?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Marketability and nutritional content are critical factors that need to be considered when a quality of orange fruit is assessed. Among the many factors affecting orange fruit quality, tree age and harvesting season are crucial once; however, the factors remained unmapped. Thus, this study was aimed to explore quality of orange fruit due to differences in tree age and harvesting season. Three trees age groups (young = 10 to 16, moderate = 20 to 27 and old = greater than 30 years) grown at Gunda Gundo monastery and its surroundings, northern Ethiopia and are harvested in October and December. The physico-chemical and antioxidant contents were characterized during two consecutive years (2017–2018). R software (R version 3.6.2) was used for the analysis. The highest single fruit weight of 218.71 g, peel thickness (3.6 mm) and pH (3.73) were obtained from young tree age in 2017. Total soluble solid of 13.4° Brix was recorded from moderate tree age in 2017, while 13.3° Brix was recorded from older trees of October harvest in 2018. High rag mass of 41.78% in October of 2017 and 38.04% in December of 2018 harvest were obtained from fruits of young trees. High vitamin C (53.95 mg 100 ml−1) and total sugar (11.1%) from the young trees; the highest juice mass (52.04%) from moderate trees age; and the highest phenolic content (9.15 mgGAEg−1), iron-reducing power (54.2 mgAAEg−1), total antioxidant (26.55 mgBHTg−1) and lower DPPH EC50 value (1.5 mg ml−1) from fruits of older tree were obtained. This experimental study results show that old trees age had high antioxidant activity.
Introduction
Citrus is one of the world’s most important economic fruit crops. It is grown commercially in more than 137 countries around the world (Ismail and Zhang, Citation2004). Different varieties are cultivated in countries with climates ranging from tropical to temperate. Seventy percent of the total production is from the northern hemisphere (UNCTAD, Citation2005). According to FAOSTAT (Citation2019), citrus ranked among the highest worth in terms of international trade. Ethiopia is relatively newcomer in citrus trade (Seifu, Citation2003). The citrus crops are grown mainly by smallholders and some commercial farmers (Mohammed, Citation2007). The total area coverage and the annual production of citrus were reported 5,947 ha and 77,087 tons, respectively (CSA, Citation2012). Although citrus production in Ethiopia fluctuated substantially in recent years (FAO, Citation2016), specifically orange production tended to increase through 1999–2018 period reaching 4000 ha and 31,000 tons production in 2017/18 (USDA, Citation2018).
Ethiopia is agro-ecologically diverse. Many parts of the country are suitable for growing sub-tropical and tropical fruits. In addition, there are also many specific microclimates where could be used to grow various fruits adapted to. In Tigray, Northern Ethiopia, different citrus fruits are being grown, among which an elite type of Gunda Gundo orange is highly valued fruit crop. The fruit is monastery-based grove. It is propagated from seed, and has vigorous growth habit. The fruit tree has long fruit-bearing life it reached up to age of 60–65 years (Monastery Elders, personal communication, 2016). Farmers and monastics around the monastery are owned the crop for long historical time and they are assertive in its unique quality. According, Wendm et al. (Citation2016) the Gunda Gundo orange fruit has got better consumers’ preference and relatively high market price than any other orange available at regional markets. However, the determinant factor for Gunda Gundo orange quality is not very well understood.
Quality of fruit is a result of complex interactions of tree physiology, biotic (cultivar), environmental factors and management (Al-Jaleel et al., Citation2005; Iglesias et al., Citation2007). In a long bearing life of fruits, tree age influences quality of fruit. However, studies to determine its effect, especially in citrus fruits are rarely investigated. Tree age affected juice content, acidity and maturity index of oranges (Storey and Treeby, Citation2002), total soluble solid (TSS), vitamin C and acidity content of guava (Asrey et al., Citation2007). Khalid et al. (Citation2012) reported that tree age of ‘Kinnow’ mandarin affected sugar content, acidity, vitamin C and TSS, where non-reducing sugar and vitamin C were higher in young 3-year-old trees, whereas total sugar, reducing sugar, TSS and acidity were higher in 18-year-old trees. Ozeker (Citation2000) also reported that ‘Marsh’ seedless grapefruit harvested from 20-year-old trees produced bigger fruit with thinner rinds compared with 34- year-old trees. Apart from tree age, harvesting season also affects fruit quality. Cardenosa et al. (Citation2015) reported that early season harvesting resulted in a better antioxidant activity, which is explained in terms of vitamin contents than late-season harvesting. It is generally known that, chemical composition of the orange juice varies with the cultivars, genetic and environment (Bermejo et al., Citation2011; Ebere, Citation2008; Gattuso et al., Citation2007).
Orange has been identified as one of the main dietary sources of bioactive compounds (Gonzalez-Molina et al., Citation2010; Hounsome et al., Citation2008; Hussain et al., Citation2004; Lorente et al., Citation2014; Marti et al., Citation2009). It contains antioxidants (Gonzalez-Molina et al., Citation2010; Lorente et al., Citation2014; Tripoli et al., Citation2007), vitamin C (Hounsome et al., Citation2008; Marti et al., Citation2009), vitamins, and minerals (Supraditareporn and Pinthong, Citation2007; Wardlaw et al., Citation2004). These contents are reported to reduce cancer (Wardlaw et al., Citation2004); obesity (Gonzalez-Molina et al., Citation2010) and protect human body from degenerative diseases (Chen et al., Citation2007). The relative abundance of these active compounds determines the contribution of specific fruit juice to improve human health. However, different studies have shown that the chemical constituents are influenced by the plant species, tree age, harvest seasons, and by other factors such as, soil nutrients, geographical location, and climate conditions (Azmir et al., Citation2013; Campbell et al., Citation2013; Crespo et al., Citation2010; Garcia-Salas et al., Citation2014). The study of Meena and Asrey (Citation2018) on Mangifera indica fruit and Vazquez-Leon et al. (Citation2017) on Moringa oleifera leaves indicated that carotenoids contents increased with tree age, whereas the ascorbic acid contents decreased. In apple fruit, antioxidant capacity and phenolic compounds were affected by harvesting season (Kevers et al., Citation2011; Renard et al., Citation2007).
Changes in the bioactive compounds and physico-chemical content of Gunda Gundo orange with respect to the tree ages and harvesting season have not been reported before. Even though, there can be other several factors that affect the fruit quality, this research focused to assess the effect of tree age and harvesting season on physico-chemical and antioxidant potential of the Gunda Gundo orange.
Materials and Methods
Study Area
The experiment was conducted at Gunda Gundo monastery areas of Tigray, Northern Ethiopia. The research was conducted during 2017 and 2018 years. The experimental site is located between 14° 22ʹ01” N and 39° 37ʹ40” E with an altitude range between 1160 and 1345 m above sea level. The area is characterized by high-rugged mountains, deep gorges, and incised river valley. The soil is sandy loam in texture, containing total nitrogen (0.13%), available phosphorus (3.57 mg/kg), electrical conductivity (EC; 0.21dsm−1) pH (8.17), organic matter (OM; 2.03%) and cation exchange capacity (CEC; 14.67 cmolkg−1). The soil nutritional content of the area indicated that, total nitrogen, OM, CEC, are within the range of critical medium to medium, while available phosphorus and EC are at very low rate. The area requires soil fertility improvement to get the potential benefits from the tree.
Experimental Design
The experiment was conducted on an established citrus grove, consisting of different tree age groups. The orchard was stratified into three blocks along the gorge based on aspects. East faced environment strata were considered. Treatments were tree age category (young = 10 to 16, moderate = 20 to 27, old = greater than 30 years of age) and two harvest seasons (beginning of October) and (end of December). Randomized complete block design with three replications was used. Three trees from each age group of the stratum were selected and 10 uniformly mature fruits were harvested from each tree. Immediately after harvest fruits were transported to the lab for further analysis.
Physical Fruit Quality
Fruit mass (g) was determined by weighing using digital balance. Peel thickness (mm) was measured from the outside edge to the inner edge of the white albedo tissue with the Vernier caliper (Digital caliper (0–150 mm), resolution 0.01 mm, Japan). Number of seeds per fruit were counted and weighed. Fruit firmness (kg cm−2) was measured using penetrometer (EFFE GI, FT 011, Italy). Juice (% w/w) and fruit rag (% w/w) were determined according to Saleem et al. (Citation2008) and Grewal et al. (Citation2000), respectively.
Biochemical Fruit Quality
Total soluble solid (TSS, °Brix) was determined using Abbe’s refractometer (NAR- 1 T, Atago Japan) (Grewal et al., Citation2000). Juice pH was measured with a pH meter (pH 016, Belgium). Titratable acidity (TA, %) was determined as percentage of citric acid by titration with sodium hydroxide and using phenolphthalein 1% as indicator according Srivastava and Sanjeev (Citation2003). Maturity index (MI) was calculated as the ratio of TSS (°Brix) to TA (%) (Crisosto et al., Citation2003). Reducing, non-reducing and total sugar percentage was measured as per the official Lane- Eynon AOAC method (Aurelie et al., Citation2016).
Total Antioxidant Compounds Determination
Total phenolic content (TPC) was determined according to method described by Shan et al. (Citation2005). A 0.1 mL of the juice (1 mg mL−1) and 1 mL Folin-Ciocalteu reagent (dilute ten times) was mixed and the mixture was left for 5 min and then 1 mL (75 g L−1) of sodium carbonate was added. The absorbance of the resulting blue color was measured at 765 nm with a UV-visible spectrophotometer after incubation for 90 min at room temperature. The total phenolic content was estimated from gallic acid (1–100 mg mL−1) calibration curve (y = 0.015x + 0.081, R2 = 0.99) and results was expressed as mg gallic acid equivalent per gram of juice (mgGAEg−1).
Vitamin C was determined using titration method 20 mL of orange filtrate, 25 mL of distilled water and 1 mL of 0.5% starch solution (indicator) were mixed and immediately titrated to the end-point with the standardized 0.005 molL−1 iodine solution. Results were repeated in triplicates and expressed in mg of vitamin C per 100 g of juice.
Determination of Antioxidant Activity (AA)
DPPH Radical Scavenging Assay
The 2, 2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity of the orange juice was determined as described by Katerere and Eloff (Citation2005) with slight modification. Different concentrations (0.5 to10 mg mL−1) of the juice were taken in different test tubes. DPPH solution (2 mL, 0.006%, and w/v) in ethanol was added in each of the test tubes containing 1 mL of the juice. The reaction mixture and the reference standard of vitamin C were vortexes and left to stand at room temperature in the dark for 30 min. The absorbance of the resulting solution was then taken at 517 nm. Ethanol was used as blank. The ability to scavenge the DPPH radical was calculated using the following equation;
Where, Abs control – is absorbance of DPPH, Abs sample-is absorbance of the sample (mixture of DPPH and the juice). The juice concentration allowing a 50% scavenging activity (EC50) was determined and expressed in mg mL−1.
Ferric Ion Reducing Power
The ferric reducing antioxidant power (FRAP) measures the presence of antioxidants in the juice causes the reduction of the yellow ferric/ferricyanide complex to the ferrous form which can be monitored by measuring the formation of Perl’s Prussian blue at 700 nm (Amarowicza et al., Citation2004). The reducing power was expressed as mg of vitamin C equivalents per gram of juice (mgAAE g−1) using the following equation based on L-ascorbic acid calibration curve (y = 0.006x + 0.120, R2 = 0.98).
Phosphomolybdenum Assay
The total antioxidant activity (TAC) of the orange juice was determined according to the method (Prieto et al., Citation1999). The antioxidant activity was expressed as mg equivalent of butylated hydroxytoluene per gram of orange juice (mgBHTE g−1) using standard graph (y = 0.0094x + 0.112, R2 = 0.99).
Data Analysis
Two-way nested ANOVA in mixed-effects model was implemented. Harvesting season was assigned as fixed effect. Age is nested within tree and nested within block as random effects. Statistical analysis was performed with R (version 3.6.2) software (R Core Team, Citation2019). Least significant difference tests were used for mean separation at p < .05. Linear regression analysis was used to calculate EC50 value of the DPPH. Correlations between parameters were established using Pearson´s coefficient. Data of two-year for physico-chemical quality factor, one-year data of 2018 harvest for bioactive compounds (phenolic and vitamin C content, FRAP, DPPH, and phosphomolybdenum assay) and sugar content were reported.
Results
Physico-chemical Responses of Fruit Attributes to Tree Age and Harvesting Season
Fruit mass was significantly (p < .05) affected by tree age and harvesting season in both years (2017 and 2018). In 2017 the highest fruit mass (217.71 g) was obtained from the interaction of young tree age and October harvest, while the lowest (176.34 g) from the interaction of old tree age and December harvest (). In the young tree age, fruits of less in number, large fruit size and thick peel were observed (data not shown). In 2017, older tree age and October harvest interaction gave significantly the highest seed number per fruit (15) and seed mass percentage recorded 3.37% (). On the other hand, in 2018 the highest seed number (18.2) was obtained from moderate tree age during the October harvest. Fruit from younger tree age harvested in 2017 during December was significantly less firm (4.41 kg cm−2). Whilst, high firmness values (6.87 kg cm−2 in 2017, and 6.63 kg cm−2 in 2018) were obtained from trees of older age groups harvested in October ().
Table 1. Physio-chemical fruit quality of orange as affected by tree age and harvesting season
Acidity and pH were significantly (p < .05) affected by interaction of tree age and harvest season (). The highest acidity expressed in citric acid (1.21% in 2017, 1.1% in 2018) was obtained from trees of old age. High pH (3.73 in 2017, 3.69 in 2018) was obtained from young trees during October harvest.
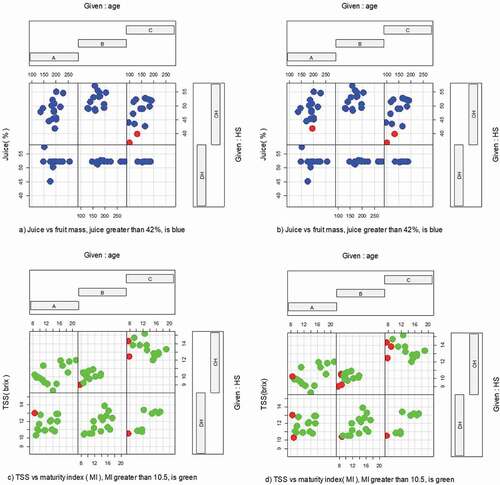
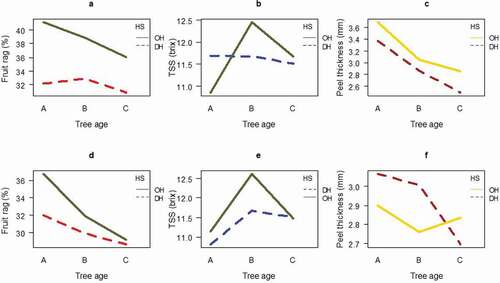
The highest fruit rag mass percentage (41.78% in 2017 and 38.04% in 2018) of October harvest was recorded from young trees ((,)). In both years (2017 and 2018), October harvest recorded the highest TSS in trees of moderate age ((,)). Peel thickness was highest in trees of young age, harvested in October (4.63 mm in 2017), and in December (4.31 mm in 2018), while the lowest (2.06 mm in 2017, 2.02 mm in 2018) was obtained from trees of old age harvested in December () in 2017, ) in 2018). Juice content of orange was significantly (p = .003) affected by interaction of tree age and harvest season. In both years of harvest, the highest juice content (52%) was recorded from moderate tree age, harvested in December. The lowest juice content (40.3%) was obtained from the tree of old age.
Figure 1. Interaction effect of tree age and harvest season on fruit rag, TSS and peel thickness (a-c 2017), (d-f 2018), A = young B = moderate, C = older trees, HS = harvesting season, O = October, D = December
Juice content, relative to fruit mass () in 2017, ) in 2018) was lower at trees of old age and in October harvest. Similarly, the maturity index and TSS interaction (), 2017, ), 2018) were shown lower result at the older trees in October harvest.
Tree Age Effect on Sugar Content
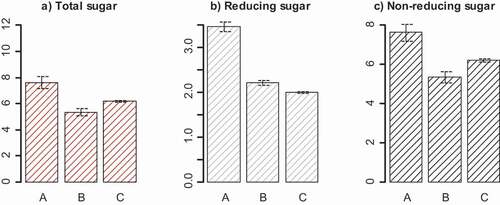
Tree age significantly (P < .05) influenced sugar content. The highest total sugar (11.11%), reducing sugar (3.48%) as well as non-reducing sugar (7.63%) were recorded from the younger trees (A) in 2018 harvest ((a–c)).
Total Phenolic and Vitamin C Content
Total phenolic content was significantly (p < .05) varied among age groups and ranges from 8.25 to 9.15 mgGAEg−1 (). The highest phenolic content (9.15 mgGAEg−1) was observed from the older trees. Vitamin C content was significantly (p < .05) affected by tree age (). The highest vitamin C (53.95 mg100 g−1) was obtained from the trees of young age.
Table 2. DPPH activity EC50, total phenolic and Vitamin C contents of orange affected by tree age
Antioxidant Activity (Aa)/capacity
The bioactive compounds were assessed using three common methods (FRAP, DPPH and phosphomolybdenum assay). The values of antioxidant activity of individual method are depicted in . The DPPH radical scavenging effect of orange juice was affected by tree age ()). At juice concentration (0.5, 1, 2.5, 5, 10 mg mL−1), the young tree (GA1) DPPH scavenging indicated 33.98, 39.17, 51.96, 68.56, and 93.37%, respectively. While, slightly higher scavenging 40.65, 45.76, 56.54, 74.22, and 95.08%, respectively, were observed in the older trees (GA3). The L-ascorbic acid used as standard indicator is significantly higher than any concentration of the juice. The EC50 values was calculated from graph of percentage scavenging activity against concentration and showed significant (P < .5) difference ().
Figure 4. DPPH radical scavenging activity (a), Ferric reducing power (mg of ascorbic acid per gram of juice) (b) and total antioxidant capacity of orange juice at different concentrations using phosphomolybdenum method butylated hydroxytoluene equivalent (mg BHTE g−1), (c) from different tree ages (GA1 = young, GA2 = moderate and GA3 = old, trees age). Values are average of triplicate measurements (mean ±SD)
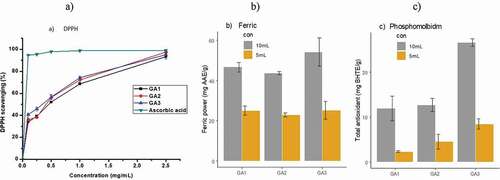
The iron reducing power of orange juice from different tree ages is depicted in ). At the concentration of 5 mg mL−1 of orange juice, iron-reducing power was not significant (p > .05) among age groups. At concentration of 10 mg mL−1 the older trees showed significant scavenging activity from the trees of young and moderate age. The reducing effect of the juice increases with the increase in concentration. This dose-dependent manner may be due to proportional increment of secondary metabolites with concentration.
Total antioxidant capacity was determined, based on the reduction of Mo (VI) to Mo (V) in the presence of antioxidant compound and subsequent formation of a green phosphate/Mo (V) complex at acidic pH and at higher temperature (Huda-Faujan et al., Citation2009). The total antioxidant activity of orange juice affected by tree age is shown in ). No significant difference (p > .05) was found between the young and moderate trees age groups in the given concentration of 5 and 10 mL−1. The older trees showed significant difference from the trees of young age in both concentrations (5 and 10 mg mL−1).
Correlation Analysis
The dependence of antioxidant activity obtained by different assays, in relation to the total phenolic and vitamin C contents were valued (). The total phenolic content correlated with DPPH radical scavenging (r = 0.73), iron-reducing power (r = 0.88), and total antioxidant assay (r = 0.84). Similarly, a positive correlation of vitamin C was found between DPPH (r = 0.79), iron reducing power (r = 0.72) and total antioxidant (r = 0.85).
Table 3. Correlations between antioxidant activities assays on total phenolic and vitamin C content
Discussion
Fruit size has a commercial importance in citrus fruits marketing. It is generally considered that, with excessive increase in fruit mass and size the quality is impaired, while on the other side, small-sized fruits are of low quality (Nawaz et al., Citation2008). Fruit mass and size are quantitative parameters, environmental conditions probably affected fruit growth and development (Corelli-Grappadelli and Lakso, Citation2004; Leibhard et al., Citation2003). In the present study, higher fruit mass was observed in trees of young age, harvested in early season (October), while smaller and less mature fruit left on the trees for the second harvest (December). High fruit size was also found in the young age trees and strongly correlated with fruit mass (data not shown). In young trees, the fewer number of fruits per tree (less crop load), the higher was assimilates accumulation and the larger the fruit mass. Ozeker (Citation2000) also reported similar finding, in that 20-year-old trees of seedless grape fruit to have bigger fruit when compared to 34-year-old trees.
The number of seeds per fruit and seed mass percentage were significantly high in the old age trees, harvested in October of 2017. Orange fruits which have high number of seeds per fruit (20–30 seeds/fruit) are not suitable for processing industry for juice extraction (Anwar and Ibrahim, Citation2004). Generally, citrus fruits with few numbers of seeds per fruit are preferred for processing and fresh consumption. The seed number recorded in all tree age groups are below the limit (< 20). In that sense Gunda Gundo orange has good quality for processing as well as for fresh consumption.
High acid content was recorded in the older trees. Koo (Citation1988) reported increased fruit acidity with increase in nitrogen content of a tree. It is postulated that N is one of phloem mobile nutrients and as tree becomes old, phloem mobile nutrients are favored (Storey and Treeby, Citation2002). This gives an opportunity for older trees to take up better nitrogen. On the one hand, young fruit trees have better chance of xylem mobility and the conductivity of xylem decreases with increase in age (Hubbard et al., Citation1999; Loescher et al., Citation1990). The physiological functioning difference in the age groups might be attributed to nitrogen absorption and acidity accumulation. In the current study high fruit acidity from old age trees, might be arisen due to better accumulation of nitrogen in the trees of older age. Although, the soil fertility status of the orchard was low (total nitrogen content of 0.13%), which is rated low to medium (Tekalign et al., Citation1991), the older trees might have better chance of nitrogen absorption via phloem, which in turn, led to have more acid in the fruits of older trees.
Fruit from older trees showed slightly inferior quality (i.e., low juice content and high acidity), as compared to those harvested from trees of moderate age. This result is consistent with that of Khalid et al. (Citation2012) who reported, the highest TSS (10.4 °Brix) from trees of 18 years of age as compared to trees of age 3, 6, and 35 years in ‘Kinnow’ mandarin. Tahir et al. (Citation2007) reported a similar trend in apples. With regard to seasonal harvest, the highest TSS was recorded in early season harvest (October). Higher total soluble solids (TSS) in the October harvest might be attributed to shortage of water supply to the orchard that occurred at the pre-harvest of September–October than the progressing harvesting season of December. The September and October are months during which rainfall usually stops and irrigation not provided, and as a result fruit trees experience water shortage. Different researchers reported association between reduced water supply and higher TSS and titratable acidity (TA). Results of the present study are in close agreement with results of these researchers in that, TSS and TA were higher in October comparing to late season (December) picking. In contrast to this, Diaz-Mula et al. (Citation2008) reported early season harvest of plum to have lower TSS than late season harvest. Fruits which were harvested from the young trees of Gunda Gundo orange had significantly higher sugar content. Schab et al. (Citation2015) reported total reducing sugar (8.71%) and direct reducing sugar (4.32%) in Washington navel orange, which sugar content of Gunda Gundo orange is also consistent with. Khalid et al. (Citation2012) reported similar result in that the highest non-reducing sugar was obtained from younger trees (3-year-old), while contrary to our findings, the total sugar and reducing sugar in ‘Kinnow’ mandarin were high in old trees (35-year-old).
European Union, UN, FAO, and USDA stated that orange varieties having a minimum of 35% juice, 11.8 °Brix TSS and 9.5 sugar-to-acid ratio are rated as grade “A” (EU, Citation2011; FAO/WHO, Citation2005; UN, Citation2010; USDA, Citation1997). This may vary with orange varieties and specific countries, for example, Navel orange in Australia is considered acceptable when the ratio of TSS: acid is above 8 and TSS 8 °Brix with a minimum juice content (33%). In USA orange varieties with TSS: acidity ratio of 8 and TSS of 9 to 10.5 °Brix are considered to have good consumer acceptance (Ladaniya, Citation2008). TSS: acid ratio of 13 is preferred in India (Khokar and Sharma, Citation1984). European countries have set minimum ratio of 6.5 maturity index for all orange varieties, as it influences sweetness and astringency (Wardy et al., Citation2009). While juice content should be at least 30%, 33%, and 35% in blood oranges, Navel and other varieties, respectively. Findings of the present research, in terms of average juice content (47.2%), TSS (11.86 °Brix) as well as sugar-to-acid ratio (12.2) indicates that quality standards of the Gunda Gundo orange falls to grade “A”.
Results reported by Khalid et al. (Citation2012) and Valpuesta and Botella (Citation2004) are consistent with the current findings in that high vitamin C obtained in younger fruit trees, which decreased with increases in tree age. The vitamin C content of Gunda Gundo orange is in agreement with the range reported by various researchers (Arena et al., Citation2001; Bungau et al., Citation2011; Farnworth et al., Citation2001; Sanusi et al., Citation2008) who reported vitamin C content ranges from 20.4 to 54.2, mg 100 g−1. High amount of vitamin C recorded in the Gunda Gundo orange is an indicator of quality orange and vitamin C is one of the important components of antioxidant. The antioxidant nature of vitamin C is getting more and more attention in recent years, especially among health-conscious. Hence, consumers demand to the fruit of Gunda Gundo orange becomes high, because of the contribution to health-protective aspect by activating scavenging potential. Victor et al. (Citation2013) indicated that, 50% of the free radical scavenging activity quantified by the DPPH assay of orange juice as a result of vitamin C. Vitamin C is highly and positively correlated (r = 0.85) with antioxidant. The result is in agreement with reports of Guo et al. (Citation2003) and Yoo et al. (Citation2004) in that contents of vitamin C and antioxidant are closely correlated.
High phenolic content was obtained from older trees. Phenolic substances are synthesized during normal development of the plant and production increases in response to environmental stress. This is because phenolics are functioning as defense mechanism to protect damaged caused by pathogen, wounding or excess UV light (Azmir et al., Citation2013; Pollastri and Tattini, Citation2011; Treutter, Citation2006; Winkel-Shirley, Citation2002). The highest phenolic content in the older trees of present study indicates that, older trees might have encountered, mineral nutrient imbalance, water stress due to xylem function efficiency reduction and wounding stresses. Hence, such condition is leading to trigger production of high phenolic compounds to protect such pressure from the surrounding environment. These results are in agreement with the findings of Beh et al. (Citation2012) and Belitz et al. (Citation2009) who reported that phenolic compounds are affected by age and greater concentration of total phenols found in older orange trees. DPPH is a stable nitrogen-centered-free radical commonly used for testing radical scavenging activity of the compound or plant extracts. When the stable DPPH radical accepts an electron from the antioxidant compound, the violet color of the DPPH radical was reduced to yellow colored diphenylpicryl hydrazine radical which was measured colorimetrically. Substances which can perform this reaction can be considered as antioxidants and therefore radical scavengers (Dehpour et al., Citation2009). The lower DPPH value of EC50 from the older trees also indicated orange juice of the older trees showing the highest scavenging potential. Gunda Gundo oranges showed strong EC50 DPPH activity when compared to Cardenosa et al. (Citation2015) report, in which orange varieties evaluated for their DPPH content exhibited within the range of 9–11 mg mL−1. As the concentration of juice increased, the percent inhibition of DPPH radical also increased. This is in line with reports of Huang et al. (Citation2005). Iron reduction (Fe3+ to Fe2+) is often used as an indicator of electron-donating activity, which is an important mechanism of phenolic antioxidant reaction (Hinneburg et al., Citation2006). The better reducing effect of the juice increases with the increase in the concentration. This may be due to the increase in the concentration of the secondary metabolites in the fruit.
The antioxidant activities obtained by different assays were showed strong correlation. The higher TPC and total antioxidant resulted in higher antioxidant activity, which was similarly to that reported by Ruanma et al. (Citation2010) in different herbal extracts. Therefore, these results suggest that DPPH, FRAP, and phosphomolybdenum assays are recommended methods to asses antioxidant activity in orange. Data in the literature about the relation between concentration of phenolic compounds and antioxidant activity are contradictory. While some authors have observed high correlation (Schab et al., Citation2015), others find no direct correlation or only a very weak correlation.
Conclusions
It was found that as tree age increases, the physical fruit parameters (fruit mass, fruit rag, and juice content) decrease. On the other hand, seed number per fruit and acid content showed increment with trees age. Higher iron reducing power, total antioxidant using phosphomolbidium, and lower DPPH EC50 value indicated concurrent evidence in that fruits from the older trees showed better antioxidant activity. When comparing the results of consecutive two years, changes observed in 2017 showed well-marked differences among the studied orange quality parameters. The late harvest season (December) seems preferably a better option for, high juice content, maturity index, and low acid content.
Acknowledgments
This work was supported by the Open Society Foundation-Africa Climate Change Adaptation Initiative (OSF-ACCAI Grant No. OR2014-18350). We are grateful for getting permission to conduct this study in a grove of orange owned by farmers and monastic staff of Gunda Gundo monastery.
Disclosure Statement
The authors declare no conflict of interest.
Additional information
Funding
Literature Cited
- Al-Jaleel, A., M. Zekri, and H. Yahia. 2005. Yield, fruit quality, and tree health of ‘Allen Eureka’ lemon on seven rootstocks in Saudi Arabia. Sci. Hortic. 105:457–465. doi: https://doi.org/10.1016/j.scienta.2005.02.008.
- Amarowicza, R., R. Pegg, P. Rahimi-Moghaddame, B. Barld, and J. Weilc. 2004. Free radical scavenging capacity and antioxidant activity of selected plant species from Canadian prairies. Food Chem. 84:551–562. doi: https://doi.org/10.1016/S0308-8146(03)00278-4.
- Anwar, R., and M. Ibrahim. 2004. Is Kinnow better than orange? Proc. the 1st Int. Conf. Citriculture, p. 196–199. Faisalabad, Pakistan, University of Agriculture.
- Arena, E., B. Fallico, and E. Maccarone. 2001. Evaluation of antioxidant capacity of blood orange juices as influenced by constituents, concentration process and storage. Food Chem. 74:423–427. doi: https://doi.org/10.1016/S0308-8146(01)00125-X.
- Asrey, R., R.K. Pal, V.R. Sagar, and V.B. Patel. 2007. Impact of tree age and canopy position on fruit quality of guava. Acta Hortic. 735:259–262. doi: https://doi.org/10.17660/ActaHortic.2007.735.34.
- Aurelie, C.R., V.J. Braesco, J. Chupin, and L. Bouillo. 2016. Nutritional composition of orange juice: a comparative study between French Commercial and home-made juices. Food Nutr. Sci. 7:252–261.
- Azmir, J., I.S.M. Zaidul, M.M. Rahman, K.M. Sharif, A. Mohamed, F. Sahena, M.H.A. Jahurul, K. Ghafoor, N.A.N. Norulaini, and A.K.M. Omar. 2013. Techniques for extraction of bioactive compounds from plant materials: a review. J. Food Eng. 117(4):426–436. doi: https://doi.org/10.1016/j.jfoodeng.2013.01.014.
- Beh, L.K., Z. Zakaria, B.K. Beh, W.H. Yong, S.Y. Keong, and N.B.M. Alitheen. 2012. Comparison of total phenolic content and antioxidant activities of freeze-dried commercial and fresh fruit juices. J. Med. Plant Res. 6(48):5857–5862.
- Belitz, H.D., W. Grosch, and P. Schieberle. 2009. Fruits and fruit products, Springer, Berlin, p. 807–861. Food chem.
- Bermejo, A., M.J. Losa, and A. Cano. 2011. Analysis of bioactive compounds in seven citrus cultivars. Food Sci. Tech. Int. 17:55–62. doi: https://doi.org/10.1177/1082013210368556.
- Bungau, S., A. Fodor, D.M. Tit, and I. Szabo. 2011. Studies on citrus species fruits ascorbic acid content using kinetic, spectrophotometric and iodometric methods. Anal. Univ. Oradea Fascicula 16:212–217.
- Campbell, O.E., I.A. Merwin, and O.I. Padilla-Zakour. 2013. Characterization and the effect of maturity at harvest on the phenolic and carotenoid content of Northeast USA Apricot (Prunus armeniaca) varieties. J. Agric. Food Chem. 61(51):12700–12710. doi: https://doi.org/10.1021/jf403644r.
- Cardenosa, V., C.M. Joao, L.B. Barreira, J. Francisco, J.M. Arenas-Arenas, I.C. Moreno-Rojas, and F.R. Ferreira. 2015. Variety and harvesting season effects on antioxidant activity and vitamins Content of Citrus sinensis Macfad. Molecules 20:8287–8302. doi: https://doi.org/10.3390/molecules20058287.
- Chen, L., C. Vigneault, G.S.V. Raghavan, and S. Kubow. 2007. Importance of the phytochemical content of fruits and vegetables to human health. Stewart Postharvest Rev. 3:2.
- Corelli-Grappadelli, L., and A.N. Lakso. 2004. Fruit development in deciduous fruit tree crops as affected by physiological factors and environmental conditions (key note). Acta Hortic. 636:425–441. doi: https://doi.org/10.17660/ActaHortic.2004.636.52.
- Crespo, P., J.G. Bordonaba, L.A. Terry, and C. Carlen. 2010. Characterization of major taste and health-related compounds of four strawberry genotypes grown at different Swiss production sites. Food Chem. 122(1):16–24. doi: https://doi.org/10.1016/j.foodchem.2010.02.010.
- Crisosto, C.H., G.M. Crisosto, and P. Metheney. 2003. Consumer acceptance of ‘Brooks’and ‘Bing’ cherries is mainly dependent on fruit SSC and visual skin color. Post Bio. Tech. 28:159–167. doi: https://doi.org/10.1016/S0925-5214(02)00173-4.
- CSA (Central Statistical Agency). 2012. Agricultural sample survey: Report on area and production of major crops for private peasant holding. Vol. 1. Statistical Bulletin. CSA, Addis Abeba, Ethiopia.
- Dehpour, A.A., M.A. Ebrahimzadeh, S.F. Nabavi, and S.M. Nabavi. 2009. Antioxidant activity of methanol extract of Ferula assafoetida and its essential oil composition. Grasas Aceites 60(4):405–412. doi: https://doi.org/10.3989/gya.010109.
- Diaz-Mula, H.M.D., P.J. Zapata, F. Guillen, S. Castillo, D. Martinez-Romero, and D. Valero Serrano. 2008. Changes in physico chemical and nutritive parameters and bioactive compounds during development and on-tree ripening of eight plum cultivars: A comparative study. J. Sci. Food Agric. 88:2499–2507. doi: https://doi.org/10.1002/jsfa.3370.
- Ebere, O.D. 2008. Citrus fruits: A rich source of phyto-chemicals and their roles in human health. Int. J. Chem. Soc. 6:451–471.
- EU (European Union). 2011. EU. Reglamento de ejecucion (UE) Nº 543/2011 de la Comision de 7 de junio. Off. J. Eur. Union L157:71–75. Available in 24 different languages.
- FAO. 2016. Citrus fruit fresh and processed statistical bulletin. 10, 2020. http://www.fao.org/3/a-i8092e.pdf
- FAO/WHO. 2005. Codex Alimentarius. Joint FAO/WHO food standards programme. Codex Alimentarius Commission. Food and Agriculture Organization of the United Nations/World Health Organization, Rome, Italy.
- FAOSTAT. 2019. Citrus: World markets and trade. 20 Jul. http://www.fao.org/economic/est/est-commodities/citrus-fruit/en/
- Farnworth, E.R.M., R. Lagacé, V.Y. Couture, and B. Stewart. 2001. Thermal processing, storage conditions, and the composition and physical properties of orange juice. Food Res. Int. 34:25–30. doi: https://doi.org/10.1016/S0963-9969(00)00124-1.
- Garcia-Salas, P., A.M. Gómez-Caravaca, A. Morales-Soto, A. Segura -Carretero, and A. Fernandez-Gutierrez. 2014. Identification and quantification of phenolic compounds in diverse cultivars of eggplant grown in different seasons by high-performance liquid chromatography coupled to diode array detector and electrospray-quadrupole-time of flight-mass spectrometry. Food Res. Int. 57:114–122. doi: https://doi.org/10.1016/j.foodres.2014.01.032.
- Gattuso, G., D. Barreca, C. Gargiulli, U. Leuzzi, and C. Caristi. 2007. Flavonoid composition of citrus juices. Molecules 12:1641–1673. doi: https://doi.org/10.3390/12081641.
- Gonzalez-Molina, E., R. Domínguez-Perles, D.A. Moreno, and C. Garcia-Viguera. 2010. Natural bioactive compounds of citrus limon for food and health. J. Pharma. Biomed. Anal. 51:327–345. doi: https://doi.org/10.1016/j.jpba.2009.07.027.
- Grewal, A.G., I.A. Hafiz, A.H. Chaudhary, M.I. Khan, and M.I. Chaudhary. 2000. Quality estimation during marketing of kinnow and feutrell’s early. Int. J. Agric. Biol. 2:328–330.
- Guo, C., J. Yang, J. Wei, Y. Li, J. Xu, and Y. Jiang. 2003. Antioxidant activities of peel, pulp and seed fractions of common fruits as determined by FRAP assay. Nutr. Res. 23:1719–1726. doi: https://doi.org/10.1016/j.nutres.2003.08.005.
- Hinneburg, I., H.J. Damien Dorman, and R. Hiltunen. 2006. Antioxidant activities of extracts from selected culinary herbs and spices. Food Chem. 97:122–129. doi: https://doi.org/10.1016/j.foodchem.2005.03.028.
- Hounsome, L., B. Hounsome, D. Tomos, and G. Edwards-Jones. 2008. Plant metabolites and nutritional quality of vegetables. J. Food Sci. 73:48–65. doi: https://doi.org/10.1111/j.1750-3841.2008.00716.x.
- Huang, D., B. Ou, and R.L. Prior. 2005. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 53:1841–1856. doi: https://doi.org/10.1021/jf030723c.
- Hubbard, R.M., B.J. Bond, and M.G. Ryan. 1999. Evidence that hydraulic conductance limits photosynthesis in old Pinus ponderosa trees. Tree Physiol. 19:165–172. doi: https://doi.org/10.1093/treephys/19.3.165.
- Huda-Faujan, N., A. Noriham, A.S. Norrakiah, and A.S. Babji. 2009. Antioxidant activity of plants methanolic extracts containing phenolic compounds. Afr. J. Biotechnol. 8:484–489.
- Hussain, I., M. Asif, M. Ahmed, M. Khan, and I. Shakir. 2004. Effect of uni-packaging on the post-harvest behavior of citrus fruits. Pak. J. Nutr. 3:336–339. doi: https://doi.org/10.3923/pjn.2004.336.339.
- Iglesias, D.J., M. Cercos, J.M. Colmenero-Flores, M.A. Naranjo, G. Rios, E. Carrera, O. Ruiz-Rivero, I. Lliso, R. Morillon, F.R. Tadeo, et al. 2007. Physiology of citrus fruiting. Braz. J. Plant Physiol. 19:333‒362. doi: https://doi.org/10.1590/S1677-04202007000400006.
- Ismail, M., and J. Zhang. 2004. Post-harvest citrus diseases and their control. Outlooks Pest Manage. 15:29–35. doi: https://doi.org/10.1564/15feb12.
- Katerere, D., and J. Eloff. 2005. Antibacterial and antioxidant activity of Sutherlandia frutescens (Fabaceae), a reputed Anti-HIV/AIDS. Phytomed. Phytother. Res. 19:779–781. doi: https://doi.org/10.1002/ptr.1719.
- Kevers, C., J. Pincemail, J. Tabart, J. Defraigne, and J. Dommes. 2011. Influence of cultivar, harvest time, storage conditions, and peeling on the antioxidant capacity and phenolic and ascorbic acid contents of apples and pears. J. Agric. Food Chem. 59:6165–6171. doi: https://doi.org/10.1021/jf201013k.
- Khalid, S., U.M. Aman, A.S. Basharat, S.K. Ahmad, S.K. Muhammad, and A. Muhammad. 2012. Tree age and canopy position affect rind quality, fruit quality and rind nutrient content of ‘Kinno w’ mandarin (Citrus nobilis Lour × Citrus deliciosa Tenora). Sci. Hortic. 135:137–144. doi: https://doi.org/10.1016/j.scienta.2011.12.010.
- Khokar, U.U., and R. Sharma. 1984. Maturity indices for sweet orange cv Blood Red. Haryana J. Hortic. Sci. 13:22–25.
- Koo, R.C.J. 1988. Fertilization and irrigation effects of fruit quality, p. 97. In: J.J. Ferguson and W.F. Wardowski (eds.). Factors affecting fruit quality citrus short course. Proceedings. Univ. of Florida, Coop.
- Ladaniya, M.S. 2008. Growth, maturity, grade standards and physic-mechanical characteristics of fruit, p. 191–212. In: M.S. Ladaniya (ed.). Citrus fruit, biology, technology and evaluation. Academic Press, USA.
- Leibhard, R., M. Kellerhals, W. Pfammatter, M. Jertmini, and C. Gessler. 2003. Mapping quantitative physiological traits in apple (Malus × domestica Borkh). Plant Mol. Biol. 52:511–526. doi: https://doi.org/10.1023/A:1024886500979.
- Loescher, W.H., T. McCamant, and J. Keller. 1990. Carbohydrate reserves, translocation, and storage in woody plant roots D. Hortscience 25:3–281. doi: https://doi.org/10.21273/HORTSCI.25.3.274.
- Lorente, J., S. Vegara, N. Martí, A. Ibarz, L. Coll, J. Hernandez, M. Valero, and D. Saura. 2014. Chemical guide parameters for Spanish lemon (Citrus limon (L.) Burm.) Jucies. Food Chem. 162:186–191. doi: https://doi.org/10.1016/j.foodchem.2014.04.042.
- Marti, N., P. Mena, J.A. Canovas, V. Micol, and D. Saura. 2009. Vitamin C and the role of citrus juices as functional food. Nat. Prod. Commun. 4:677–700. doi: https://doi.org/10.1177/1934578X0900400506.
- Meena, N.K., and R. Asrey. 2018. Tree age affects postharvest attributes and mineral content in Amrapali Mango (Mangifera indica) Fruits. Hortic. Plant J. 4(2):55–61. doi: https://doi.org/10.1016/j.hpj.2018.01.005.
- Mohammed, Y. 2007. Distribution and management of Phaeoramularia leaf and fruit spot disease of citrus in Ethiopia. Fruits 62:99–106. doi: https://doi.org/10.1051/fruits:2007003.
- Nawaz, M.A., W. Ahmad, S. Ahmad, and M.M. Khan. 2008. Role of growth regulators on preharvest fruit drop, yield and quality in kinnow mandarin. Pak. J. Bot. 40:1971–1981.
- Ozeker, E. 2000. Determination of fruit characteristics of ‘Marsh seedless’ grapefruit cultivar in Izmir (Turkey). Pak. J. Biol. Sci 3:69–71. doi: https://doi.org/10.3923/pjbs.2000.69.71.
- Pollastri, S., and M. Tattini. 2011. Flavonols: Old compounds for old roles. Ann. Bot. 108:1225–1233. doi: https://doi.org/10.1093/aob/mcr234.
- Prieto, P., M. Pineda, and M. Aguilar. 1999. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of Vitamin E. Anal. Biochem. 269:337–341. doi: https://doi.org/10.1006/abio.1999.4019.
- R Core Team. 2019. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
- Renard, C., N. Dupont, and P. Guillermin. 2007. Concentrations and characteristics of procyanidins and other phenolics in apples during fruit growth. Phytochemistry 68:1128–1138. doi: https://doi.org/10.1016/j.phytochem.2007.02.012.
- Ruanma, K., L. Shank, and G. Chairote. 2010. Phenolic content and antioxidant properties of green chilli paste and its ingredients. Maejo Int. J. Sci. Technol. 4:193–200.
- Saleem, B.A., A.U. Malik, M.A. Pervez, A.S. Khan, and M.N. Khan. 2008. Spring application of growth regulators affects fruit quality of ‘Blood red’ sweet orange. Pak. J. Bot. 40:1013–1023.
- Sanusi, R.A., Y. Ogunro, and S. Nwozoh. 2008. Effect of storage time on ascorbic acid content of some selected “Made in Nigeria” fruit preserves. Pak. J. Nutr. 7(6):730–732. doi: https://doi.org/10.3923/pjn.2008.730.732.
- Schab, M.C., M.M. Ferreyra, C.V. Davies, A. Stefan, M.C. Cayetand, L.M. Gerard, and R.F. Gdnzaiez. 2015. Effects of orange winemaking variables on antioxidant activity and bioactive compounds. Food Sci. Tech. 35(3):407–413. doi: https://doi.org/10.1590/1678-457X.6571.
- Seifu, G.-M. 2003. Status of commercial fruit production in Ethiopia. Ethiopian Agricultural Research Organization (EARO), Addis Ababa, Ethiopia.
- Shan, B., Y. Cai, M. Sun, and H. Corke. 2005. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J. Agric. Food Chem. 53:7749–7759. doi: https://doi.org/10.1021/jf051513y.
- Srivastava, R.P., and K. Sanjeev. 2003. Fruit and vegetable preservation principles and practices: important methods for analysis of fruits and vegetables and their products. 3rd ed. International Book distribution Co., Lucknow, India, p. 363.
- Storey, R., and M.T. Treeby. 2002. Nutrient uptake into navel oranges during fruit development. J. Hortic. Sci. Biotechnol. 77:91–99. doi: https://doi.org/10.1080/14620316.2002.11511463.
- Supraditareporn, M., and R. Pinthong. 2007. Physical, chemical and microbiological changes during storage of orange juices cv. Sai Nam Pung and cv. Khieo Waan in Northern Thailand. Int. J. Agri. Biol 9:726–730.
- Tahir, I., I.E. Johansson, and M.E. Olsson. 2007. Improvement of quality and storability of apple cv. Aroma by adjustment of some pre-harvest conditions. Sci. Hortic. 112:164–171. doi: https://doi.org/10.1016/j.scienta.2006.12.018.
- Tekalign, M., I. Hague, and E.A. Aduayi. 1991. Soil, plant, water, fertilizer, animal manure and compost analysis manual. Plant science division working document 13. ILCA, Addis Abeba, Ethiopia.
- Treutter, D. 2006. Significance of flavonoids in plant resistance: A review. Environ. Chem. Lett. 4:147–157. doi: https://doi.org/10.1007/s10311-006-0068-8.
- Tripoli, E.L.A., M. Guardia, S. Giammanco, D. Di Majo, and M. Giammanco. 2007. Citrus flavonoids: Molecular structure, biological activity and nutritional properties: A review. Food Chem. 104:466–479. doi: https://doi.org/10.1016/j.foodchem.2006.11.054.
- UN. 2010. UN. Unece Standard FFV-14. 2010. Citrus fruit. United Nations, New York and Geneva, p. 12.
- UNCTAD. 2005. Citrus fruit market. Market information in the commodities area. 12 Mar., 2020 www.unctad.org/infocommIntergovernmentalGrouponCitrusFruits
- USDA. 1997. United States Standards for Grades of Florida Oranges and Tangelos. USDA; Feb.
- USDA. 2018. Assessment of commodity and trade issues made by USDA staff and not necessarily statement of officials U.S. Government policy. Gain Report Number ET1827. Global Agriculture Information Network.
- Valpuesta, V., and M.A. Botella. 2004. Biosynthesis of L-ascorbic acid in plants: New pathways for an old antioxidant. Trends Plant Sci. 9(12):573–577. doi: https://doi.org/10.1016/j.tplants.2004.10.002.
- Vazquez-Leon, L.A., D.E. Paramo-Calderon, V.J. Robles-Olvera, O.A. Valdés-Rodríguez, A. Perez-Vazquez, M.A. Garcia-Alvarado, and G.C. Rodriguez-Jimenes. 2017. Variation in bioactive compounds and antiradical activity of Moringa oleifera leaves: Influence of climatic factors, tree age, and soil parameters. Eur. Food Res. Technol. 243:1593–1608. doi: https://doi.org/10.1007/s00217-017-2868-4.
- Victor, R.P., H. Lan-Anh, and P. Vinood. 2013. Diet quality: an evidence-based approach. Vol. I. New York: Humana Press.
- Wardlaw, G.M., J.S. Hampl, and R.A. Di Sivelstro. 2004. Perspectives in Nutrition. 6th ed. McGraw Hill Companies, Inc., New York.
- Wardy, W., F. Saalia, M. Steiner-Asiedu, A. Budu, and S. Sefa-Dedeh. 2009. A comparison of some physical, chemical and sensory attributes of three pineapples (Ananas comosus) varieties grown in Ghana. Afr. J. Food Sci. 3(1):022–025.
- Wendm, Y., G. Hruy, N. Aregay, M. Hadush, E. Abrha, and K. Gebrehiwot. 2016. Evaluation of fruit quality of Gunda Gundo orange in contrast to other orange fruits. J. Drylands 6(1):429–433.
- Winkel-Shirley, B. 2002. Biosynthesis of flavonoids and effects of stress. Curr. Opin. Plant Biol. 5:218–223. doi: https://doi.org/10.1016/S1369-5266(02)00256-X.
- Yoo, K.M., K.W. Lee, J.B. Park, H.W. Lee, and K. Hwang. 2004. Variation in major antioxidant and total antioxidant activity of Yuzu (Citrus junos Sieb ex Tanaka) during maturation and between cultivars. J. Agric. Food Chem. 52(19):5907–5913. doi: https://doi.org/10.1021/jf0498158.