phylogenetic comparisons (Filiz et al., Citation2018). In this study, we performed a genetic diversity analysis using RAPD, ISSR, and cpDNA (trnL-F region) markers for some Prunus armeniaca genotypes grown in the Malatya region of Turkey.
Figure 1. cpDNA tmL-F region and primer e and primer f (Taberlet et al, Citation1991)
Materials and Methods
Plant Materials, Genomic DNA Isolation and PCR
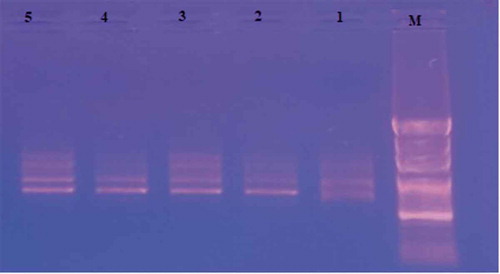
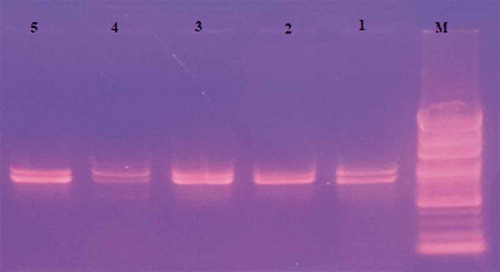
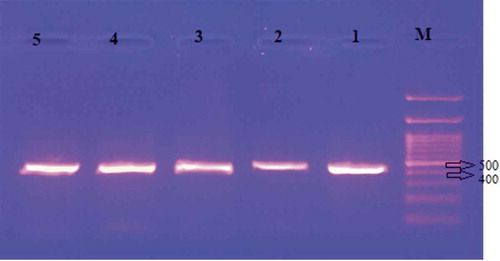
The leaf samples of five cultivars (belonging to Prunus armeniaca (Apricot)) genotypes used in this study were collected from Malatya/Turkey. The collected leaf samples were brought to the laboratory and prepared for genomic DNA isolation. A commercial kit (GeneMark) was used for the genomic DNA isolation of the plants. The obtained gDNA samples were stored at −20°C. RAPD and ISSR-PCR amplifications and cpDNA trnL-F region PCR amplifications using trnLe and trnLf primers are presented in –, respectively. A Gradient Thermocycler device was used for PCR applications. Both RAPD and ISSR-PCR amplifications in PCR tubes that contained 2 µL genomic DNA (20–100 ng), 10 µM primer 5 μL master mix (Cat. No: RP02-II-400, RP02-II-2000, 0.75 U of Taq DNA polymerase, reaction buffer, 2 mM MgCl2, 250 µM dNTPs, and enzyme stabilizer) and 17 μL ddH2O. For (cpDNA) trnLF regions were amplified in PCR tubes that contained 2 µL genomic DNA (20–100 ng), 10 µM primers, trne and trnf primers, 5 μL master mix (Cat. No: RP02-II-400, RP02-II-2000, 0.75 U of Taq DNA polymerase, reaction buffer, 2 mM MgCl2, 250 µM dNTPs and enzyme stabilizer) and 16 μL ddH2O. PCR products were analyzed by electrophoresis on 1% agarose gel and the amplified products were detected after staining with ethidium bromide. Some gel pictures of RAPD, ISSR markers and trnL-F region shown in –.
Table 1. Primers used in the RAPD-PCR reactions and their Tm degrees
Table 2. Primers used in the ISSR-PCR reactions and their Tm degrees
Table 3. cpDNA trnL-F primers sequences and PCR reactions
RAPD and ISSR Analyses
Following the RAPD and ISSR-PCR analyses, DNA bands were scored as ‘1ʹ in the presence of DNA,’0ʹ in the absence of DNA and a ‘?’ for missing data. The genetic relationship of the local Prunus armeniaca genotypes used in the research was analyzed using the PAUP 4.0b10 (Swofford, Citation2001) and UPGMA (unweighted pair group method with arithmetic mean) phylogenetic tree using the same software was constructed based on the arithmetic mean of the family trees.
cpDNA trnL-F Sequences
BioEdit (Hall, Citation1999) and Finch TV programs were used to process the sequences of the trnL-F gene region to be in ABI3430XL prism format. Contigs from forward and reverse sequences of each type were constructed, but only that of trnf was obtained for some samples. Some bases that were read incorrectly by the device performing the sequencing reactions, were visually corrected by hand using BioEdit and FinchTV programs based on the strength and cleanness of the signals (peaks) in the chromatogram. Finally, the contig sequences were confirmed manually. Maximum likelihood phylogenetic tree was generated by using MEGA 6.0 program (Tamura et al., Citation2013) to infer the phylogenetic relationships among Prunus armeniaca genotypes, sequences of which were obtained. Also, the genetic distance matrix between the genotypes was also performed using the same software.
Results and Discussion
Molecular marker techniques are effective tools used to analyze genetic diversity among plants (Wu et al., Citation2019). Molecular systematic information provides deeper insight into genetic structure in addition to analysis of genetic diversity through the classification of varieties (Kiani and Siahchehreh, Citation2017). In previous studies, genetic diversity of Prunus species has been analyzed using cpDNA (atpB-rbcL, trnL-trnF and rps16-trnQ) region (Batnini et al., Citation2019), internal transcribed spacer (ITS) region (Gilani et al., Citation2010), cpDNA trnL-F region (Gilani et al., Citation2011), ISSR (Li et al., Citation2013) and RAPD (Casas et al., Citation1999), SSR technique (Bakır et al., Citation2019).
RAPD and ISSR Analysis
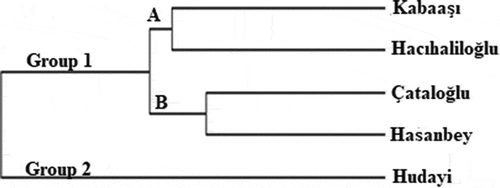
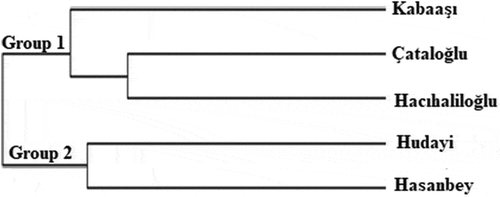
In the RAPD-PCR analysis, eleven primers were used and a total of 46 bands were obtained. Among these, 18 were polymorphic and the rate of polymorphism was around 39.13%. In the study, the highest number of bands was obtained from OPA-07 primer. According to the PAUP analysis, the closest genetic distance was found between ‘Çataloğlu’ and ‘Hasanbey’ genotypes with a value of 0.10, and the farthest genetic distance were between ‘Kabaaşı’ and ‘Hudayı,’ ‘Hudayı,’ and ‘Hacıhaliloğlu,’ ‘Hasanbey,’ and ‘Hudayı’ genotypes with a value of 0.26 (). UPGMA tree constructed via PAUP 4.0b10 phylogenetic analysis program yielded 2 groups. Group 1 consisted of subgroups; Subgroup A consist of ‘Kabaaşı’ and ‘Hacıhaliloğlu’ genotypes, while subgroup B consisted of ‘Çataloğlu’ and ‘Hasanbey’ genotypes. Group 2 only consist of ‘Hudayı’ genotype (). In ISSR-PCR analysis, a total of 95 bands were obtained. Of these, 41 were polymorphic and the rate of polymorphism was about 43.15%. According to PAUP analysis, the closest genetic distance was 0.15, while the farthest genetic distance was 0.28 (). The UPGMA tree constructed using PAUP 4.0b10 phylogenetic analysis program consisted of 2 groups (). Group 1 consisted of ‘Kabaaşı,’ ‘Hacıhaliloğlu,’ and ‘Çataloğlu’ genotypes. Group 2 consisted of ‘Hasanbey,’ and ‘Hudayı’ genotypes were related to this subgroup. In past studies; Ercisli et al. (Citation2009) used RAPD-PCR to determine genetic relationships among 23 apricot varieties cultivated in Turkey. In this study, 12 RAPD primers revealed polymorphism, 121 bands were obtained in total, and 118 of them were found polymorphic. In the UPGMA phylogenetic tree obtained in this study, ‘Hacıhaliloğlu’ and ‘Kabaaşı’ genotypes were detected in a group. While ‘Hasanbey’ genotype was found in the same group with ‘Şekerpare’ and ‘Ethem Bey’ genotypes, ‘Çataloğlu’ genotype was found in a different group with ‘İsmailağa,’ ‘Hacı Kız,’ and ‘Cölöğlu’ genotypes. In the present RAPD study, ‘Hacıhaliloğlu,’ ‘Kabaaşı,’ ‘Çataloğlu,’ and ‘Hasanbey’ genotypes were found in group 1. In ISSR analysis, on the other hand, while the ‘Kabaaşı’ and ‘Çataloğlu’ genotypes were found in a group 1, the and ‘Hasanbey’ genotype was found in a group 2. In general, RAPD and ISSR results were found partially compatible. The ISSR technique is based on determining the random distribution of DNA nucleotides in plant genomes, independent of chromosome regions. Compared to RAPD technique, it is much more sensitive and reproducible (Güngör et al., Citation2020). Yılmaz et al. (Citation2012) investigated morphological diversity among genotypes using 57 morphological and 13 pomological characters of 93 apricot genotypes located in different regions of Turkey. ‘Hacıhaliloğlu,’ ‘Çataloğlu,’ ‘Kabaaşı,’ and ‘Hasanbey’ genotypes were found in the same group in the UPGMA tree obtained with morphological and pomological characters. In the RAPD analysis conducted within the scope of the present study, ‘Hacıhaliloğlu,’ ‘Kabaaşı,’ ‘Çataloğlu,’ and ‘Hasanbey’ genotypes were found in group 1. In the ISSR analysis, ‘Hacıhaliloğlu,’ ‘Çataloğlu,’ and ‘Kabaaşı’ were found in the same group while the ‘Hasanbey’ genotype was found in a different group. Bakır et al. (Citation2019) selected 44 genotypes in Kapadokya Nevsehir (Turkey) having superior characteristics such as: Flowering later than wild apricots, resistant to late spring frosts, having large fruits, and delayed fruit ripening. Genetic relationship between the selected wild apricot genotypes were investigated with 13 SSR primers and they found that genetic similarity values among wild apricot genotypes ranged from 12% to 96%. Li et al. (Citation2013) investigated the genetic variability of 14 wild Prunus armeniaca populations using ISSR markers. In the study, 15 ISSR primers were used, and 155 bands corresponding to an average of 10.3 bands per primer were detected and obtained 147 polymorphic loci from them. Polymorphism rate (94.84%) was found in the study.
Table 4. Pairwise genetic distance matrix obtained from RAPD primers
Table 5. Pairwise genetic distance matrix obtained from ISSR primers
cpDNA trnL-F Sequences Analysis
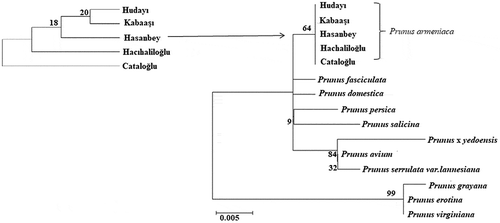
For cpDNA trnL-F sequences, the average base length ranged from 398 to 403 nucleotides among five Prunus armeniaca genotypes. Genetic distance based on trnL-F set was performed with MEGA 6.0 software. The genetic distance between the five apricot genotypes is 0.00 (). Average nucleotide composition of trnL-F was 31.0% T, 14.4% C, 37.4% A and 17.2% G. The maximum likelihood tree formed based on trnL-F sequences of five apricot genotypes could not be resolved. The trnL-F sequence analysis was not efficient in revealing the genetic relationship among Prunus armeniaca genotypes. The cpDNA has uniparental mode of inheritance and low mutation rate of construction and sequence (Hu et al., Citation2014). Additional information of Prunus fasciculata (JX414446.1), Prunus domestica (HQ244023.1), Prunus grayana (HQ244032.1), Prunus avium (HQ243988.1), Prunus x yedoensis (AF429927.1), Prunus serotina (AY864829.1), Prunus serrulata var. lannesiana (AF318659.1), Prunus persica (AF429937.1), Prunus salicina (AF429946.1) and Prunus virginiana (AF318695.1) received from NCBI database. The phylogenetic tree was generated using the maximum likelihood method by taking the cpDNA trnL-F sequences of the genotypes. The phylogenetic tree indicating Prunus armeniaca genotypes distribution in Turkey was supported by a 64% bootstrap value to be monophyletic (). Batnini et al. (Citation2019) evaluated the genetic diversity and phylogenetic structure of 42 Tunisian Prunus armeniaca genotypes using three regions of the cpDNA (atpB-rbcL, trnL-trnF, and rps16-trnQ). As a result of their work, they obtained a total of 2333 cpDNA sequences and found that in general, the rps16-trnQ region was the most appropriate candidate to be a barcode for the analysis of the data set and yielded a general variation at the cross-species level. Gilani et al. (Citation2011) revealed the phylogenetic relationship between species by looking at the cpDNA trnL-F sequence polymorphism of 12 Prunus species found in Pakistan. In the study, 541 constant, 25 parsimony uninformative, and 18 parsimony informative characters of 584 characters from the aligned sequences were identified. Gilani et al. (Citation2010) determined the phylogenetic relationship between the species by looking at the nrDNA ITS sequence polymorphism of 23 Prunus species found in different regions of Pakistan. From the sequences aligned in the study, they detected 124 parsimony uninformative and 87 parsimony informative characters from 716 characters. In the strict consensus tree they created based on sequence results, P. armeniaca species was in the same group as P. mume and P. mexicana species. Chloroplast DNA has small genome size and slow mutation in plants, and it has been proven by scientists to be very useful in understanding plant phylogenetic studies and clearer taxonomic levels (Liang et al., Citation2020). Chloroplast DNA trnL-F region can be used to address related questions between closely related species and genera (Choulak et al., Citation2017). There have been similar studies in the past with sequences and populations of important plant species, trnL-F such as; Turkish Olea europaea subsp. europaea (Kaya et al., Citation2018), Turkish rice (Oryza sativa) (Filiz et al., Citation2018), Turkish cotton (Gossypium hirsutum L.) (Hocaoglu-Ozyigit et al., Citation2020), Turkish apple (Malus L.) genotypes (Sevindik et al., Citation2019), Laurus nobilis populations from Turkey (Sevindik and Okan, Citation2019), Turkish some Centaurea L. (Kalmer and Tekpınar, Citation2017), Turkish Urtica ssp. (Kolören and Eker, Citation2018), Fritillaria were collected from different regions of Turkey (Türktaş et al., Citation2012). Orchis Species from Turkey (Dizkırıcı et al., Citation2016).
Table 6. Pairwise genetic distance matrix obtained from cpDNA trnL-F sequences
Conclusion
Approximately 39.13% and 43.15% polymorphism were detected among Prunus armeniaca genotypes based on RAPD and ISSR analyses, respectively. However, trnL-F sequence analyses were not found efficient in revealing the genetic relationship among Prunus armeniaca genotypes. Overall, the outcomes of this research, which aimed to analyze the genetic diversity of Prunus armeniaca genotypes through RAPD, ISSR, and trnL-F sequence comparisons, will be used for the development of more efficient new varieties particularly for breeding, through the association with many different specific characters of Prunus armeniaca genotypes.
References
- Abbasi, F., A. Khadivi, M. Taghizadeh, and B. ValizadehKaji. 2019. Micropropagation of Prunus scoparia, a suitable rootstock for almond under drought conditions. Int. J. Fruit Sci. 19(2):221–230. doi: 10.1080/15538362.2018.1539695.
- Asma, B.M. 2011. Her Yönüyle Kayısı. Uyum Ajans, Ankara.
- Asma, B.M., T. Kan, and O. Birhanlı. 2007. Characterization of promising apricot (Prunus armeniaca L.) genetic resources in Malatya, Turkey. Genet. Resour. Crop Evol. 54(1):205–212. doi: 10.1007/s10722-005-3809-9.
- Bakır, M., H. Dumanoğlu, V. Erdoğan, C. Ernim, and T. Macit. 2019. Characterization of wild apricot (Prunus armeniaca L.) genotypes selected from Cappadocia region (Nevşehir-Turkey) by SSR markers. J. Agric. Sci. 25(4):498–507. doi: 10.15832/ankutbd.457850
- Batnini, M.A., H. Bourguiba, N. Trifi-Farah, and L. Krichen. 2019. Molecular diversity and phylogeny of Tunisian Prunus armeniaca L. by evaluating three candidate barcodes of the chloroplast genome. Sci. Hortic. 245:99–106. doi: 10.1016/j.scienta.2018.09.071.
- Casas, A.M., E. Igartua, G. Balaguer, and M.A. Moreno. 1999. Genetic diversity of Prunus rootstocks analyzed by RAPD markers. Euphytica 110(2):139–149. doi: 10.1023/A:1003745311408.
- Choulak, S., K. Chatti, Z. Marzouk, and N. Chatti. 2017. Genetic differentiation and gene flow of some Tunisian pistachio (Pistacia vera L.) varieties using chloroplastic DNA. J. Res. Biol. Sci. 2:35–41.
- Deng, Y., Y. Luo, Y. He, X. Qin, C. Li, and X. Deng. 2020. Complete chloroplast genome of Michelia Shiluensis and a comparative analysis with four Magnoliaceae species. Forests 11(3):267. doi: 10.3390/f11030267.
- Dizkırıcı, A., S. İşler, and O. Yiğit. 2016. Determining phylogenetic relationships among Orchis species in Van province using trnL Intron and trnL-F regions. J. Inst. Nat. Appl. Sci. 21(2):83–91.
- Ercisli, S., A. Guleray, N. Yildirim, A. Esitken, and E. Orhan. 2009. Identification of apricot cultivars in Turkey (Prunus armeniaca L.) using RAPD markers. Rom. Biotechnol. Lett. 14(4):4582–4588.
- Filiz, E., M.E. Uras, I.I. Ozyigit, U. Sen, and H. Gungor. 2018. Genetıc diversity and phylogenetic analyses of Turkısh rice varieties revealed by ISSR markers and chloroplast trnL-F region. FEB 27(12):8351–8358.
- Gilani, S.A., R.A. Qureshi, A.M. Khan, and D. Potter. 2010. A molecular phylogeny of selected species of genus Prunus L. (Rosaceae) from Pakistan using the internal transcribed spacer (ITS) spacer DNA. Afr. J. Biotechnol. 9(31):4867–4872.
- Gilani, S.A., R.A. Qureshi, M. Khan, F. Ullah, Z. Nawaz, I. Ahmad, and D. Potter. 2011. A molecular phylogeny of selected species of genus Prunus L. (Rosaceae) from Pakistan using the trn-L & trn-F spacer DNA. Afr. J. Biotechnol. 10(22):4550–4554.
- Güngör, H., M.A. Akbudak, E. Filiz, İ. Yüce, and Z. Dumlupınar. 2020. Genetic diversity in sodium azide (NaN3) induced barley mutants using ISSR markers. Derim 7(1):27–32. doi: 10.16882/derim.2020.630502.
- Gürcan, K., N. Çetinsağ, H. Pınar, and T. Macit. 2019. Molecular and biological assessment reveals sources of resistance to Plum pox virus-Turkey strain in Turkish apricot (Prunus armeniaca) germplasm. Sci. Hortic. 252:348–353. doi: 10.1016/j.scienta.2019.04.003.
- Hagen, L.S., J. Chaib, B. Fady, V. Decroocq, J.P. Bouchet, P. Lambert, and J.M. Audergon. 2004. Genomic and cDNA microsatellites from apricot (Prunus armeniaca L.). Mol Ecol. Notes 4(4):742–745. doi: 10.1111/j.1471-8286.2004.00802.x.
- Hall, T.A. 1999. Bioedit: A user-friendly biological sequence alignment editor and analyses program for windows 95/98/NT. Nucleic Acids Symp. 41:95–98.
- Hao, D.C., S.L. Chen, and P.G. Xiao. 2010. Sequence characteristics and divergent evolution of the chloroplas psbA-trnH noncoding region in gymnosperms. J. Appl. Genet. 51(3):259–273. doi: 10.1007/BF03208855.
- Hocaoglu-Ozyigit, A., B. Ucar, V. Altay, and I.I. Ozyigit. 2020. Genetic diversity and phylogenetic analyses of Turkish cotton (Gossypium Hirsutum L.) lines using ISSR markers and chloroplast trnL-F regions. J. Nat. Fibers 1–14. doi: 10.1080/15440478.2020.1788493.
- Hu, D., P. Zhang, Y.L. Sun, S. Zhang, Z. Wang, and C. Chen. 2014. Genetic relationship in mulberry (Morus L.) inferred through PCR-RFLP and trnD-trnT sequence data of chloroplast DNA. Biotechnol. Biotechnol. Equip. 28(3):425–430. doi: 10.1080/13102818.2014.928980.
- Hürkul, M.M., and A. Köroğlu. 2019. Etnobotanik Bir Derleme: Amygdaloideae (Rosaceae) Alt Familyası. FABAD J. Pharm. Sci. 44(1):35–46.
- Ipek, M., Ş. Arıkan, L. Pırlak, and A. Eşitken. 2019. Phenological, morphological and molecular characterization of some promising walnut (Juglans regia L) genotypes in Konya. Erwerbs-Obstbau 61(2):149–156. doi: 10.1007/s10341-018-0411-9.
- Jafari, S.H., A. Sepehry, H. Soltanloo, and A.A. Karimian. 2019. Genetic differentiation between bitter and sweet asafetida plants using ISSR markers. Mol. Biol. Rep. 46(1):1069–1078. doi: 10.1007/s11033-018-4565-1.
- Kala, M., S.C. Kala, K.V.N. Reddi Ratnakar, A.C. Sekhar, and P.C.O. Reddy. 2017. Assessment of genetic diversity of Canthium parviflorum (Lam.) by RAPD and ISSR markers. Ann. Plant Sci. 6(11):1775–1783. doi: 10.21746/aps.2017.11.1.
- Kalmer, A., and A.D. Tekpınar. 2017. Determination of phylogenetic relationships of five species of Centaurea genus by using trnT-LF cpDNA region. Cumhuriyet Sci. J. 38(4):52–59. doi: 10.17776/csj.363294.
- Kaya, E., R. Vatansever, and E. Filiz. 2018. Assessment of the genetic relationship of Turkish olives (Olea europaea subsp. europaea) cultivars based on cpDNA trnL-F regions. Acta Bot. Croat. 77(1):88–92. doi: 10.1515/botcro-2017-0019.
- Kiani, G., and M. Siahchehreh. 2017. Diversity in squash varieties assessed by ISSR markers. Int. J. Veg. Sci. 23(5):430–437. doi: 10.1080/19315260.2017.1319005.
- Kolören, O., and S. Eker. 2018. Genetic diversity of Urtica species in Ordu province of Turkey based on chloroplast DNA trnL-F intergenic spacer regions. Anadolu Tarim Bilimleri Dergisi 33(3):202–208.
- Li, M., Z. Zhao, and X.J. Miao. 2013. Genetic variability of wild apricot (Prunus armeniaca L.) populations in the Ili valley as revealed by ISSR markers. Genet. Resour. And Crop Evol. 60(8):2293–2302. doi: 10.1007/s10722-013-9996-x.
- Liang, C., L. Wang, J. Lei, B. Duan, W. Ma, S. Xiao, S. Guo, H. Qi, Z. Wang, Y. Liu, et al. 2019. A comparative analysis of the chloroplast genomes of four Salvia medicinal plants. Engineering 5(5):907–915. doi: 10.1016/j.eng.2019.01.017.
- Liang, H., Y. Zhang, J. Deng, G. Gao, C. Ding, L. Zhang, and R. Yang. 2020. The complete chloroplast genome sequences of 14 Curcuma species: Insights into genome evolution and phylogenetic relationships within zingiberales. Front. Genet. 11:802. doi: 10.3389/fgene.2020.00802.
- Marimuthu Somasundaram, S., U. Subbaraya, S.G. Durairajan, S. Rajendran, J. Gopalakrishnan, B. Shahul Hameed, D. Palani, and B. Suthanthiram. 2019. Comparison of two different electrophoretic methods in studying the genetic diversity among plantains (Musa spp.) using ISSR markers. Electrophoresis 40(9):1265–1272. doi: 10.1002/elps.201800456.
- Mekapogu, M., O.K. Kwon, K.J. Lee, M.S. Ahn, J.T. Park, and J.A. Jung. 2020. Identification of standard type cultivars in Chrysanthemum (Dendranthema grandiflorum) using SSR markers. Hortic. Environ. Biotechnol. 61:153–161. doi: 10.1007/s13580-019-00201-0.
- Özbek, S. 1978. Temperature fruits. Cukurova University, Faculty of Agriculture publications: 111. Ankara Univ. Press, Ankara. ( in Turkish).
- Ozdemir, F.A., and N. Gur. 2018. In vitro propagation of cataloglu apricot (Prunus armeniaca L.) cultivar using apical node as explant. Prog. Nutr. 20(1–S):176–181. doi: 10.23751/pn.v20i1-S.5576.
- Potter, D., F. Gao, P.E. Bortiri, S.-H. Oh, and S. Baggett. 2002. Phylogenetic relationships in Rosaceae inferred from chloroplast mat K and trn L- trn F nucleotide sequence data. Plant Syst. Evol. 231(1–4):77–89.doi.10.1007/s006060200012.
- Ramzan, F., H.T. Kim, K.K. Shim, Y.H. Choi, A. Younis, and K.-B. Lim. 2018. Genetic diversity and relationship assessment of Lilium lancifolium × Asiatic hybrid ‘Chianti’ progeny by ISSR markers. .Eur J Hortic Sci 83(3):142–150. doi: 10.17660/eJHS.2018/83.3.3.
- Ruiz-Chután, J.A., J. Salava, D. Janovská, J. Žiarovská, M. Kalousová, and E. Fernández. 2019. Assessment of genetic diversity in Sorghum bicolor using RAPD markers. Genetika 51(3):789–803. doi: 10.2298/GENSR1903789R.
- Sevindik, E., and K. Okan. 2019. Genetic diversity and phylogenetic analyzes of Laurus nobilis L. (Lauraceae) populations revealed chloroplast (cpDNA) trnL Intron and trn L-F region. Int. J. Fruit Sci. 1–12. doi: 10.1080/15538362.2019.1707745.
- Sevindik, E., Z.T. Murathan, S. Filiz, and K. Yalcin. 2019. Molecular characterization based on chloroplast (trnL-F) DNA sequence of the apple genotypes in Ardahan/Turkey. Bangladesh J. Bot. 48(4):1099–1106. doi: 10.3329/bjb.v48i4.49058.
- Shaw, J., E.B. Lickey, E.E. Schilling, and R.L. Small. 2007. Comparison of whole chloroplast genome sequences to choose noncoding regions for phylogenetic studies in angiosperms: The tortoise and the hare III. Am J. Bot. 94(3):275–288. doi: 10.3732/ajb.94.3.275.
- Swofford, D.L. 2001. PAUP. phylogenetic analysis using parsimony (and other methods), version 4.0b10. Sinaeur Associates, Sunderland.
- Taberlet, P., L. Gielly, G. Pautou, and J. Bouvet. 1991. Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Mol. Biol. 17:1105–1109.
- Tamura, K., G. Stecher, D. Peterson, A. Filipski, and S. Kumar. 2013. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol Biol. Evol. 30:2725–2729. doi:10.1093/molbev/mst197.
- TUIK. 2020. 20 Feb. 2020. https://biruni.tuik.gov.tr/medas/?kn=92&locale=tr
- Türktaş, M., M. Aslay, E. Kaya, and F. Ertuğrul. 2012. Molecular characterization of phylogenetic relationships in Fritillaria species inferred from chloroplast trnL-trnF sequences. Turk. J. Biol. 36(5):552–560. doi: 10.3906/biy-1201-30.
- Wu, W., F. Chen, K. Yeh, and J. Chen. 2019. ISSR analysis of genetic diversity and structure of plum varieties cultivated in southern China. Biology 8(1):2.
- Yılmaz, K.U., S.P. Kargı, and S. Kafkas. 2012. Morphological diversity of the Turkish apricot (Prunus armeniaca L.) germplasm in the Irano-Caucasian ecogeographical group. Turk. J. Agric. For. 36(6):688–694. doi: 10.3906/tar-1111-14.
- Zarei, A., J. Erfani-Moghadam, and M. Mozaffari. 2017. Phylogenetic analysis among some pome fruit trees of Rosaceae family using RAPD markers. Biotechnol. Biotechnol. Equip. 31(2):289–298. doi:10.1080/13102818.2016.1276414.
- Zhang, Q., C. Feng, W. Li, Z. Qu, M. Zeng, and W. Xi. 2019a. Transcriptional regulatory networks controlling taste and aroma quality of apricot (Prunus armeniaca L.) fruit during ripening. BMC Genomics 20(1):1–15. doi:10.1186/s12864-019-5424-8.
- Zhang, X., Q. Zhang, X. Sun, X. Du, W. Liu, and W. Dong. 2019b. Differential expression of genes encoding phenylpropanoid enzymes in an apricot cultivar (Prunus armeniaca L.) with cleavable endocarp. Trees 33(6):1695–1710. doi: 10.1007/s00468-019-01890-x.
- Zhou, T., M. Ruhsam, J, Wang, H. Zhu, W. Li, X. Zhang, Y. Xu, F. Xu, X. Wang. 2019. The complete chloroplast genome of Euphrasia regelii, pseudogenization of ndh genes and the phylogenetic relationships within Orobanchaceae. Front. Genet. 10:444. doi:10.3389/fgene.2019.00444.