?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In this study, the antioxidant effects, protein contents and phytochemical structure content of jujube, oleaster fruits and their seeds were investigated. Methanol extraction of fruits and seeds was prepared and volatile organic components were examined by GC-MS and phenolic components were examined by LC-MS/MS. Antioxidant activity was determined by DPPH and NO scavenging (%). In addition, mineral content was defined with ICP-MS. DPPH scavenging activity (%) was observed most in jujube fruit (80.36%) then it continued as oleaster seed (79.33%) and oleaster fruit (72.00%) and jujube seed (66.26%) respectively. According to protein results, there was a 2.19-fold difference between jujube fruit and seed. This difference was found 0.78 times between oleaster fruit and seed. 4-hydroxybenzoic acid, gallic acid and vanillic acid contents of phenolic compounds were found in high amounts. P (phosphorus) contents in jujube, oleaster, jujube seed and oleaster seed, respectively; 722.67, 736.91, 453.74 and 687.10 mg/kg. In the jujube and jujube seed samples, the contents of Ca (calcium) were found 1261.02 and 2228.24 mg/kg. K (potassium) contents were determined 7351.16 and 7688.42 mg/kg in jujube and oleaster, respectively.
Introduction
Jujube (Zizyphus jujuba Mill.), a fruit of the dicotyledonous Rhamnaceae plant, is widely distributed in Asia, Europe and America (Wang et al., Citation2016a). Ziziphus jujuba is grown economically in many countries. Fruits are consumed fresh, dried or processed (jam, bread, cake, jelly, etc.) (Pareek, Citation2002). Jujube fruit is very rich in polysaccharides, phenolics, flavonoids and saponins. These molecules are responsible for biological activities, such as reduced proliferation of cancer cells, regulation of immune function and decreased blood triglycerides (Dahiru and Obidoa, Citation2008; Li et al., Citation2011). It has been used as a medicine against diseases such as palpitations, insomnia, anemia, splenic insufficiency, diarrhea, insufficiency, hepatotoxicity and fever in traditional Chinese medicine (Mahajan and Chopda, Citation2009). In a study, some phenolics such as chlorogenic acid, caffeic acid, catechin, epicatechin and routine were isolated from jujube fruit (Hudina et al., Citation2008). Phenolic compounds have been reported to suppress stress factors in plants and improve fruit quality. In addition, phenolic compounds have antioxidant activity, so they also help remove free radicals (Li et al., Citation2005). In a study among 62 fruits, Jujube has been reported to have a high phenolic content. It has been suggested to be a good dietary source to be less affected by oxidative stress due to its high phenol content (Fu et al., Citation2011). In several studies of fruits of various maturity, it has been shown that the phenolic content changes significantly and this change also affects the antioxidant capacity.(Acosta-Montoya et al., Citation2010)
Elaeagnus angustifolia L. (oleaster, Russian olive, wild olive) belongs to the genus Elaeagnus of Elaeagnacea (Araliaceae) family (Sahan et al., Citation2013). There are more than 90 species of this plant, which is mostly grown in Asia, Europe and North America in the world (Saboonchian et al., Citation2014a). The oleaster fruit is sweet and edible, although it has a dry and floury structure. It contains flavonoids, terpenoids, glucose, fructose and phenolic acids (such as 4-hydroxybenzoic acid, 4-hydroxycinnamic acid, benzoic acid, caffeic acid, ferulic acid and vanillic acid). From oleaster extract, 4(+)-catechin, (−)-epicatechin, (+)-gallocatechin, (−)-epigallocatechin, kaempferol, quercetin, luteolin, isorhamnetin and isorhamnetin-3-0-β-D-galactopyranoside were isolated (Okmen and Turkcan, Citation2014). Its seed is good for diseases such as urinary diseases, diarrhea, nausea, vomiting, asthma and bloating (Chaiklahan et al., Citation2014; Faramarz et al., Citation2017; Farzaei et al., Citation2015). Flavonoids are mainly found in fruits and vegetables. Due to their phenolic hydroxyl groups, they have properties such as metal chelation, reduction of lipid peroxidation and free radical scavenging and show high antioxidant activity (Okmen and Turkcan, Citation2014).
Plants produce a vast and diverse assortment of organic compounds, the great majority of which do not appear to participate directly in growth and development. These substances traditionally referred to as secondary metabolites. Their functions, many of which remain unknown, are being elucidated with increasing frequency (Bonofigilo et al., Citation2016). Phytochemicals form the medicinal properties of plants. Plants can synthesize a large number of secondary metabolites. However, only 10% of them have been investigated and isolated (Uysal et al., Citation2018). Plant secondary metabolites have been used in traditional medicine for centuries because of their effective biological activities.
In this study, jujube, oleaster fruits and their seeds were studied. Jujube and oleaster fruits are physiologically similar, although they belong to different genera (Ziziphus-Elaeagnus) and families (Rhamnaceae-Elaeagnaceae). It is also used in the treatment of common diseases in traditional medicine. Therefore, the common phytochemical content and antioxidant effects of these two fruits and seeds were compared.
Materials and Methods
Extraction Process
The dried fruit of jujube and oleaster was obtained from Manisa in Turkey. Before the extraction process, jujube (1), jujube seed (2), oleaster (3) and oleaster seed (4) samples were washed in pure water and air-dried at room temperature for 3 weeks. For analysis, dried samples were pulverized in a high-speed plant mill and prepared for extraction. Powdered plant samples of 2 g were extracted in 40 mL of 99.9% methanol in ultrasonic bath for 30 min then left at room temperature. The final extraction concentrate was then adjusted to 50 mg/mL. Extraction yields of samples are between 0.8 and 12 mg/mL
Antioxidant Test
DPPH and NO Scavenging Activity
The antioxidant activity of the extracts was tested using the DPPH radical with minor modifications (Zhang et al., Citation2011). A 2.10−4 M DPPH solution was prepared in methanol for this method. Receive 0.5 mL of the sample and 4 mL the DPPH solution was added.The mixture was shaken and kept at room temperature for 60 min in the dark. Eighty percent methanol was used as a blind and samples were read at 517 nm by spectrophotometer (TECAN Infinite M200 microplate reader). IC50 values were calculated as 5.2, 32.31, 17.81 and 7.74 mg/mL for jujube, oleaster, jujube seed and oleaster seed, respectively. The results were calculated according to the following equation.
Nitric oxide scavenging activity was determined according to the following method (Marcocci et al., Citation1994). Four milliliters of each of the extracts prepared in different concentrations was taken and 1 mL of sodium nitroprusside solution (25 mM) was added to the mixture and incubated for 2 h at 37°C. After incubation, 0.5 mL of the solutions was removed and mixed with 0.3 mL of Griess reagent (5% H3PO4 in 1% sulfanilamide and 0.1% naphthylethylenediamine dihydrochloride). The absorbance of the formed chromophore was read against the blind at 570 nm on the spectrophotometer (TECAN Infinite M200 Microplate reader). The results were calculated.
Determination of Phenolic Compounds by LC-MS/MS
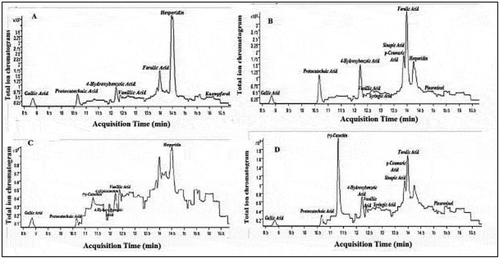
Determination of phenolic profiles of plant extracts, high-performance liquid chromatography–mass spectrometer – mass spectrometer (Agilent 1260 Triple Quadrupole MS/MS) was used. Each analysis was performed with three replications. HPLC column C18 ODS used in the analyses (25 x 4.6 mm x 5 µm) was used. Injection volume for analysis: 2 µL. Water/0.1% formic acid (A), methyl alcohol (99.9%) (B) was used as a carrier phase. The gradient method as follows: 3 min 2% B, 6 min 25% B, 10 min 50% B, 14 min 95% B, 17.5 min 2% B. Flow rate: 0.4 mL/min. The identification of compounds was performed in positive and negative modes (Gören et al., Citation2009). LC-MS/MS total ion chromatograms of phenolic compounds of the samples are shown .
Determination of Volatile Organic Molecules by GC-MS
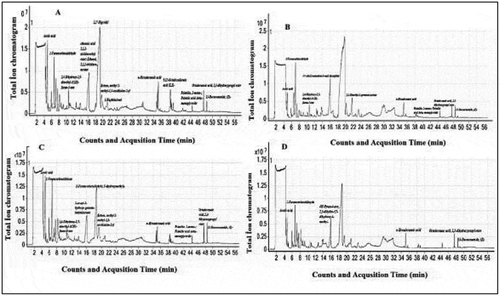
Volatile molecules in the extract were qualitatively analyzed in electron ionization (EI) mode with Agilent Technology 7890A Gas Chromatography (GC) Mass spectrometer (MS). Chromatographic column Agilent HP-5 MS, capillary column (30 mx 0.25 mm, film thickness of 0.25 mm). The furnace temperature was started at 40°C, followed by standing for 5 min, then at 5°C min−1 at 280°C and held for 5 min. Helium gas (99.999%) was used as the carrier gas. The constant flow rate is 1.5 mL min−1 and the injector temperature is 250°C. The extract was injected in splitless mode with 1 mL. Interpretation of the mass spectrum was performed according to the National Institute of Standards and Technology (NIST) database. GC-MS total ion chromatograms of volatile organic molecules of the samples are shown .
ICP-MS Analysis
In this study, dried fruit of jujube and oleaster and jujube and oleaster seeds were used. These samples were washed with ultrapure water then dried in an oven at 80 degrees. Dried samples of 0.5 g were weighed and 10 mL of nitric acid was added. Microwave decomposition was performed in 200°C temperature, 15 min ramp, 20 min hold and 20 min cooling program. After microwave decomposition process, Na, Mg, P, K, Ca, Mn, Fe, Cu, Zn were analyzed by ICP-MS (Agilent-7700) device.
Protein Assay
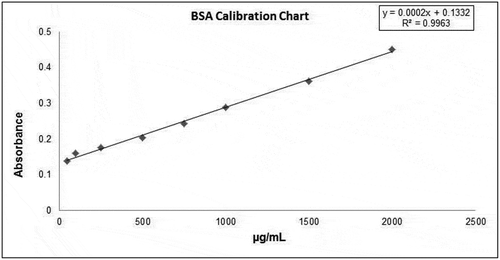
The total protein amount was made according to Bradford (Citation1976) and using BSA (Bovine Serum Albumin) standards. Standards were prepared in the range of 0.05–2 mg/mL. The calibration graph of BSA standards is given in (Bradford, Citation1976).
Statistical Analysis
Data were subjected to Analysis of Two-way ANOVA using GraphPad Prism 8.4.2. Means were separated from each other by Bonferroni’s multiple comparisons test (p < .05). The analysis was performed in triplicate.
Results and Discussion
Antioxidant Activity
According to the results obtained, in the amount of 50 mg/mL sample, DPPH scavenging activity (%) was observed most in jujube fruit (80.36%) then it continued as oleaster seed (79.33%), oleaster fruit (72.00%) and jujube seed (66.26%) respectively (). It was determined that DPPH-free radical scavenging activity increased as the concentration increased. Although the antioxidant capacity of jujube fruit is higher than the oleaster fruit, the relationship in the seeds was found to be opposite. In the amount of 50 mg/mL sample, NO scavenging activity (%) was observed most in oleaster seed (90.80%) then it continued as jujube (86.51%) and oleaster fruit (79.99%) and jujube seed (74.04%) respectively (). It is notable that jujube contains a lot of polyphenols (260 mg GAE/100 g DW) with well-known antioxidant activity (Wang et al., Citation2016b). High antioxidant power may be attributed to the presence of flavonoids, such as flavan-3-ols and flavonols. The antioxidant potency of flavonoids is approximately proportional to the total number of hydroxyl groups and is positively affected by the presence of an ortho-dihydroxy moiety in the B-ring (Re et al., Citation1999). In a study, it was stated that the DPPH antioxidant activity of the oleaster plant was higher in the fruit than the leaf shell and flower parts (Incilay, Citation2014). Leaf and flower extracts of E. angustifolia L. contain phenolic and flavonoid compounds that have antioxidant properties and protect cells from oxidative damage and delays or reduce the risk of many degenerative diseases (Saboonchian et al., Citation2014b).
Phenolic Compound Content
According to the results of the phenolic compound, 4-hydroxybenzoic acid, gallic acid and vanillic acid contents of phenolic compounds were found in high amounts. While Hesperidin is especially high in jujube fruit, ferulic acid is also high in oleaster fruit. +Catechin in jujube and oleaster seeds was highly detected. Ferulic acid was detected more in fruits and seeds of the oleaster plant, and kaemferol and hyperoside in jujube fruit. Protocatheic acid is higher in the fruits of both plants than its seeds. Total phenolic compound contents are jujube fruit, oleaster fruit, oleaster seed and jujube seed from high to low, respectively (). In a study conducted in different parts of the oleaster plant, it was determined that the total phenolic content was quite high in the fruits (Incilay, Citation2014). As a result of the experiments, it is seen that antioxidant activity and total phenolic content have been changed as parallel in jujube seed. However, in other examples, no correlation was fully observed. Some studies have shown a relationship between phenolic content and antioxidant capacity (Yang et al., Citation2002). However, in our study, in many studies, no correlation was found in jujube fruits in antioxidant capacity and total phenolic compound content (Li et al., Citation2005). An important part of the total antioxidant activity is also due to the presence of other phytochemical compounds such as ascorbic acid, alpha-tocopherol and pigments and their synergistic effects. For example, jujube contains abundant ascorbic acid (192–359 mg 100 g−1), which is known to be a strong reducing agent and may contribute to antioxidant activity by reducing the oxidized state of antioxidant compounds (Li et al., Citation2007a).
Table 1. LC-MS/MS phenolic compound content in samples
Volatile Organic Molecule Content
In GC-MS volatile organic molecule contents, jujube and oleaster fruits have been determined more components than seeds (). While lauric acid was also observed in jujube fruit, it was not detected in other samples. Octadecanoic acid, 2,3-dihydroxypropyl ester, which is an octadecenoic acid derivative was detected in all sample extracts. Palmitic acid.beta.-monoglyceride which is a palmitic acid derivative was detected in other samples except oleaster seed (). In a study on the leaves and flowers of the Oleaster plant, 65.75% (flowers), 49.16% (leaves) esters were determined from the volatile components obtained in the GC-MS analysis (Torbati et al., Citation2016). Zhao et al. detected oleic and linolic acid at a high rate and palmitic acid at a low rate in various jujube fruits. The reason for this is that jujube fruits with different genotypes are used in the study (Zhao et al., Citation2006). In the study, some fatty acids such as lauric, tridecanoic, myristic, pentadecanoic, palmitic, palmitoleic, heptadecanoic, linoleic, linolenic, oleic, stearic, eicosanoic and docosanoic acids were identified in the extract of the fruit of oleaster (Yıldırım et al., Citation2015).
Table 2. GC-MS volatile organic compounds of jujube fruit methanol extract
An abundance of palmitoleic acid in fruit skin and a high amount of linoleic acid and palmitic acid in seeds of oleaster was reported in the study, which might be explained through the differences in genotypes, climatic condition and soil composition variation (Sahan et al., Citation2015).
Mineral Content
According to mineral content results; Mg, P, K and Ca contents were determined in a very high amount. P contents in jujube, oleaster, jujube seed and oleaster seed, respectively; 722.67, 736.91, 453.74 and 687.10 mg/kg. Ca content of jujube and jujube seed is 1261.02 and 2228.24 mg/kg. K contents are 7351.16 and 7688.42 mg/kg in jujube and oleaster, respectively (). The studies have shown that the different parts of E. angustifolia L. contain different concentrations of minerals. The roots, barks, branches and leaves of the plant contain iron, lead, copper, cadmium, zinc, chromium, nickel and cobalt (Khan et al., Citation2016). In one study, they reported that the most common mineral in the oleaster plant is K, then it is Na and P (Hamidpour et al., Citation2017). According to our results, although the plant contains the most K and P minerals in the fruit and its seed, the Na content is low. This provides a more preferred food possibility. Li et al. reported that the Ca content in jujube species ranged between 45.6 and 118 mg/100 g (Li et al., Citation2007a). Several clinical and other studies have also shown calcium to be effective pressure-lowering agents (Zemel, Citation2009). The content of Zn in jujube fruit and seed was low (2.18–1.89 mg/kg). However, it is higher in oleaster fruit and seed (3.89–5.84 mg/kg). Zinc is important in nutrition for many reasons for the organism. It plays a role in immune system, insulin secretion, vitamin A secretion and the synthesis of key enzymes such as superoxide dismutase (Boron et al., Citation1988; Chausmer, Citation1998; Hwang et al., Citation2002).
Table 3. GC-MS volatile organic compounds of oleaster fruit methanol extract
Table 4. GC-MS volatile organic compounds of jujube seed methanol extract
Table 5. GC-MS volatile organic compounds of oleaster seed methanol extract
Table 6. ICP-MS metal content of samples
Protein Content
According to protein results, there was a 2.19-fold difference between jujube fruit and seed. This difference was found 0.78 times between oleaster fruit and seed. Although there was no significant difference between jujube and oleaster fruits, a 1.57 fold was found between the seeds. It was observed that the protein content was richer than the seeds compared to the fruits (). Russian olive fruits have high nutritional values and contain proteins, sugar, vitamins and minerals (Fonia et al., Citation2009; Taheri et al., Citation2010). In addition to polysaccharides and insoluble fibers, jujubes contain other components such as proteins, polyphenols and trace elements (Li et al., Citation2007b).
Table 7. Protein content
Conclusion
Although they belong to different plant families, jujube and oleaster fruit and seeds were investigated in this study because they are very similar in appearance and used in the treatment of similar diseases in traditional medicine. These fruits can be used as a good natural preservative in food preservation with its high antioxidant capacities and low metal contents. When the phytochemical components are evaluated as a result of the experiments, it can be said that these fruits and seeds can be used as a good food source and may lead to future pharmacological studies.
Figure 1. A: DPPH scavenging activity (%) in samples with methanol extract, B: NO scavenging activity (%) in samples with methanol extract (Data represent the means ± SE, *,*:p<0.05, **:p<0.01 , n: 3)
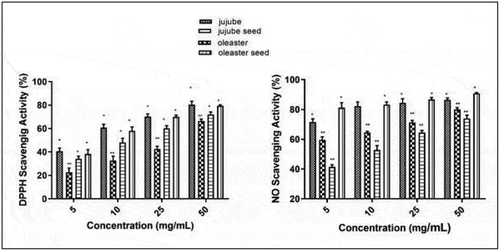
Figure 2. LC-MS/MS total ion chromatograms of phenolic compounds (A: jujube, B: oleaster, C: jujube seed, D: oleaster seed)
Declaration of competing interests
The authors declare they have no conflict of interest.
Acknowledgments
The experiments in the study were carried out in Manisa Celal Bayar University, Application Science and Research Center (ASRC), Turkey.
References
- Acosta-Montoya, Ó., F. Vaillant, S. Cozzano, C. Mertz, A.M. Pérez, and M.V. Castro. 2010. Phenolic content and antioxidant capacity of tropical highland blackberry (Rubus adenotrichus Schltdl.). During Three Edible Maturity Stages. Food Chem. 119:1497–1501. doi: 10.1016/j.foodchem.2009.09.032.
- Bonofiglio, D., C. Giordano, F. De Amicis, M. Lanzino, and S. Ando. 2016. Natural Products as Promising Antitumoral Agents in Breast Cancer: Mechanisms of Action and Molecular Targets.. Mini-Reviews Med. Chem. 16(8):596–604. doi: 10.2174/1389557515666150709110959.
- Boron, B., J. Hupert, D.H. Barch, C.C. Fox, H. Friedman, T.J. Layden, and S. Mobarhan. 1988. Effect of zinc deficiency on hepatic enzymes regulating vitamin A status. J. Nutr. 118(8):995–1001. doi: 10.1093/jn/118.8.995.
- Bradford, M.M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72(1–2):248–254. doi: 10.1016/0003-2697(76)90527-3.
- Chaiklahan, R., N. Chirasuwan, P. Triratana, S. Tia, and B. Bunnag. 2014. Effect of extraction temperature on the diffusion coefficient of polysaccharides from Spirulina and the optimal separation method. Biotechnol. Bioprocess Eng. 19(2):369–377. doi: 10.1007/s12257-013-0733-2.
- Chausmer, A.B. 1998. Zinc, insulin and diabetes. J. Am. Coll. Nutr. 17(2):109–115. doi: 10.1080/07315724.1998.10718735.
- Dahiru, D., and O. Obidoa. 2008. Evaluation of the antioxidant effects of Ziziphus mauritiana lam. leaf extracts against chronic ethanol-induced hepatotoxicity in rat liver. African J. Tradit. Complement. Altern. Med. 5:39–45. doi: 10.4314/ajtcam.v5i1.31254.
- Faramarz, S., G. Dehghan, and A. Jahanban Esfahlan. 2017. Antioxidants in different parts of oleaster as a function of genotype. BioImpacts 5(2):79–85. doi: 10.15171/bi.2015.09.
- Farzaei, M.H., R. Bahramsoltani, Z. Abbasabadi, and R. Rahimi. 2015. A comprehensive review on phytochemical and pharmacological aspects of Elaeagnus angustifolia L.. J. Pharm. Pharmacol. 67(11):1467–1480. doi: 10.1111/jphp.12442.
- Fonia, A., I.R. White, and J.M.L. White. 2009. Allergic contact dermatitis to Elaeagnus plant (Oleaster). Contact Derm. 60(3):178–179. doi: 10.1111/j.1600-0536.2008.01485.x.
- Fu, L., B.-T. Xu, X.-R. Xu, R.-Y. Gan, Y. Zhang, E.-Q. Xia, and H.-B. Li. 2011. Antioxidant capacities and total phenolic contents of 62 fruits. Food Chemistry 129(2):345–350. doi: 10.1016/j.foodchem.2011.04.079.
- Gören, A.C., S. Çikrikçi, M. Çergel, and G. Bilsel. 2009. Rapid quantitation of curcumin in turmeric via NMR and LC–tandem mass spectrometry. Food Chemistry 113(4):1239–1242. doi: 10.1016/j.foodchem.2008.08.014.
- Hamidpour, R., S. Hamidpour, M. Hamidpour, M. Shahlari, M. Sohraby, N. Shahlari, and R. Hamidpour. 2017. Russian olive (Elaeagnus angustifolia L.): From a variety of traditional medicinal applications to its novel roles as active antioxidant, anti-inflammatory, anti-mutagenic and analgesic agent. Journal of Traditional and Complementary Medicine 7(1):24–29. doi: 10.1016/j.jtcme.2015.09.004.
- Hudina, M., M. Liu, R. Veberic, F. Stampar, and M. Colaric. 2008. Phenolic compounds in the fruit of different varieties of Chinese jujube (Ziziphus jujuba Mill.). J. Hortic. Sci. Biotechnol. 83(3):305–308. doi: 10.1080/14620316.2008.11512382.
- Hwang, C.-S., G.-E. Rhie, J.-H. Oh, W.-K. Huh, H.-S. Yim, and S.-O. Kang. 2002. Copper- and zinc-containing superoxide dismutase (Cu/ZnSOD) is required for the protection of Candida albicans against oxidative stresses and the expression of its full virulence. Microbiology 148(11):3705–3713. doi: 10.1099/00221287-148-11-3705.
- Incilay, G. 2014. Volatile Composition, Antimicrobial and Antioxidant Properties of Different Parts from Elaeagnus angustifolia L.. Journal of Essential Oil Bearing Plants 17(6):1187–1202. doi: 10.1080/0972060X.2014.929044.
- Khan, S.U., A. Khan, A.-U.-H.A. Shah, S.M. Shah, S. Hussain, M. Ayaz, and S. Ayaz. 2016. Heavy metals content, phytochemical composition, antimicrobial and insecticidal evaluation of Elaeagnus angustifolia. Toxicology and Industrial Health 32(1):154–161. doi: 10.1177/0748233713498459.
- Li, J., Y. Liu, L. Fan, L. Ai, and L. Shan. 2011. Antioxidant activities of polysaccharides from the fruiting bodies of Zizyphus Jujuba cv. Jinsixiaozao. Carbohydr. Polym. doi: 10.1016/j.carbpol.2010.11.051.
- Li, J.-W., L.-P. Fan, S.-D. Ding, and X.-L. Ding. 2007a. Nutritional composition of five cultivars of chinese jujube. Food Chemistry 103(2):454–460. doi: 10.1016/j.foodchem.2006.08.016.
- Li, J.-W., L.-P. Fan, S.-D. Ding, and X.-L. Ding. 2007b. Nutritional composition of five cultivars of chinese jujube. Food Chemistry 103(2):454–460. doi: 10.1016/j.foodchem.2006.08.016.
- Li, J.-W., S.-D. Ding, and X.-L. Ding. 2005. Comparison of antioxidant capacities of extracts from five cultivars of Chinese jujube. Process Biochemistry 40(11):3607–3613. doi: 10.1016/j.procbio.2005.03.005.
- Mahajan, R.T., and M.Z. Chopda.2009. Phyto-pharmacology of Ziziphus jujuba mill - A plant review. Pharmacogn. Rev. 3 (6): 320-329. .
- Marcocci, L., J.J. Maguire, M.T. Droylefaix, and L. Packer. 1994. The nitric oxide-scavenging properties of Ginkgo biloba extract EGb 761. Biochem. Biophys. Res. Commun. 16(2):748–755. doi: 10.2174/1389557515666150709110959.
- Okmen, G., and O. Turkcan. 2014. A study on antimicrobial, antioxidant and antimutagenic activities of elaeagnus angustifolia l. LEAVES. Complement Altern. Med. 11:116–120. doi: 10.4314/ajtcam.v11i1.17.
- Pareek, O.P. 2002. Medicinal and Aromatic Plants of the World - Africa - Google Kitaplar, volume 3. ed. Springer Nature, The Netherlands.
- Re, R., N. Pellegrini, A. Proteggente, A. Pannala, M. Yang, and C. Rice-Evans. 1999. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine 26(9–10):1231–1237. doi: 10.1016/S0891-5849(98)00315-3.
- Saboonchian, F., R. Jamei, and S. Hosseini Sarghein. 2014a. Phenolic and flavonoid content of Elaeagnus angustifolia L. (leaf and flower). Avicenna J. Phytomedicine. 4:231–238. doi: 10.22038/ajp.2014.1975.
- Saboonchian, F., R. Jamei, and S. Hosseini Sarghein. 2014b. Phenolic and flavonoid content of Elaeagnus angustifolia L (leaf and flower). Avicenna J. Phytomedicine. 4:231–238. doi: 10.22038/ajp.2014.1975.
- Sahan, Y., A. Dundar, and E. Aydin., 2013, Characteristics of cookies supplemented with oleaster (Elaeagnus angustifolia L.) Flour. I physicochemical, sensorial and textural properties. academia.edu
- Sahan, Y., D. Gocmen, A. Cansev, G. Celik, E. Aydin, A.N. Dundar, D. Dulger, H.B. Kaplan, A. Kilci, and S. Gucer. 2015. Chemical and techno-functional properties of flours from peeled and unpeeled oleaster (Elaeagnus angustifolia L.). J. Appl. Bot. Food Qual. 88:34–41. doi: 10.5073/JABFQ.2015.088.007.
- Taheri, J., F. Anbari, and Z. Maleki, 2010. Efficacy of Elaeagnus angustifolia topical gel in the treatment of symptomatic oral lichen planus. ncbi.nlm.nih.gov
- Torbati, M., S. Asnaashari, and F.H. Afshar. 2016. Essential oil from flowers and leaves of Elaeagnus angustifolia (Elaeagnaceae): Composition, radical scavenging and general toxicity activities. Adv. Pharm. Bull. 6(2):163–169. doi: 10.15171/apb.2016.023.
- Uysal, S., Z. Aumeeruddy-Elalfi, G. Zengin, A. Aktumsek, P.Z. Mašković, J.M. Vujić, and M.F. Mahomoodally. 2018. In vitro antioxidant, cytotoxicity and chemical profile of different extracts from Acanthus hirsutus Boiss used in Anatolian folk medicine. Eur. J. Integr. Med. 17:135–140. doi: 10.1016/j.eujim.2017.12.009.
- Wang, B., Q. Huang, C. Venkitasamy, H. Chai, H. Gao, N. Cheng, W. Cao, X. Lv, and Z. Pan. 2016a. Changes in phenolic compounds and their antioxidant capacities in jujube (Ziziphus jujuba Miller) during three edible maturity stages. LWT - Food Sci. Technol. doi: 10.1016/j.lwt.2015.10.005.
- Wang, B., Q. Huang, C. Venkitasamy, H. Chai, H. Gao, N. Cheng, W. Cao, X. Lv, and Z. Pan. 2016b. Changes in phenolic compounds and their antioxidant capacities in jujube (Ziziphus jujuba Miller) during three edible maturity stages. LWT - Food Sci. Technol. 66:56–62. doi: 10.1016/j.lwt.2015.10.005.
- Yang, J.-H., H.-C. Lin, and J.-L. Mau. 2002. Antioxidant properties of several commercial mushrooms. Food Chemistry 77(2):229–235. doi: 10.1016/S0308-8146(01)00342-9.
- Yıldırım, I., Z. Gökçe, and Ö. Yılmaz.2015. THE INVESTIGATION OF BIOCHEMICAL CONTENT OF Elaeagnus angustifolia. J. Turkish Chem. Soci. JOTCSA. 2(1):34-41
- Zemel, B. 2009. Dietary Pattern and Hypertension: The DASH Study. Nutr. Rev. 55(8):303–305. doi: 10.1111/j.1753-4887.1997.tb05489.x.
- Zhang, G., L. He, and M. Hu. 2011. Optimized ultrasonic-assisted extraction of flavonoids from Prunella vulgaris L. and evaluation of antioxidant activities in vitro. Innovative Food Science & Emerging Technologies 12(1):18–25. doi: 10.1016/j.ifset.2010.12.003.
- Zhao, J., S.P. Li, F.Q. Yang, P. Li, and Y.T. Wang. 2006. Simultaneous determination of saponins and fatty acids in Ziziphus jujuba (Suanzaoren) by high performance liquid chromatography-evaporative light scattering detection and pressurized liquid extraction. Journal of Chromatography A 1108(2):188–194. doi: 10.1016/j.chroma.2005.12.104.