ABSTRACT
Decrease in photosynthesis results from high leaf temperature, photosynthetic photon flux density, and UV-B. Here, we investigated whether a reflective material (kaolin) could improve photosynthetic performance and yield of clementine (Citrus clementina Hort. ex Tan., “Comune” cultivar) in southern Italy. Kaolin was sprayed on tree canopies every month during two summers; gas exchange, fluorimetry, and qualitative and productive parameters were measured. Kaolin increased the total average yield per tree and improved fruit color. PCA indicated that the best performances were obtained with kaolin in the last harvest. Kaolin affected the final fruit price by 3%, justifying its use.
KEYWORDS:
Introduction
Stomatal opening can be insufficient for the cooling of the leaves (Nahal, Citation1981) when leaves are overheated by a massive influx of solar radiation. This phenomenon is currently exacerbated by global warming and by the reduction of the ozone layer, which has led to an increase in the amount of UV radiation on Earth.
However, high photosynthetic photon flux density (PPFD) radiation causes damages beyond photoinhibition; it leads to the impairment of photosynthetic proteins, causing (photo) destruction of the photosynthesizing pigments (Powles, Citation1984) via oxidation (Caffarri et al., Citation2014; Hendry et al., Citation1987; Powles, Citation1984) in a phenomenon known as photooxidation (Foyer et al., Citation1994). The problem lies in the fact that photo-inhibition and photo-oxidation adversely affect photosynthesis; therefore, the productivity of plants is compromised, both in qualitative and quantitative terms.
Furthermore, heat stress caused by high infrared solar radiation is known to exacerbate photoinhibition and photooxidation. Indeed, recent studies have shown the deactivation of Rubisco under heat stress, as a secondary consequence of perturbations in the thylakoid membrane (Salvucci and Crafts-Brandner, Citation2004). Moreover, heat stress is known to inhibit the repair of damages caused to PSII (Allakhverdiev et al., Citation2008; Sharkey, Citation2005), the photosynthetic apparatus most sensitive to high temperature (Berry and Bjorkman, Citation1980; Havaux, Citation1996; Yordanov et al., Citation1986). Additionally, stress caused by other environmental factors (water and nutritional deficiency) can further accentuate the negative effect of high Photosynthetically Active Radiation (PAR) and ultraviolet light (UV-B) (Balakumar et al., Citation1993; Takeuchi et al., Citation2002; Tevini, Citation1996).
In the outer layer of the canopy, an excess of visible light leads to cellular damage (Li et al., Citation2009) and a partial loss of capacity to convert radiant energy into dry material, resulting in low growth and production of the plant (Krause et al., Citation1995; Laing et al., Citation1995; Long et al., Citation2006). Environments with high radiation fluxes, especially when plants are exposed to additional environmental stresses, may cause photoinhibition (Long et al., Citation1994; Maxwell and Johnson, Citation2000), which is the anti-stress protection mechanism of plants, involving the antioxidant system (Brosché and Strid, Citation2003; Fedina et al., Citation2007; Frohnmeyer and Staiger, Citation2013). Dynamic photoinhibition is a short-term reversible regulatory process for the controlled dissipation of excessive light energy. In contrast, chronic photoinhibition is a gradual, long-term reversible process that may occur following prolonged exposure to extreme photon fluxes and under other environmental stress conditions (Werner et al., Citation2002).
Citrus species have a natural habitat in the shaded environment of the forest understory (Davies and Albrigo, Citation1994; Spiegel-Roy and Goldschmidt, Citation1996; Syvertsen and Lloyd, Citation1994). The citrus leaf has a low light saturation point (Kriedemann, Citation1968; Sinclair and Allen, Citation1982). However, citrus trees are cultivated in a subtropical climate where high solar radiation leads to high temperature and high PPFD availability for several hours on numerous summer days. The PPFD is higher on the canopy surface compared to the inner layers of the canopy because of shading from the overlying canopy layers (Jackson, Citation1980).
Reflective materials have been tested on many fruit trees such as Malus domestica Borkh (Glenn et al., Citation2001), Vitis vinifera L. (Shellie et al., Citation2013a), Prunus amygdalus L. (Rosati et al., Citation2006), and also on several citrus species such as Citrus sinensis (L.) Osbeck (Ali and El Zayat, Citation2019), Citrus reticulata L. (El-Tanany et al., Citation2019; Ennab et al., Citation2017; Zaghloul et al., Citation2017), and Citrus paradisi L. (Jifon and Syvertsen, Citation2003). However, each species has a different response to the use of reflective materials. Indeed, canopy architecture can influence reflective material distribution in the canopy; the resulting effect on leaf physiology must then be analyzed in order to understand plant response to the applied material.
The aim of this work was to verify the distribution and effects of kaolin in the canopy of the clementine citrus tree and to evaluate its impact on the whole tree physiology. In particular, in the warm climatic condition of Southern Italy, we tested if kaolin could improve the photosynthetic performance of leaves and increase yield per tree, and further tested its effect on fruit quality.
Materials and Method
Orchard
The experiment was conducted over two years (2017–2018) on the farm of Cooperative COPPI (CZ), Italy (38°51ʹ42.8” N, 16°15ʹ45.8” E). The “Comune” cultivar of clementine trees (Citrus clementina Hort. ex Tan.) had been grafted onto sour orange rootstock and planted in the spring of 1998 in sub-acidic (pH 6) sandy soil containing 2.23% organic matter and 1.5 g·kg−1 nitrogen content. The plants had been spaced 6 m × 4 m apart (417 plants ha−1) in a north to south row orientation, and the trees had been trimmed to a globe shape. Twenty-four trees with canopy volume of about 30 m3 and similar fruit load were selected for the experiment. The canopy volume was calculated following the methodology proposed by Barrett and Brown (Citation2012).
Treatment and Experimental Design
Kaolin was used in its commercial formulation, containing 95% kaolinite (Surround WP, Serbios Srl, Rovigo, Italy). The product has good properties for use as a reflective foliar coating. It was applied at concentrations of 50 g.L−1 following the manufacturer-recommended dosage for other citrus species.
Trees sprayed with kaolin were compared with control trees sprayed with only water. A total of 24 trees, three blocks of eight plants with four plants per treatment, were selected in a randomized block design.
The reflective material, sprayed with 3 L of suspension per tree, covered the leaves of the treatment trees uniformly. The treatments were applied every month, from the beginning of the summer season to the beginning of the autumn season (i.e., 30 June, 30 July, 30 August, and 30 September) during each year. During this time, the precipitation was higher during the second year ()
Table 1. Rainfall during the June, July, August, September, and October months during two observation years. (2017–2018). Kaolin was sprayed on 30th day of June, July, August and September months
Canopy and Field Measurements
Light and Temperature, Wihteness, Gas Exchange, and Fluorescence Measurements of the Canopy
The PAR and UV radiation were monitored on each of the three trees per block using sensors installed onto two layers of the canopy: the outer layer (O), within 0–15 cm from the canopy surface, and the inner layer (I), within 15–45 cm from the canopy surface. The PAR Light Sensor sensors 3668I (Spectrum Technologies Inc., Aurora, Illinois, USA) and UV Light Sensors 36761 (Spectrum Technologies Inc., Aurora, Illinois, USA) were connected to a logger that recorded the measurements every minute throughout the summer months during two years.
The leaf temperature was also monitored every minute throughout the summer months during to years with fine wire thermocouples (GMR Instruments, Firenze, Italy, UE) connected to a data logger (Spectrum Technologies Inc., Aurora, Illinois, USA). The thermocouples were pressed against the abaxial leaf surface and held in place using lightweight clips.
Furthermore, the amount of kaolin (g.m−2) sprayed on the leaves in the outer and inner layers of the canopy was determined using a methodology adopted from Jifon and Syvertsen (Citation2003). Ten testers (polymethyl methacrylate plate, 3 cm × 3 cm) were weighed and randomly hung on each layer of the treated and untreated trees. They were removed and reweighed after treatment when the reflective materials had dried. The amount of reflective material was determined by the weight difference of the testers before and after treatment. Both coated and control (treated only with water) testers were successively exposed to sunlight at midday (noon), during a sunny day. The PPFD (double asterisks (**) in indicates measurements done on the tester in open field) and UV radiation was recorded using PAR and UV light sensors (Spectrum Technologies Inc., Aurora, Illinois, USA), respectively. These sensors were placed 1 cm below the testers. Furthermore, the PPFD measurements were also performed in the outer and inner layers of the canopy (one asterisk (*) in indicates measurements done on the tree). Therefore, the PAR and UV-B sensors were also installed on each layer of four trees per treatment.
Table 2. The kaolin amount on tester, PPFD and leaf gas exchange parameters measured on the outer (O) and inner (I) layers of the canopy tree treated with kaolin (TK) and untreated (control; TCN)- (* indicates measurements done on the tree; ** indicates measurements done on tester in the open field; the average on two years)
Leaf reflection (whiteness) was measured by using a spectrophotometer (CR700; Minolta Cor., Ramsey, New Jersey, USA) on six leaves randomly chosen from two layers of canopy (outer and inner) of three random plants each from the three blocks (six leaves × two layers × three plants × three blocks = 108 leaves). The whiteness measurements (L values ranging from 0 to 100, where black = 0 and white = 100) of the adaxial leaf surfaces were recorded before treatment when the leaves were clean, and after treatment, when the leaves were coated with kaolin.
Gas exchange measurements [net assimilation of CO2, (An), stomatal conductance (gs), and internal CO2 concentration (Ci)] were performed on the leaves of the outer and inner layers of trees (six leaves × two layers × three plants × three blocks). The measurements were taken using a portable photosynthesis system (Li-Cor 6400XT; LI-COR Biosciences, Lincoln, Nebraska, USA).
Photoinhibition was estimated using a chlorophyll fluorometer (Li-Cor 6400–40; LI-COR Biosciences, Lincoln, Nebraska, USA). Measurements were taken in light-adapted and dark-adapted (using leaf clips, for 30 min) leaves. The same leaves used for the gas exchange measurements were used (three light-adapted leaves + three dark-adapted leaves x two layers × three plants × three blocks). On the leaves adapted to darkness (three leaves × two layers × three plants × three blocks), the quantum yield of PSII was calculated as the ratio of variable fluorescence to maximum fluorescence (Fv/Fm). This value represents the maximum quantum efficiency. In leaves adapted to light, immediately after the gas exchange measurements, the PSII efficiency values (ΦPSII), relative photosynthetic electronic transport rate (ETR), photochemical extinction (qP), and non-photochemical extinction (NPQ) were determined.
Subsequently, leaves from the two layers were detached, washed with distilled water, and the SPAD 502 (Spectrum Technologies Inc., Aurora, Illinois, USA) index was measured. The PAR spectrum light was measured at midday (12:00) using a spectroradiometer (PS-300; Apogee Instruments Inc., Logan, Utah, USA). An average of four measurements for each layer was calculated.
The gas exchange, fluorimetry, and PAR spectrum measurements were carried out during a clear sunny summer day (from 11:00 to 13:00) on four days during the last week of the summer months (June, July, August) in both years.
Soil water potential was maintained between 0.03 MPa and field capacity during the experiment in all treated and control trees. Soil water potential was continuously monitored with Watermark® (Irrometer Company, Inc., Riverside, USA) sensors positioned at a distance of 60 cm from the trunk and at a depth of 30 cm. In the 0.15–0.30 m soil layer, greater amounts of fine roots were found (data not shown), in accordance with another experiment on citrus trees.
Fruit Quality Parameters
These observations were carried out at the “Colture Arboree” Laboratory, of AGRARIA Department, at the Mediterranean University of Reggio Calabria. For each tree, five fruits were randomly sampled 10th, 20th and 30th of November, 2017, and 12th and 22nd of November and 2nd of December, 2018 (20 fruits x 2 layers x 3 trees x 3 times = 360 fruits per treatment per block per three sampling event per year). They were immediately used to determine the following: the color of the skin and pulp, in terms of CIELab and HSB color spaces, using a spectrophotometer (CR700; Minolta Cor., Ramsey, New Jersey, USA); and height (H), diameter (D), relative length (H/D), fresh weight (FW), and dry weight (DW) using an electronic balance (Mettler-Toledo, Grelfensee, Switzerland). The juice of the fruits was extracted and was used to measure the total soluble solids (TSS), using a handheld digital refractometer (PR-1; Atago, Tokyo, Japan), and titratable acidity (TA), using 10 mL of juice diluted with distilled water (1:1) and titrated to pH 8.2 with 0.1 N NaOH (%). The TSS/TA ratio was calculated. Finally, ascorbic acid (AA) content was determined, using the procedure based on the reduction of the dye 2.6-dichlorophenolindophenol (DIP) by ascorbic acid (mg ascorbic acid 100 g−1 FW)(Hughes, Citation1983).
For each fruit, the analyses of total polyphenol content (TPC) and total antioxidant capacity (TAC) were performed. The pulp portion (20 g), removed before juice extraction, was homogenized using an Ultraturrax blender (20.000 rpm, T 25 Basic; IKA, Werke, Germany, UE). The TPC and TAC were analyzed separately using a Lambda 35 spectrophotometer (PerkinElmer Corporation, Waltham, Massachusetts, USA). Before measuring the TPC and TAC, standard curves were prepared for each test. The TPC (mg gallic acid equivalents g−1 FW) was determined using the Folin–Ciocalteu method (Slinkard and Singleton, Citation1997). The TAC was determined using the modified TEAC assay and expressed as mmol Trolox, equivalent to g−1 FW (Pellegrini et al., Citation1999; Re et al., Citation1999). The TEAC assay included both the hydrophilic and the lipophilic contributions (Scalzo et al., Citation2005) of the orange samples.
Yield
The yield per tree (in weight and fruits number) was determined at harvest (during the first ten days of December in both years).
Statistical Analysis
All data were subjected to ANOVA tests, and the means were compared by using Tukey’s test when the ANOVA indicated significant (P < .05) variable effects. All data are reported as the means from both years.
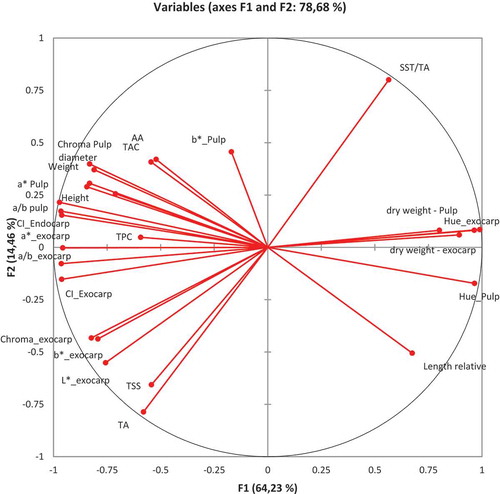
All data analyses were performed using IBM® SPSS® Statistic, version 22 (SPSS Inc. IBM Company, Armonk, New York, USA). The variables were then used in a Principal Component Analysis (PCA) to discriminate treatment as a function of harvest times (TK1,TK2, TK3 for the kaolin treatment; TCN1, TCN2, TCN3 for the control), which allowed us to assess the effect of the date of collection and treatment on all the analyzed parameters. All variables of the PCA were projected in the plane of F1 and F2 (). In this way, the relationships between variables and the formation of factors could be analyzed.
Results and Discussion
In our experiment, the amount of kaolin sprayed on the leaf surfaces was approximately 10 g.m−2 on the outer canopy layer, and 50% less on the inner canopy layer ().
The precipitation was very low in both years and generally occurred well in advance before the treatment was implemented ().
3.1 Light and temperature, lightness, gas exchange, and fluorescence measurements of the canopy The average PPFD from 10:00 to 17:00 was 18% lower in treated trees compared to control trees (). Kaolin successfully reduced the PPFD in the outer layer as a result of its reflective property, presenting an advantage for these leaves during the hours when the PPFD is high because citrus leaves have a low light saturation point (Kriedemann, Citation1968; Sinclair and Allen, Citation1982).
The PPFD in the inner layer was lower compared to that of the outer layer. However, in the treated trees, it was approximately 71% higher than in the control trees (). The amount of reflective material deposited on the leaves in the inner layer was lower compared to the outer layer because the latter acted as a barrier, reducing the kaolin amount in the inner layer. Therefore, the kaolin amount on the leaves was reduced from the outer to the inner layer, as seen in the . However, the higher PPFD recorded in the inner layer of the treated trees compared to the control trees is attributable to the reflected light coming from the outer layer. This light reflection allowed for an increased and improved PPFD distribution within the canopy () where light is generally low; this was in accordance with the results obtained by Rosati et al. (Citation2006).
The UV-B radiation was also 18% lower in the outer layer of the treated trees compared to the outer layer of the non-treated trees. On the contrary, the UV-B radiation did not change significantly between inner layers of treated and control tree canopies ().
The average leaf temperature from 10:00 to 16:00 was strongly influenced by kaolin treatment (). Indeed, the leaf temperature of the outer canopy layer of control trees was approximately 32°C, with a peak of 36°C at noon (); in contrast, kaolin treatment lowered the leaf temperature of the outer layer canopy to under 30°C (). The leaf temperature in the inner layers of the canopies was not influenced by the treatment, and was optimal (~26°C) in both treated and control trees; it is known that the best temperature for p15 hotosynthesis in the citrus leaf is approximately 25°C (Ribeiro et al., Citation2005).
Table 3. SPAD index, lightness and leaf temperature measured on the outer (O) and Inner (I) layers of the canopy tree treated with kaolin (TK) and untreated (TCN = control) (average on two years)
During nighttime, no differences were observed between treated and control trees in terms of the leaf temperature of both canopy layers (data not shown). Thus, our results showed that long-wave emittance was unaffected by kaolin, in agreement with observations made by Jifon and Syvertsen (Citation2003).
The light reflected, i.e. whiteness [lightness (L*)], from the leaves coated with reflective material was 55% and 17% higher in the outer and inner canopy layers, respectively, compared to the outer and inner layer of control trees (); similar results were reported by Glenn et al. (Citation1999).
Stomatal opening is the response to four independent variables: light, CO2, temperature, and Vapor pressure deficit (VPD) (Draper and Smith, Citation1996). Stomatal conductance (gs) was significantly higher – by 35% and 42% in the outer and inner layers, respectively – in treated as compared to control trees (). Our result showed that the treatment allowed for better stomatal opening in the leaves of the outer canopy layer compared to those of the control canopy, in agreement with reports by Cantore et al. (Citation2009) and Shellie and King (Citation2013b). Therefore, our experiment showed that kaolin did not obstruct stomata (Rosati et al., Citation2006; Wünsche et al., Citation2004); this is probably because the amount of kaolin found on the lower part of the leaf was negligible. This can be attributable to the distribution mode of the reflective material; indeed, in our experiment, the jet of kaolin was sprayed from the top to the bottom of the canopy by a machine that moved among rows, facilitating the covering of the adaxial surface of the leaves and reducing the risk of stomatal obstruction.
Net assimilation (An) was statistically higher – by 33% and 43% in the outer and inner layers, respectively – in the treated tree canopies compared to the control tree canopies (5.1 µmol CO2 m−2s−1 ± 0.2 and 6.8 µmol CO2 m−2s−1 ± 0.7) (), in agreement with the results of Chamchaiyaporn et al. (Citation2013) and Jifon and Syvertsen (Citation2003). In the outer layer of the treated tree, this result can be attributed to the reflection action of kaolin. Kaolin also reflects the Infrared (IR) solar component – which is responsible for the heating of leaves – thus, reducing the value of the IR component from a very high to a moderate level for citrus leaves during hot summer months. Higher temperature influences the photosynthesis process negatively because it directly encourages stomatal closure. Furthermore, because the opening of stomata in citrus plants is also extremely sensitive to evaporative demand, the thermal increase on the outer canopy leaves also causes an increase in VPD to the extent that it induces stomatal closure (Bonal and Guehl, Citation2011) in control plants as compared to the outer canopy leaves of the treated plants. Therefore, stomatal conductance (gs) was significantly higher – by 35% and 42% in the outer and inner layers, respectively – in treated compared to control tree canopies (). Our result showed that treated leaves of the outer layer allowed for better stomatal opening compared to the control, in agreement with results obtained in other studies (Cantore et al., Citation2009; Shellie and King, Citation2013b).
The lower stomatal conductance, induced by the excessive radiation experienced by the control trees, makes it difficult to dispose of the high photon quantity during photosynthesis. This results in a lack of a stomatal factor, which then negatively influences net assimilation (An). The fluorescence parameter also reflects this phenomenon.
The analysis of the obtained results showed that light stress had influenced the efficiency of PSII, causing a decrease in the quantum yield values of PSII (Fv/Fm and ΦPSII). A decreased in the Fv/Fm value indicates the presence of photo-inhibitory damage in response to abiotic stresses such as high temperatures (Gamon and Pearcy, Citation1989) and excess light (Ögren and Sjöström, Citation1990); the leaves of the untreated canopies showed a lower maximum quantum efficiency (Fv/Fm), whereas those of the treated canopies reached the critical value. The change in Fv/Fm values as a result of treatment with kaolin was similar to that observed in other studies on plants of Citrus paradisi (L.) and Vitis treated with an aqueous suspension of kaolin at 6% (w/v) (Correia et al., Citation2012; Dinis et al., Citation2016; Jifon and Syvertsen, Citation2003).
Furthermore, the quantum efficiency of PSII (PhiPS2) was 44% lower in the control leaves than in the treated leaves (), in agreement with Correia et al. (Citation2012); this fluorescence index (PhiPS2) allows for the tracing of the amount of light absorbed by the leaves and used to feed the photochemical reactions of the light phase of the photosynthetic process (). The photochemical quenching (qP) increased by 45% in the kaolin treatment compared to the control treatment (), which shows the state of oxidation-reduction of QA in PSII, expresses the decay of fluorescence caused by photochemical potential, and provides information on open reaction centers.
Table 4. Fluorimetric parameters detected on the outer layer of the canopy tree treated with kaolin (TK) and untreated (TCN = control)(average on two years)
Finally, the ETR (Electron Transport Rate), which provides information about electron transport, showed significant differences between treatment (). The treated canopy leaves showed better electron transport, and the value was 44% higher compared to that of the untreated canopy leaves (). The results obtained indicated that the higher light intensity in the leaves of control canopies caused greater saturation of photochemical reactions – reflected in the lower values of Fv/Fm, ΦPSII and qP – while the absorbed energy dissipated in non-photochemical processes (NPQ) was 45% higher ().
It can be said that, in clementine leaves, which have a low light saturation point, the kaolin treatment allowed the leaves to use a higher percentage of the received photons, reducing the number of unused ones (Demmig-Adams and Adams, Citation1992; Ruban and Horton, Citation1995). The SPAD index (), which expresses the chlorophyll status, increased by 13% in the leaves of treated plants, reflecting the protective action of kaolin on chlorophyll pigments.
Thus, with the kaolin treatment, exposure of plants to the high levels of irradiation on hot summer days resulted in improved photosynthetic capacity and reduced use of photonic energy in processes that damage the photosynthetic enzyme apparatus (photoinhibition) and destroy photosynthetic pigments (photooxidation) (Anderson et al., Citation1998; Bowler et al., Citation1992; Osmond et al., Citation1997).
Fruit Carpological Parameters
The measurements carried out on the fruits showed statistically significant differences as a result of kaolin treatment. Indeed, the height of the fruits was 9% higher in kaolin treated fruits compared to the control fruits (). The equatorial diameter showed increases of about 11% in the treated fruits compared to the control fruits (). The treatment did not modify the shape of the fruits; in fact, the H/D ratio (relative length) did not show statistically significant differences among treated and control fruits. The average fresh weight showed an increase of 30% in the fruits of the treated plants when compared with the fruits of the control plants (). The fruit dry weight was 20% higher in the treated plants when compared with the control plants ().
Table 5. Carpomentric parameters of citrus clementine fruit in tree treated with Kaolin (TK) and non-treated (TCN) (average on two years)
The difference in production among treated and control trees is attributable to increased fresh fruit weight because no difference was observed in the number of fruits per tree between treatments: TCN:831.17 (± 44.46 S.E).; TK = 960.83 (±131.69 S.E). Therefore, the yield per tree was significantly higher in TK compared to TCN: TK = 70.53 (± 10.19 S.E).; TK = 67.00 (±3.55 S.E). This result is due to the better performance of leaves in the treated trees. The plants are mainly engaged in the growth phase of the fruits during hot summer months; therefore, fruits are the main sinks that benefits from the better performance of leaves (source).
In recent years, the price of kaolin has significantly decreased. In our experiment, the treated fruit size increased and, with it, also its price (about 50%). Therefore, it can be estimated that the treatment has reduced only 3% of the final price.
Fruit Color Parameters
The color of the flavedo is influenced by the presence of chlorophyll, carotenoid, and anthocyanin pigments (Lancaster et al., Citation1997). The colorimetric parameters detected on the flavedo allowed for the evaluation of the influence of the reflective product on the pigmentation of the flavedo. It is possible to see how the flavedo lightness showed a lower value in the kaolin treated fruits compared to the control fruits (). Furthermore, a* chromatic component was higher in the kaolin treated fruits, the color shifting toward an intense red tint in the fruits of the treated trees, in agreement with the results reported by other authors (Chamchaiyaporn et al., Citation2013; Glenn et al., Citation2001; Shellie and King, Citation2013a, Citation2013b; Yazici and Kaynak, Citation2009) (). The chromatic component b* showed a lower value in the fruits of the kaolin treated plants compared to those of the control ().
Table 6. Main colorimetric indices of the exocarp (E) and the pulp (P) of fruit in trees treated with kaolin (TK) and untreated (TCN = control) – (average on two years)
The hue angle confirmed a change in the flavedo toward red for the fruits of the treated trees and toward yellow for those of the control trees. The chroma value was higher in the control compared to the fruits of the treated plants. The colorimetric index a/b showed higher values in the fruits of the treated plants compared to the fruits of the control plants ().
As far as the colorimetric parameters of the pulp, the trend was markedly similar to that of the flavedo. Lightness showed significantly lower values in the fruits of the treated plants (). The chromatic component a* was 12% higher in the fruits of the treated plants, revealing a tendency for orange coloration of the fruit pulp (). Regarding the chromatic component b*, there were no statistically significant differences between the fruits of the control and the kaolin treated plants (). A similar trend was reported for the chroma (). The hue angle confirmed how the treatment with reflective material also influenced the pigmentation of the pulp (). The tint in the fruits of the treated plants showed a value of that surpasses that of the control tree fruits by approximately three points (). Finally, the colorimetric index a/b showed higher values in the fruits of the treated plants, meaning a more orange pigmentation of the pulp in fruits treated with kaolin ().
Table 7. Maturation indices in the fruit of citrus clementine tree treated with Kaolin (TK) and non-treated (TCN = control) -(average on two years)
The reduction of the daily temperature, induced by the screen created by the reflective material on the fruits, reduces the activation of the enzymes that degrade chlorophyll, accentuating the carotenoids that accumulate during the ripening process (Alquezar et al., Citation2008; Fattahi et al., Citation2011).
Maturation Indexes and Nutraceutical Parameters
In terms of maturation indexes, TSS was not influenced by kaolin and was not significantly different among treated and control trees (), in agreement with the results of a report by Chamchaiyaporn et al. (Citation2013); in contrast, titratable acidity (TA) was lowered by the treatment (). The values obtained were in agreement with those reported by other authors for clementine (Hussain et al., Citation2012; Poiroux-Gonord et al., Citation2012). Therefore, the fruits were not conditioned by kaolin treatment during the maturation process.
It is known that the antioxidant capacity of fruits requires the synergistic activity carried out by several bio-molecules present in varying amounts, depending on the fruit species. It is possible to include polyphenols, chlorophyll pigments (chlorophyll a, chlorophyll b, and carotenoids), and ascorbic acid among these biomolecules. The ascorbic acid content was not significantly different between the treated fruits and the control fruits (). Concerning the total polyphenol content (TPC) and the total antioxidant capacity (TAC), the differences between the treatments did not appear to be statistically significant either (). However, the values were higher or similar to those found by other authors for clementine (Cano et al., Citation2008; Rapisarda et al., Citation2009).
Table 8. Nutraceutic parameters in the fruit of citrus clementine tree treated with Kaolin (TK) and non-treated (TCN = control) -(average on two years)
PCA
Factors F1 and F2 of the PCA accumulated 64.23% and 14.46% of the variance, respectively (), and totaled 78.68% of the initial variability (). The correlations between variables and factors are reported in . In particular, L*, a*, b*, Chroma, °Hue, a/b of exocarp, L*, a*, Chroma, °Hue, a/b of pulp, fresh weight, height and transversal diameter of fruit, relative length, dry weight exocarp, dry weight pulp, and two nutraceutical variables (TPC and AA) participated in the F1 factor formation (); all variables of the maturation index, i.e., SST, TA, and TSS/TA, b* pulp, contributed to the F2 factor formation of the PCA (); TAC contributed to the F3 factor formation ().
Table 9. Correlations between variables and factors in PPCA
Figure 2. Bi-plot. Projection of the variable vectors and centroids treatment in function of harvest date
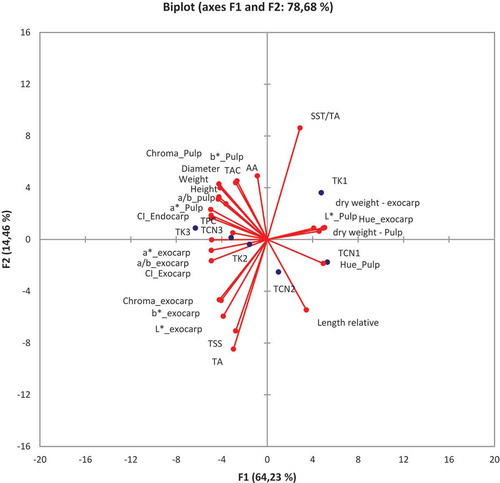
The results of the PCA can be explained by the positioning of the parameters relative to the centroid on the biplot (). The biplot enables one to look at the observations on a two- dimensional map and to identify trends (). It shows the observations with the centroids of the two treatments on the three harvest times. On the first harvest date, the fruit was of lower quality, and the kaolin treatment improved TSS/TA ratio. On the second harvest date, the fruit quality improved in the treated plants, whereas, in the control, the TSS/TA worsened. It can be seen that the latter harvest had better fruit fresh weight, pulp and exocarp coloration, and vitamin C and polyphenols content (). The kaolin treatment clearly enhanced all these parameters compared to those in the control trees.
Conclusion
This study shows that, in Southern Italy, where high radiation can limit photosynthetic capacity, kaolin can improve leaf carbon uptake potential in the outer canopy layers of clementine trees. This reflective material also improves light penetration inside the canopy, where low light is the limiting factor for the photosynthetic process as a result of shading by upper canopy leaves. Therefore, kaolin increases the net productivity (An) also in the inner layer of the canopy. Cumulatively, this has led to an increase in productivity of trees treated with kaolin, such as increase in fruit size compared to control trees. Moreover, treatment with kaolin has improved the fruit quality levels of the clementine, Comune cv., in this area, especially during late harvest (December). Finally, it is necessary to state that the product was easily removed with normal fruit washing operation before packaging. The decreasing costs of reflective materials in the last few years, together with the increase in fruit size and tree yield, justify the use of reflective materials for this high-income citrus species.
Declaration of Interest Statement
This work was supported by the MIUR under Project PON03PE_00090_03 “Sustainable models and new technologies for the valorisation of Mediterranean vegetable chains”.
Acknowledgments
We would like to thank the OP FruJt and COPPI cooperatives for their cooperation.
Additional information
Funding
References
- Ali, M.S.M., and H.E. El Zayat. 2019. Effect of some biological stimulants and kaolin particles sprays on fruit retention, productivity and fruit quality of Washington Navel orange trees. Hortscience J. Suez Canal Univ 8(1):69–78. doi: 10.21608/hjsc.2019.59855.
- Allakhverdiev, I.S., V.D. Kreslavski, V.V. Klimov, A.D. Los, R. Carpentier, and P. Mohanty. 2008. Heat stress: An overview of molecular responses in photosynthesis. Photosynth. Res. 98:541. doi: 10.1007/s11120-008-9331-0.
- Alquezar, B., M.J. Rodrigo, and L. Zacarías. 2008. Carotenoid biosynthesis and their regulation in citrus fruits. Tree For. Sci. Biotech. 2:23–35.
- Anderson, J.M., Y.I. Park and W.S. Soon. 1998. Unifying model for the photoinactivation of photosystem II in vivo under steady-state photosynthesis. Photosynth. Res. 56: 1–13.
- Balakumar, T., V. Hani Babu Vincent, and K. Paliwal. 1993. On the interaction of UV‐B radiation (280–315 nm) with water stress in crop plants. Physiol. Plant 87(2):217–222. doi: 10.1111/j.1399-3054.1993.tb00145.x.
- Barrett, A.S., and L.R. Brown. 2012. A novel method for estimating tree dimensions and calculating canopy volume using digital photography. Afr. J. Range For. Sci. 29(3):153–156.
- Bonal, D., and J.M. Guehl. 2011. Contrasting patterns of leaf water potential and gas exchange responses to drough in seedlings of tropical rain-forest species. Functional Ecology, 15:490–496.
- Berry, J., and O. Bjorkman. 1980. Photosynthetic response and adaptation to temperature in higher plants. Annu. Rev. Plant Physiol. 31:491–543. doi: 10.1146/annurev.pp.31.060180.002423.
- Brosché, M., and A. Strid. 2003. Molecular events following perception of ultraviolet-B radiation by plants. Physiol. Plant. 117:1–10. doi: 10.1034/j.1399-3054.2003.1170101.x.
- Bowler, C., D. Inze, and M. Montagu. 1992. Superoxide dismutases and stress tolerance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 43:83–116.
- Caffarri, S., T. Tibiletti, R.C. Jennings, and S. Santabarbara. 2014. Comparison between plant photosystem I and photosystem II architecture and functioning. Curr. Protein Pept. Sci. 15(4):296–331. doi: 10.2174/1389203715666140327102218.
- Cano, A., A. Medina, and A. Bermejo. 2008. Bioactive compounds in different citrus varieties. Discrimination among cultivars. J. Food Compos. Anal. 21:377–381.
- Cantore, V., B. Pace, and R. Albrizio. 2009. Kaolin-based particle film technology affects tomato physiology, yield and quality. Environ. Exp. Bot. 66:279–288. doi: 10.1016/j.envexpbot.2009.03.008.
- Chamchaiyaporn, T., K. Jutamanee, P. Kasemsap, P. Vaithanomsat, and C. Henpitak. 2013. Effects of kaolin clay coating on mango leaf gas exchange, fruit yield and quality. Kasetsart J. Nat. Sci. 47:479–491.
- Correia, C., L.T. Dinis, R. Pinheiro, H. Fraga, H. Ferreira, I. Gonçalves, J. Costa, A. Oliveira, A. Malheiro, and J. Moutinho-Pereira. 2012. Climate change and adaptation strategies for viticulture. J. Int. Sci. Publ: Agric. Food 2:424–429.
- Davies, F.S., and L.G. Albrigo. 1994. Citrus. CAB International, Wallingford.
- Demming-Adams, B., W.W. III Adams 1992. Photoprotection and other responses of plants to high light stress. Annu. Rev. Plant. Phisiol. Plant Mol. Biol. 43: 599–626.
- Dinis, L.T., H. Ferreira, G. Pinto, S. Bernardo, C.M. Correia, and J. Moutinho-Pereira. 2016. Kaolin-based, foliar reflective film protects photosystem II structure and function in grapevine leaves exposed to heat and high solar radiation. Photosynthetica. 54:47–55. doi: 10.1007/s11099-015-0156-8.
- Draper, N.R., and H. Smith. 1996. Applied regression analysis. John Wiley and Sons, New York.
- El-Tanany, M.M., A.M.A. Kheder, and H.R. Abdallah. 2019. Effect of some treatments on reducing sunburn in Balady Mandarin fruit trees (Citrus reticulata, Blanco). Middle East J. Agric. Res. 08(3):889–897.
- Ennab, H.A., S.A. El-Sayed, and M.M.S. Abo El-Enin. 2017. Effect of kaolin applications on fruit sunburn, yield and fruit quality of Balady Mandarin (Citrus Reticulata, Blanco). Menoufia J. Plant Prod. 2:129–138.
- Fattahi, J., Y. Hamidoghli, R. Fotouhi, M. Ghasemnejad, and D. Bakhshi. 2011. Assessment of fruit quality and antioxidant activity of three citrus species during ripening. Hortic. Biol. Environ. 2(2):113–128.
- Fedina, I., M. Velitchkova, K. Georgieva, K. Demirevska, and L. Simova. 2007. L. UV-B response of green and etiolated barley seedlings. Biol. Plant. 51:699–706. doi: 10.1007/s10535-007-0145-2.
- Foyer, C.H., M. Lelandais, and K.J. Kunert. 1994. Photooxidative stress in plants. Physiol. Plant. 92:696–717. doi: 10.1111/j.1399-3054.1994.tb03042.x.
- Frohnmeyer, H., and D. Staiger. 2013. Ultraviolet-B radiation-mediated responses in plants. Balancing damage and protection. Plant Physiol. 133:1420–1428. doi: 10.1104/pp.103.030049.
- Gamon, J.A., R.W. Pearcy. 1989. Photoinhibition in Vitis californica: interactive effects of sunlight, temperature and water status. Plant Cell Environ. 13: 267–275.
- Glenn, D.M., G.J. Puterka, S. Drake, T.R. Unruh, A.L. Knight, P. Baherle, E. Prado, and T. Baugher. 2001. Particle film application influences apple leaf physiology, fruit yield and fruit quality. J. Amer. Soc. Hort. Sci. 126:175–181. doi: 10.21273/JASHS.126.2.175.
- Glenn, D.M., G.J. Puterka, T. Vanderzwet, R.E. Byers, and C. Feldhake. 1999. Hydrophobic particle films: A new paradigm for suppression of arthropod pests and plant diseases. J. Econ. Entomol. 92(4):759–771. doi: 10.1093/jee/92.4.759.
- Havaux, M. 1996. Short-term responses of Photosystem I to heat stress. Photosynth. Res. 47:85–97. doi: 10.1007/BF00017756.
- Hendry, G.A.F., J.D. Houghton, and S.B. Brown. 1987. The degradation of chlorophyll – A biological enigma. New Phytol. 107:255–302.
- Hughes, D.E. 1983. Titrimetric determination of ascorbic acid with 2,6-dichlorophenol indophenol in commercial liquid diets. J. Pharm. Sci. 72:126. doi: 10.1002/jps.2600720208.
- Hussain, S., F. Curk, C. Dhuique-Mayer, L. Urban, P. Ollitrault, F. Luro, and R. Morillon. 2012. Autotetraploid trifoliate orange (Poncirus trifoliata) rootstocks do not impact clementine quality but reduce fruit yields and highly modify rootstock/scion physiology. Sci. Hortic. 134:100–107. doi: 10.1016/j.scienta.2011.11.008.
- Jackson, J.E. 1980. Light interception and utilization by orchard systems. Hortic. Rev. 2:208–267.
- Jifon, J.L., and J.P. Syvertsen. 2003. Kaolin particle film application can increase photosynthesis and water use efficiency of “Ruby Red” grapefruit leaves. J. Amer. Soc. Hort. Sci. 128:107–112. doi: 10.21273/JASHS.128.1.0107.
- Krause, G.H., A. Virgo, and K. Winter. 1995. High susceptibility to photoinhibition of young leaves of tropical forest trees. Planta. 197:583–591. doi: 10.1007/BF00191564.
- Kriedemann, P.E. 1968. Photosynthesis in vine leaves as a function of light intensity, temperature, and leaf age. Vitis 7:213–220.
- Laing, W.A., D.H. Greer, and T. Schnell. 1995. Photoinhibition of photosynthesis causes a reduction in vegetative growth rates of dwarf bean (Phaseolus vulgaris) plants. Austral. J. Pl. Physiol. 22:511–520.
- Lancaster, J.E., C.E. Lister, P.F. Reay, and C.M. Triggs. 1997. Influence of pigment composition on skin color in a wide range of fruit and vegetables. J. Amer. Soc. Hort. Sci. 122:594–598. doi: 10.21273/JASHS.122.4.594.
- Li, Z., S. Wakao, B.B. Fischer, and K.K. Niyogi. 2009. Sensing and responding to excess light. Annu. Rev. Plant Biol. 60:239–260. doi: 10.1146/annurev.arplant.58.032806.103844.
- Long, S.P., S. Humphries, and L. Falkowski. 1994. Photoinhibition of photosynthesis in nature. Annu. Rev. Plant Physiol. Plant Mol. Biol. 45:633–662. doi: 10.1146/annurev.pp.45.060194.003221.
- Long, S.P., X.G. Zhu, S.L. Naidu, and D.R. Ort. 2006. Can improvement in photosynthesis increase crop yields? Plant Cell Environ. 29:315–330. doi: 10.1111/j.1365-3040.2005.01493.x.
- Maxwell, K., and G.N. Johnson. 2000. Chlorophyll fluorescence—a practical guide. J. Exp. Bot. 51:659–668. doi: 10.1093/jexbot/51.345.659.
- Nahal, I. 1981. The Mediterranean climate from a biological viewpoint, p. 63–86. In: F. Di Castri, D.W. Goodall, and R.L. Specht (eds.). Ecosystems of the World II: Mediterranean-type shrublands. Elsevier Scientific Publishing Company, Amsterdam.
- Osmond, B., Badger, M., Maxwell, K., Björkman, O., Leegood R. 1997. Too many photons: photorespiration, photoinhibition and photooxidation. Trends Plant Sci. 2: 119–121.
- Pellegrini, N., R. Re, M. Yang, and C. Rice Evans. 1999. Screening of dietary carotenoids and carotenoid-rich fruit extracts for antioxidant activities applying the ABTS+ radical cation decolorization assay. Methods Enzymol. 299:589–603.
- Poiroux-Gonord, F., A.L. Fanciullino, L. Berti, and L. Urban. 2012. Effect of fruit load on maturity and carotenoid content of clementine (Citrus clementina Hort. ex Tan.). J. Sci. Food Agric. 92(10):2076–2083. doi: 10.1002/jsfa.5584.
- Powles, S.B. 1984. Photoinhibition of photosynthesis induced by visible light. Annu. Rev. Plant Physiol. 35:15–44. doi: 10.1146/annurev.pp.35.060184.000311.
- Rapisarda, P., S. Fabroni, S. Peterek, G. Russo, and H.P. Mock. 2009. Juice of new citrus hybrids (Citrus clementina Hort. ex Tan. X C.sinensis L. Osbeck) as a source of natural antioxidants. Food Chem. 117:212–218. doi: 10.1016/j.foodchem.2009.03.101.
- Ribeiro, R.V, Machado M.C., and M.C.. De Santos. 2005. Leaf temperature in sweet orange plants under field condition: influence of meteorological elements. Rev. Bras. Agrometeorologia 13:353–368.
- Re, R., N. Pellegrini, A. Proteggente, A. Pannala, A.M. Yang, and C. Rice Evans. 1999. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3.
- Rosati, A., S.G. Metcalf, R.P. Buchner, A.E. Fulton, and B.D. Lampinen. 2006. Physiological effects of kaolin applications in well-irrigated and water-stressed walnut and almond trees. Ann. Bot. 98:267–275. doi: 10.1093/aob/mcl100.
- Ruban, A.N., P. Horton. 1995. Regulation of non-photochemical quenching of chlorophyll fluorescence in plant. Aust. J. Plant Physyol. 22: 221–230.
- Salvucci, M.E., and S.J. Crafts-Brandner. 2004. Relationship between the heat tolerance of photosynthesis and the thermal stability of rubisco activase in plants from contrasting thermal environments. Plant Physiol. 134:1460. doi: 10.1104/pp.103.038323.
- Scalzo, J., A. Politi, N. Pellegrini, B. Mezzetti, and M. Battino. 2005. Plant genotype affects total antioxidant capacity and phenolic contents in fruit. Nutrition 21(2):207–213. doi: 10.1016/j.nut.2004.03.025.
- Sharkey, T.D. 2005. Effects of moderate heat stress on photosynthesis: Importance of thylakoid reactions, rubisco deactivation, reactive oxygen species, and thermo tolerance provided by isoprene. Plant Cell Environ. 28:269–277. doi: 10.1111/j.1365-3040.2005.01324.x.
- Shellie, K., and B.A. King. 2013a. Kaolin particle film and water deficit influence red wine grape color under high solar radiation in an arid climate. Am. J. Enol. Vitic. 64:214–222. doi: 10.5344/ajev.2013.12067.
- Shellie, K., and B.A. King. 2013b. Kaolin-based foliar reflectant and water deficit influence Malbec leaf and berry temperature, pigments, and photosynthesis. Am. J. Enol. Vitic. 64:223–230. doi: 10.5344/ajev.2012.12115.
- Sinclair, T.R., and L.H. Allen Jr. 1982. Carbon dioxide and water vapor exchange of leaves on field-grown citrus trees. J. Expt. Bot. 33:1166–1175. doi: 10.1093/jxb/33.6.1166.
- Slinkard, K., and V.L. Singleton. 1997. Total phenol analysis: Automation and comparison with manual methods. Am. J. Enol. Vitic. 28:49–55.
- Spiegel-Roy, P., and E.E. Goldschmidt. 1996. Biology of Citrus. Cambridge University Press, Cambridge.
- Syvertsen, J.P., and J. Lloyd.1994. Citrus. p. 65–99. In: eds. B. Schaffer and P.C. Andersen. Handbook of Environmental Physiology of Fruit Crops. Vol. II, CRC Press, Boca Raton.
- Takeuchi, A., T. Yamaguchi, J. Hidema, A. Strid, and T. Kumagai. 2002. Changes in syntreatmentand degradation of Rubisco and LHCII with leaf age in rice (Oryza sativa L.) growing under supplementary UV‐B radiation. Plant Cell Environ. 25:695–706. doi: 10.1046/j.1365-3040.2002.00844.x.
- Tevini, M. 1996. Erhöhte UV‐B‐Strahlung: Ein Risiko für Nutzpflanzen? Biol. Unserer Zeit 26(4):245–254. doi: 10.1002/biuz.19960260411.
- Werner, C., O. Correia, and W. Wolfram Beyschlag. 2002. Characteristic patterns of chronic and dynamic photoinhibition of different functional groups in a Mediterranean ecosystem. Funct. Plant Biol. 29:999–1011. doi: 10.1071/PP01143.
- Wünsche, J.N., L. Lombardini, and D.H. Greer. 2004. Surround particle film applications effects on whole canopy physiology of apple. Acta Hortic. 636:565–571. doi: 10.17660/ActaHortic.2004.636.72.
- Yazici, K., and L. Kaynak. 2009. Effects of kaolin and shading treatments on sunburn on fruit of Hicaznar of pomegranate (Punica granatum L., cv Hicaznar). Acta Hortic. 81:167–174. doi: 10.17660/ActaHortic.2009.818.24.
- Yordanov, I.S., R. Dilova, T. Petkova, V. Pangelova, V. Goltsev, and K.H. Süss. 1986. Mechanisms of the temperature damage and acclimation of the photosynthetic apparatus. Photobiochem. Photobiophys. 12:147–155.
- Zaghloul, A.E., H.A. Ennab, and M.A. El-Shemy. 2017. Influence of kaolin sprays on fruit quality and storability of balady Mandarin. Alexandria Sci. Exch. J. 38(4):661–670. doi: 10.21608/asejaiqjsae.2017.4102.
- Ögren, E., M. Sjöström. 1990. Estimation of the effect of photoinhibition on the carbon gain in leaves of willow canopy. Planta. 181: 560–567.