?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The present study was conducted to determine the composition of pomegranate seed oils according to fruit cracking and maturity stage. Quality attributes and fatty acid profile of seed oils of intact and cracked fruits from two pomegranate varieties (Gabsi and Chelfi) were studied at two different harvest dates (1 September and mid October). The variety, harvest date, and fruit cracking were proved to affect significantly seed oil quality. ‘Gabsi’ variety exhibited higher seed oil content and total phenolic content compared to those in ‘Chelfi’ fruits. However, seed oils from ‘Chelfi’ fruits were characterized by the highest amounts of palmitic, palmitoleic, linoleic, and stearic acids. Seeds from cracked fruits exhibited higher oil content than those in intact fruits. Fruit cracking increased the peroxide value, phenol concentration, and the contents of linoleic, linolenic, catalpic, and erucic acids while decreased the β-carotene content. Changes occur in analytical parameters and fatty acid profile as the ripening process progressed. A significant decrease in free acidity content was noted while the peroxide value, the palmitic and linoleic acid contents increased with the delay of harvest. Therefore, the variety, fruit cracking, and harvest date contribute largely to the characteristics of pomegranate seed oils.
Introduction
Over the past few years, scientific investigations have laid a credible basis for the traditional remedy applications of different anatomical parts of the plant. There has been a virtual explosion of interest in the pomegranate as a medicinal and nutritional product because of its multifunctionality and its great benefit in the human diet and health (Jaiswal et al., Citation2010). Pomegranate (Punica granatum L.) is an ancient fruit that is mainly consumed as fresh fruit, juice, jams, and jellies (Martinez et al., Citation2006; Zaouay et al., Citation2014). The arils (pulp and seeds) represent 40% to 70% of the total fruit weight (Zaouay et al., Citation2012). Pomegranate seed, the by-product of pomegranate juice processing that may represent up to about 5% to 15% of the edible part, contains a range of nutraceutical components such fibers, proteins, and lipids (Elfalleh et al., Citation2012; Liu et al., Citation2009). Otherwise, pomegranate fruit is subject to cracking that is one of the serious physiological disorders in many fruit species. Cultivar sensitivity, day and night temperatures, soil moisture variation, relative humidity, irrigation, peel pliability as well as calcium and boron deficiency are some of the major factors that contribute to pomegranate fruit cracking (Khadivi-Khub, Citation2014; Singh et al., Citation2014). It seems to be a problem that lessens the marketing of pomegranate fruit to a great extent and consequently it generates an important quantity of wastes including seeds. These by-products also obtained from food-processing industries have considerable phytochemical interest as it is a source of bioactive compounds with nutritional and therapeutic values. Thus, the application of appropriate technologies for the commercial processing of seed wastes into valuable end products is essential to improve the sustainability of the pomegranate value-chain and hence the income-generating capacity of the pomegranate industry. Pomegranate seed oil represents between 12% and 20% of the seed total weight and is considered as a good source of vitamin E, sterols, and polyunsaturated fatty acids, especially linoleic and punicic acid have a broad spectrum of biological activities (Caligiani et al., Citation2010; Fernandes et al., Citation2015b). As a source of nutrients and antioxidants, pomegranate seed oils should be exploited for their potent health benefits. Nevertheless, the composition of seed oil in most oil crops is influenced by several factors such as variety, growing environment, seed processing, and extraction method (Abbasi et al., Citation2008; Curt et al., Citation2002; Liu et al., Citation2009; Were et al., Citation2006). Several studies have focused on technological issues, such as optimization of oil extraction, phytochemical composition, and health beneficial effects of pomegranate seed oil (Abbasi et al., Citation2008; Amri et al., Citation2017; Boroushaki et al., Citation2016; Pereira de Melo et al., Citation2016). Few works on the effect of pomegranate cultivars on seed oils composition have been published (Fernandes et al., Citation2015a; Kyralan et al., Citation2009). However, to the best of our knowledge, no prior study has evaluated the effects of preharvest factors (environmental conditions, cultural practices, maturity stage) on the components and quality of pomegranate seed oils. Thus, the main objective of the present work was to determine the impact of fruit cracking and maturity stage on oil content, quality indices, and fatty acid composition changes in the seed oils of two Tunisian pomegranate varieties.
Material and Methods
Plant Material and Sample Preparation
Two Tunisian pomegranate varieties (Gabsi and Chelfi) were selected from an ex-situ collection in High Agronomic Institute of Chott-Mariem (Longitude 10°38′ E, Latitude 35°55′ N, altitude 15 m), Tunisia. Intact and cracked fruits were collected at two harvest dates (1 September and mid-October). Arils were hand removed from fruits and seeds were separated from the pulp. Then, seeds were dried at room temperature, crushed, and sieved to obtain fine powder.
Seed Oils Extraction
Seed oils were extracted using the method described by Kornsteiner et al. (Citation2006). For each variety and each fruit sample (Intact and cracked fruits), 10 g of milled dry seeds were mixed with 300 ml of n-hexane and agitated on a magnetic stirrer for 3 hours. The solvent was removed using a rotary evaporator at 45°C. The extracted oil was dried by Na2SO4 anhydrous, filtered, and weighed. Oil content was expressed as g kg−1 of dry matter. Oil samples were stored at – 20°C until used experiments.
Determination of Quality Indices
Free acidity (FA), given as percentage of oleic acid, was determined by titration of an oil solution dissolved in ethanol (95%) (AFNOR, Citation1984).
Peroxide Value (PV) was determined by incubating a mixture of oil and chloroform/acetic acid with a solution of potassium iodide in the dark. Then, 25 ml of water and 500 μl of Amidon 1% were added and the liberated iodine was titrated with sodium thiosulfate Na2S2O3 (0.01 N). Result was expressed in milliequivalents of active oxygen per kilogram of oil (meq O2 kg-1) (AFNOR NF T60-220, 1984).
Determination of Total Phenolic Content
The total phenolic contents of the extracts were measured following the method of Abbasi et al. (Citation2008). The procedure consisted of extracting 50 mg of oil with 5.0 ml solvent (methanol/water, 50:50, v/v). Of this solution, 2 ml was then mixed with 0.5 ml Folin reagent. After 1 min of incubation, 1 ml of sodium carbonate solution (35%) was added and then placed in a 100 ml volumetric flask and diluted with distilled water to the mark. The mixture was incubated for 30 min and the absorbance was measured at 760 nm using a UV-Visible spectrophotometer (T60 PG Instruments). The total phenolic content was expressed as mg gallic acid kg−1 of oil.
Determination of β-carotene Content
Beta-carotene content was determined as described by Wolf (Citation1968), using an Ultraviolet-Visible spectrophotometer (T60 PG Instruments). Briefly, 1 g of seed oil was dissolved with petroleum ether to 20 ml. The absorbance was measured at 420 and 480 nm against petroleum ether. The β-carotene (mg kg−1) was calculated as follows:
(Where: 2.650 = extinction coefficient; A (λ max) = maximum absorbance).
Fatty Acid Composition
The fatty acid methyl esters (FAME) composition was determined by converting the oil to fatty acid methyl esters following the procedure described by ISO 5508 (Citation1990). Three aliquots of lipid extract were prepared by vigorous shaking of a solution of each pomegranate seed oil sample in n-hexane (0.2 g in 2 ml) with 2 N methanolic potassium hydroxide solution. The top layer (1 µl) was injected onto a Hewlett–Packard GC (Agilent 6890, Palo Alto, CA, USA) equipped with a flame ionization detector (FID) and a capillary column (HP-Innowax polyethylene glycol, 0.25 mm internal diameter, 30 m length, and 0.25 µm film thickness) to obtain individual peaks of fatty acid methyl esters. The detector temperature was 275°C and the column temperature was 150°C held for 1 min and increased at the rate of 15°C/min to 200°C and the rate of 2°C/min to 225°C and held for 4 min. Nitrogen was used as carrier gas at inlet pressure of 1.6 L/min. The fatty acid methyl esters peaks were identified comparing their retention times with individual standard FAME. The relative percentage of the fatty acid was calculated on the basis of the peak area of a fatty acid species to the total peak area of all the fatty acids in the oil sample. All samples were run in duplicate.
Statistical Analysis
The results are reported as the mean values (±standard deviation). Data were statistically analyzed by using the General Linear Model procedure to determine significant differences among the data. In addition, to determine whether there is any correlation between seed oil attributes data were analyzed using Pearson correlation. Statistical analysis was performed using the SPSS version 17.0.
Results and Discussion
Seed Oil Content
The oil contents, expressed in g kg−1, are reported in . Pomegranate seed oil content ranged between 172.01 and 205.89 g kg−1 at the first harvest date (beginning of September) and 165.37–193 g kg−1 at the second date (mid-October) (). These values were higher than those reported by Soetjipto et al. (Citation2010) and Peng (Citation2019). Tunisian pomegranate seeds are proved rich in oil that could be used in food industry, cosmetics, and pharmaceutical applications. Oil content was significantly affected by variety, whereas there are no significant differences according to harvest date and fruit type (). The highest oil content was obtained for the Gabsi variety at two harvesting dates and whatever the fruit type (intact or cracked fruits). The variety effect is in agreement with the results found by Parashar et al. (Citation2010), Fernandes et al. (Citation2015a), Fernandes et al. (Citation2015b) and Peng (Citation2019).
Table 1. Effects of variety, harvesting date, and fruit cracking on pomegranate seed oil characteristics
Table 2. Effects of fruit cracking and harvesting date on quality attributes of pomegranate seed oils from two varieties (Gabsi and Chelfi)
Seed Oil Quality Characteristics
As shown in , the analytical parameters were affected by variety, fruit type, and harvesting date except peroxide value which is not influenced by variety.
Free acidity is an important quality index. All studied samples showed a free fatty acids content in the range between 0.92% and 1.40%. Nevertheless, the seed oil extracted from cracked fruits of Gabsi variety harvested at mid-October was characterized by a high free acidity value (3.77%). In all cases, acidity values were in the limit (4.0%) recommended by the Codex Alimentarius Commission (Citation1999). The intact fruits from ‘Gabsi’ variety showed higher values than those from ‘Chelfi’ variety. Fruit cracking was found to be significantly affecting the acidity content. A decrease in acidity values was observed in cracked fruits except in ‘Gabsi’ fruits harvested in mid-October. Also, the acidity content varied significantly according to maturity stage. Values seem to be slightly higher during the second harvest date (mid-october). This increase in acidity during maturation can be explained by the increase in lipolytic activity (Anastasopoulos et al., Citation2011).
Peroxide value (PV) was used as an indicator of oil primary oxidation (El Qarnifa et al., Citation2019). The PV values of all oils were below the limit of 20 meq of oxygen/kg of oil (), which is accepted as the limit for extra-quality virgin oil (International Olive Oil Council (IOOC), Citation2003). These oils can be stored for a long time without deterioration, since oils become rancid when the peroxide value ranges from 20 to 40 meq (Nehdi, Citation2011). There are no significant differences among varieties. However, fruit type and harvest date significantly affected peroxide value (). Cracked fruits for the two varieties were characterized by the highest PV values at different harvesting dates. The relatively high peroxide values may be due to the increase in the degree of unsaturation of fatty acids or even to the position of instaurations in the fatty acid chain (Gharby et al., Citation2011). Enzymatic lipolysis and spontaneous lipid hydrolysis can also lead to a hydrolytic rancidity and an increase in the acidity of altered products (Rahmani, Citation2007). Also, the peroxide value increased with the delay of harvest date. Other studies reported an increase in peroxide value of olive oil along ripening (El Riachy et al., Citation2018). Cracked fruits harvested at the second date have therefore shown a high peroxide value and lower acidity content. However, when the acidity value is lower, the oil is more suitable for consumption and long-term storage (Özyurt, Citation2019).
Phenols are recognized as antioxidant compounds and their presence in oils has been related to their general properties, improving stability, nutritional value, and sensorial properties (Baccouri et al., Citation2007). The studied oils exhibited high total phenolic contents which ranged between 33.52 and 71.02 mg kg−1 of dry matter (). The phenol content shows significant differences according to the variety and the fruit type (). Cracked fruits from the two varieties, obtained at different harvest dates, showed higher phenol levels than those in seeds from intact fruits. Therefore, the seed oils from cracked fruits might serve as a rich source of phenolic compounds and occur as a stable oil under oxidative conditions. As reported by Abbasi et al. (Citation2008), the amount of total phenols shows a great variability depending on cultivar. Also, the effect of the maturity stage on phenol content was observed. For all varieties, there was a decrease in phenol content during maturation in both fruit types. These findings are consistent with those reported by El Qarnifa et al. (Citation2019) and Trentacoste et al. (Citation2019) who found that olive oil phenols decrease during ripening. The variability of phenolic contents observed in seed oils may be due to climatic conditions, in particular precipitations and temperature during growing and ripening of pomegranate fruits (Ben Temime et al., Citation2006).
The carotenoid pigments are widely studied organic compounds that are responsible for oil color. Among carotenoids, β-carotene is one of the most important with the highest vitamin A activity (Zeb and Murkovic, Citation2011). It serves as biological antioxidant and it helps in maintaining human health. This antioxidant inhibits lipid oxidation mainly in cosmetic products by stabilizing hydroperoxides and other free radicals. In this study, β-carotene content depends on variety, fruit type, and harvest date (). It ranged between 2.96 mg kg−1 for ‘Gabsi’ cracked fruits harvested at mid-october and 4.13 mg kg−1 for Gabsi intact fruits obtained at beginning of September. Fruit cracking significantly affected the β-carotene content. A significant decrease was observed in seed oils extracted from cracked fruits at the two harvest dates.
Fatty Acid Composition
Fatty acid composition is the most important quality characteristic of oilseed crops. Suitability of a vegetable oil for nutritional, industrial, or pharmaceutical applications is determined by its fatty acid composition. In the current study, 14 fatty acids were detected in pomegranate seed oils (). The saturated fatty acids (SFA) were within 3.68% and 5.50% of total fatty acids. Palmitic acid was the predominant SFA (1.32–3.07%), followed by stearic acid (1.37–1.60%), arachidic acid (0.38–0.52%), and behenic acid (0.14–0.64%). These results are in agreement with previous reports (Amri et al., Citation2017; Dadashi et al., Citation2013). The unsaturated acids predominate, accounting for 90.49% to 95.92% of total fatty acids. Major mono-unsaturated fatty acid (MUFA) was oleic acid ranging from 2.95% to 3.84%. To a lesser extent, eicosenoic (C20:1) and erucic (C22:1) acids were found in all seed oil samples and were within 0.41–0.48% and 0.19–0.82%, respectively. The palmitoleic acid (C16:1) as a MUFA was also detected in seed oils (0.03–0.05%). Polyunsaturated fatty acids (PUFAs) fraction accounted for 86.5% to 91.54% of total fatty acids. PUFAs were characterized by the presence of linoleic acid (C 18:2) and α-linolenic acid (C 18:3) with the percentages of 4.04–4.52% and 0.02–0.11%, respectively. Three isomers of 18:3 acid were registered; the major part of them and of all fatty acids is the punicic acid (18:3: 9-cis, 11-trans, 13-cis) which ranged between 72.31% and 79.57% (). These results are in accordance with those reported by Hernández et al. (Citation2000), Fernandes et al. (Citation2015b). However, lower values of punicic acid were observed in Tunisian pomegranate cultivars, previously studied by Elfalleh et al. (Citation2011) (18.22–55.45% of total fatty acids) and Amri et al. (Citation2017) (5.12% of total fatty acids). The remaining separated peaks corresponded probably to α-eleostearic (C18:3: 9-cis, 11-trans, 13-trans) and catalpic acids (C18:3: 9-trans, 11-trans, 13-cis) (Elfalleh et al., Citation2011). In terms of percentage, the α-eleostearic values varied between 7.03% and 10.73% of total fatty acids. The catalpic acid was detected in all seed oil samples except from intact Chelfi fruits harvested in September. It ranged from 1.80% to 3.02%. The saturated/unsaturated acid ratio was generally very low and it did not exceed 0.06% (). This ratio was the same or rather lower as previously reported (Elfalleh et al., Citation2011; Fadavi et al., Citation2006; Melgarejo and Artés, Citation2000; Parashar et al., Citation2010). Fatty acid types and contents depend on the variety, fruit type, and harvest date. In fact, variety exerted significant effect on palmitic (C16:0), palmitoleic, stearic (C18:0), linoleic (C18:2) and behenic (C22:0) acids, while the fruit type effect was mainly observed in palmitic (C16:0), linoleic (C18:2), linolenic (C18:3), catalpic and erucic (C22:1) acids (). The seed oils from Chelfi fruits exhibited higher levels of palmitic, palmitoleic, stearic and linoleic acids than those from Gabsi fruits (). As regarding fruit type effect, fruit cracking was proved to significantly increase the palmitic, linoleic, linolenic, catalpic and erucic acid contents for the two varieties and at different harvest dates.
Table 3. Effects of fruit cracking and harvesting date on fatty acid composition (% of total) of pomegranate seed oils from two varieties (Gabsi and Chelfi)
Harvest date effect was only observed on palmitic and linoleic acid contents (). The palmitic and linoleic acid levels increased significantly with the delay of harvest whatever the variety and the fruit type. However, oleic acid content decreased in intact fruits and increased in cracked fruits for the two varieties. These results are consistent with those observed in two Spanish olive varieties grown in Uruguay (Feippe et al., Citation2010). Palmitoleic and linoleic acid significantly increased across different maturity stages, whereas oleic acid and linolenic acid levels decreased with advancing maturity. During ripening, linoleic acid increases due to oleate desaturase that change oleic acid to linoleic acid in the olive (Gutierrez et al., Citation1999). A diversity of fatty acid composition in these seed oils is an important characteristic which leads to several uses in both human nutrition and non-food applications. Also, the acidic composition of the seed oil helps to promote the cultivation of certain varieties and the exploitation of damaged fruits. In cosmetology, a high content of punicic acid would be of great importance (Zielińska and Nowak, Citation2014). The particular presence of saturated fatty acids (palmitic and stearic acids) in cracked fruits from ‘Chelfi’ variety allows to find applications of pomegranate seed oil in the field of biolubricants and biosurfactants as well as in cosmetic and pharmaceutical products (Melo et al., Citation2014).
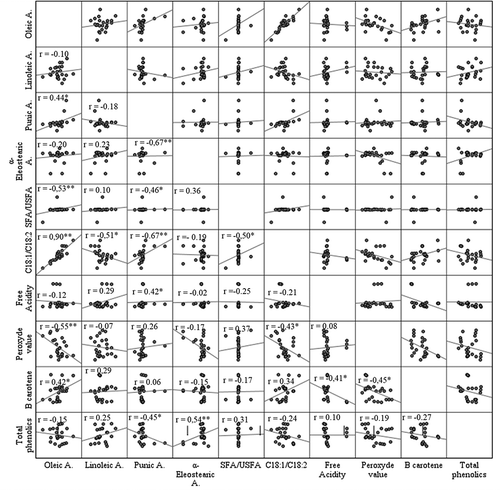
Correlation Analysis
Correlations between studied parameters are presented in . β-carotene contents in the studied samples were correlated negatively with their peroxide values, which indicate the role of β-carotene against oil oxidation (Ben Tekaya and Hassouna, Citation2006). Besides, β-carotene was found to be correlated negatively with free acidity, suggesting that low acidity in seed oil is a sign of high β-carotene amounts. A negative correlation coefficient was also observed between oleic acid and peroxide value. Gharby et al. (Citation2011) noted that the oil oxidation increases with the degree of fatty acid unsaturation. Hence, oleic acid seems to have the least effect on pomegranate seed oil alteration. Significant and negative correlation was found between punicic acid and total phenolic content. However, α-eleostearic acid may reflect the richness of pomegranate seed oil in phenolic compounds. Besides, significant negative correlations revealed that during fatty acid biosynthesis, the accumulation of punicic acid may occur at the expense of α-eleostearic and linoleic acids.
Conclusion
Pomegranate seeds are considered as a good source of oils of nutritional and economical importance. Fruit cracking of pomegranate was shown to significantly affect seed oil composition. Cracked fruits are an interesting source of seed oil with a higher peroxide value and total phenolic content as well as higher amounts of palmitic, linoleic, linolenic, catalpic and erucic acids compared to those in intact fruits. Also, a significant variation was observed in seed oil quality attributes according to variety and maturity stage. The free acidity content decreased while peroxide value increased with the delay of harvest. Progress in maturity was also accompanied by changes in the fatty acid profile which palmitic and linoleic acid contents increased, while the oleic acid (for intact fruits) decreased during ripening. The present study increases the understanding of the quality of seed oils from cracked pomegranate fruits that will have an important pharmaceutical and cosmetic potential. Also, this work opens up new avenues of research to better valorize the by-products of cracked fruits and diversify their uses, which allow pomegranate culture to remain competitive with other fruit species.
References
- Abbasi, H., K. Rezaei, Z. Emamdjomeh, and S.M. Ebrahimzadeh Mousavi. 2008. Effect of various extraction conditions on the phenolic contents of pomegranate seed oil. Eur. J. Lipid Sci. Tech. 110(5):435–440. doi: 10.1002/ejlt.200700199.
- AFNOR. 1984. Recueil des normes françaises des corps gras et graines oléagineuses et produits dérivés. Association Française de Normalisation, AFNOR, ed. Paris.
- Amri, Z., H. Lazreg-Aref, M. Mekni, S. El-Gharbi, O. Dabbaghi, B. Mechri, and M. Hammami. 2017. Oil characterization and lipids class composition of pomegranate seeds. BioMed Research International. 2017:1–8. doi: 10.1155/2017/2037341.
- Anastasopoulos, E., N. Kalogeropoulos, A.C. Kaliora, A. Kountouri, and N.K. Andrikopoulos. 2011. The influence of ripening and crop year on quality indices, polyphenols, terpenic acids, squalene, fatty acid profile, and sterols in virgin olive oil (Koroneiki cv.) produced by organic versus non‐organic cultivation method. Int. J. Food Sci. Tech. 46(1):170–178. doi: 10.1111/j.1365-2621.2010.02485.x.
- Baccouri, B., S. Ben Temime, W. Taamalli, M. M’sallem, and M. Zarrouk. 2007. Analytical characteristics of virgin olive oils from two new varieties obtained by controlled crossing on Meski variety. J Food Lipids. 14:19–34. doi: 10.1111/j.1745-4522.2006.00067.x.
- Ben Tekaya, I., and M. Hassouna. 2006. Effets des chlorophylles, du béta carotène, de l’alpha tocophérol, de leurs interactions sur la stabilité oxydative de l’huile d’olive tunisienne. OCL. 14(1):60–67. doi: 10.1051/ocl.2007.0097.
- Ben Temime, S., W. Taamalli, B. Baccouri, L. Abaza, D. Daoud, and M. Zarrouk. 2006. Changes in olive oil quality of Chetoui variety according to origin of plantation. J Food Lipids. 13:88–99. doi: 10.1111/j.1745-4522.2006.00036.x.
- Boroushaki, M.T., H. Mollazadeh, and R. Afshari. 2016. Pomegranate seed oil: A comprehensive review on its therapeutic effects. Int. J. Pharm Sci. Res. 7(2):430–442. doi: 10.13040/IJPSR.0975-8232.
- Caligiani, A., F. Bonzanini, G. Palla, M. Cirlini, and R. Bruni. 2010. Characterization of a potential nutraceutical ingredient: Pomegranate (Punica granatum L.) seed oil unsaponifiable fraction. Plant Foods Hum Nutr. 65(3):277–283. doi: 10.1007/s11130-010-0173-5.
- Codex Alimentarius Commission. 1999. Report of the sixteenth session of the Codex Committee on fats and oils. London, UK. 8–12.
- Curt, M.D., G. Sanchez, and J. Fernandez. 2002. The potential of Cynara cardunculus L. for seed oil production in a perennial cultivation system. Biomass Bioenergy. 23:33–46. doi: 10.1016/S0961-9534(02)00030-2.
- Dadashi, S., M. Mousazadeh, Z. Emam-Djomeh, and S.M. Mousavi. 2013. Pomegranate (Punica granatum L.) seed: A comparative study on biochemical composition and oil physicochemical characteristics. Int. J. Adv. Biol. Biomed. Res. 1(4):351–363. doi: 10.26655/IJABBR.2016.12.3.
- El Qarnifa, S., A. El Antari, and A. Hafidi. 2019. Effect of maturity and environmental conditions on chemical composition of olive oils of introduced cultivars in Morocco. J. Food Qual. 14(pages). doi: 10.1155/2019/1854539.
- El Riachy, M., C. Bou-Mitri, A. Youssef, R. Andary, and W. Skaff. 2018. Chemical and sensorial characteristics of olive oil produced from the Lebanese olive variety ‘Baladi’. Sustainability. 10:4630. doi: 10.3390/su10124630.
- Elfalleh, W., H. Hannachi, A. Guetat, N. Tlili, F. Guasmi, A. Ferchichi, and M. Ying. 2012. Storage protein and amino acid contents of Tunisian and Chinese pomegranate (Punica granatum L.) cultivars. Genet Resour. Crop Ev J. 59(6):999–1014. doi: 10.1007/s10722-011-9739-9.
- Elfalleh, W., M. Ying, N. Nasri, H. Sheng-Hua, F. Guasmi, and A. Ferchichi. 2011. Fatty acids from Tunisian and Chinese pomegranate (Punica granatum L.) seeds. Int J Food Sci Nutr 62(3):200–206. doi: 10.3109/09637486.2010.526932.
- Fadavi, A., M. Barzegar, and M.H. Azizi. 2006. Determination of fatty acids and total lipid content in oilseed of 25 pomegranates varieties grown in Iran. J Food Compost Anal. 19:676–680. doi: 10.1016/j.jfca.2004.09.002.
- Feippe, A., F. Ibáñez, and G.P. Altier. 2010. Fruit ripening stage effect on the fatty acid profile of ‘arbequina’ and ‘picual’ olives in Uruguay. Proc. 6th International Postharvest Symposium Eds.: M. Erkan and U. Aksoy Acta Hort. 877, ISHS.
- Fernandes, L., J.A. Pereira, I. Lopez-Cortes, D.M. Salazar, E. Ramalhosa, and S. Casal. 2015a. Lipid composition of seed oils of different pomegranate (Punica granatum L.) cultivars from Spain. Int J Food Stud. 4:95–103. doi: 10.7455/ijfs.v4i1.266.
- Fernandes, L., J.A. Pereira, I. Lopéz-Cortés, D.M. Salazar, E. Ramalhosa, and S. Casal. 2015b. Fatty acid, vitamin E and sterols composition of seed oils from nine different pomegranate (Punica granatum L.) cultivars grown in Spain. J. Food Compost. Anal. 39:13–22. doi: 10.1016/j.jfca.2014.11.006.
- Gharby, S., H. Harhar, B. Kartah, H. El Monfalouti, N. Maata, D. Guillaume, T. Ben Haddad, and Z. Charrouf. 2011. Influence de l’origine du fruit (terroir, caprin) et de la méthode d’extraction sur la composition chimique, les caractéristiques organoleptiques et la stabilité de l’huile d΄argane. In: INRA, ed.. Actes du Premier Congrès International de l’Arganier. Agadir, Morroco; 203–214.
- Gutierrez, F., B. Jimenez, A. Ruíz, and M.A. Albi. 1999. Effect of olive ripeness on the oxidative stability of virgin olive oil extracted from the varieties picual and hojiblanca and on the different components involved. J.Agri Food Chem. 44:121–127. doi: 10.1021/jf980684i.
- Hernández, F., P. Melgarejo, J.M. Olías, and F. Artés. 2000. Fatty acid composition and total lipid content of seed oil from three commercial pomegranate cultivars, p. 205–209. In: P. Melgarejo, J.J. Martínez-Nicolás, and J. Martínez-Tomé (eds.). Production, processing and marketing of pomegranate in the Mediterranean region: Advances in research and technology. CIHEAM, Zaragoza.
- International Olive Oil Council (IOOC). (2003). World olive oil consumption. Retrieved from: www.internationaloliveoil.org.
- ISO 5508. 1990. Animal and vegetable fats and oils – Analysis by gas chromatography of methyl esters of fatty acids. International Standard Organization, Geneva (Switzerland).
- Jaiswal, V., A. DerMarderosian, and J.R. Porter. 2010. Anthocyanins and polyphenoloxidase from dried arils of pomegranate (Punica granatum L). Food Chem. 118:11–16. doi: 10.1016/j.foodchem.2009.01.095.
- Khadivi-Khub, A. 2014. Physiological and genetic factors influencing fruit cracking. Acta Physiol. Plant. 37:1. doi: 10.1007/s11738-014-1718-2.
- Kornsteiner, M., K.H. Wagner, and I. Elmadfa. 2006. Tocopherols and total phenolics in 10 different nut types. Food Chem. 98:381–387. doi: 10.1016/j.foodchem.2005.07.033.
- Kyralan, M., M. Golukcu, and H. Tokgoz. 2009. Oil and conjugated linolenic acid contents of seeds from important pomegranate cultivars (Punica granatum L.) grown in Turkey. J. Am. Oil Chem.’ Soc. 86:985–990. doi: 10.1007/s11746-009-1436-x.
- Liu, G., X. Xu, Q. Hao, and Y. Gao. 2009. Supercritical CO2 extraction optimization of pomegranate (Punica granatum L.) seed oil using response surface methodology. Food Sci. Tech. 42:1491–1495. doi: 10.1016/j.lwt.2009.04.011.
- Martinez, J.J., P. Melgarejo, F. Hernandez, D.M. Salazar, and R. Martinez. 2006. Seed characterization of five new pomegranate (Punica granatum L.) varieties. Sci Horti. 110:241–246. doi: 10.1016/j.scienta.2006.07.018.
- Melgarejo, P., and F. Artés. 2000. Total lipid content and fatty acid composition of oilseed from lesser known sweet pomegranate clones. J Sci. Food Agri. 80:1452–1454.
- Melo, I.L.P., E.B.T. Carvalho, and J. Mancini-Filho. 2014. Pomegranate seed oil (Punica granatum L.): A source of punicic acid (conjugated α-linolenic cid). J. Hum. Nutr. Food Sci. 2(1):1024.
- Nehdi, I.A. 2011. Characteristics and composition of Washingtonia filifera (Linden ex André) H. Wendl. seed and seed oil. Food Chem. 126:197–202. doi: 10.1016/j.foodchem.2010.10.099.
- Özyurt, V.H. 2019. Comparison of the quality properties of some commercial cold pressed seed oils. JOTCSA 6(2):149–156. doi: 10.18596/jotcsa.496458.
- Parashar, A., N. Sinha, and P. Singh. 2010. Lipid contents and fatty acids composition of seeds oil from twenty five pomegranate varieties grown in India. J Food Sci. Tech. 2(1):12–15.
- Peng, Y. 2019. Comparative analysis of the biological components of pomegranate seed from different cultivars. Int. J. Food. Prop. 22(1):784–794.
- Pereira de Melo, I.L., E.B. Teixeira de Carvalho, A.M.O. Silva, L.T. Yoshime, J.A.G. Sattler, R.T. Pavan, and J. Mancini-Filho. 2016. Characterization of constituents, quality and stability of pomegranate seed oil (Punica granatum L.). Food Sci Tech. 36(1):132–139. doi: 10.1590/1678-457X.0069.
- Rahmani, M. 2007. Méthodes d’évaluation de la stabilité oxydative des lipides. Les technologies de laboratoires 2:18–21.
- Singh, A., U. Burman, P. Santra, and B.R. Morwal. 2014. Fruit cracking of pomegranate and its relationship with temperature and plant water status in hot arid region of India. J. Agrometeorol. 16(1):24–29.
- Soetjipto, H., M. Pradipta, and K.H. Timotius. 2010. Fatty acids composition of red and purple pomegranate (Punica granatum L) seed oil. IJCC 1:74–77.
- Trentacoste, E.R., A.P. Banco, P.N. Piccoli, and R.P. Monasterio. 2019. Olive oil characterization of cv Arauco harvested at different times in areas with early frost in Mendoza, Argentina. J Sci. Food Agri. 100:3. doi: 10.1002/jsfa.10029.
- Were, B.A., A.O. Onkware, S. Gudu, M. Welander, and A.S. Carlsson. 2006. Seed oil content and fatty acid composition in East African sesame (Sesamum indicum L.) accessions evaluated over 3 years. Field Crops Res. 97:254–260. doi: 10.1016/j.fcr.2005.10.009.
- Wolf, J.P. 1968. Manuel d’analyse des corps gras. Editions Azoulay.
- Zaouay, F., P. Mena, C. Garcia-Viguera, and M. Mars. 2012. Antioxidant activity and physico-chemical properties of Tunisian grown pomegranate (Punica granatum L.) cultivars. Ind. Crops Prod. 40:81–89. doi: 10.1016/j.indcrop.2012.02.045.
- Zaouay, F., H.H. Salem, R. Labidi, and M. Mars. 2014. Development and quality assessment of new drinks combining sweet and sour pomegranate juices. Emir J. Food Agr. 26(1):1–8. doi: 10.9755/ejfa.v26i1.14838.
- Zeb, A., and M. Murkovic. 2011. Carotenoids and triacylglycerols interactions during thermal oxidation of refined olive oil. Food Chem. 127:1584–1593. doi: 10.1016/j.foodchem.2011.02.022.
- Zielińska, A., and I. Nowak. 2014. Fatty acids in vegetable oils and their importance in cosmetic industry. Chemik 68(2):103–110.