?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Twenty-four morphological and pomological traits were characterized in a group of 47 Moroccan sweet cherry genotypes to evaluate their diversity. The fruit weight varied between 3.6 and 7.52 g, considered acceptable for the fresh market. Six genotypes showed fruit weight between 3 and 5 g, making them suitable for specific industrial uses. The statistical analysis revealed high variability among genotypes, although these variables did not allow the classification of the genotypes according to their geographical origin. Genotypes with high physical traits could be planted in modern orchards in association with commercial cultivars or incorporated in sweet cherry breeding programmes.
Introduction
Sweet cherry (Prunus avium L.) is an out-crossing, self-incompatible species belonging to the Rosaceae family. The species is commonly grown in the temperate climatic zones with cool temperatures to providing provide the chilling requirement necessary for flower induction. The sexual reproduction in sweet cherry is controlled by a Gametophytic Self-incompatibility (GSI) system (de Nettancourt, Citation2001) and open pollinated cultivars are heavily utilized in its traditional culture allowing a high genetic variability of this species around the world. It is believed that cherries originated in the area between the Black and Caspian seas in Asia Minor (Webster, Citation1996). Birds may have carried it to Europe prior to human civilization and its cultivation probably began in Roman times and spread to the US in the sixteenth century (Watkins, Citation1976). Several local varieties were selected from the natural cherry populations based on the agronomical and pomological potentialities of the genotypes. However, majority of these cultivars are self-incompatible and require the association of at least two cultivars in the commercial orchards to ensure adequate production. In the last decades, a large number of new sweet cherry cultivars with valuable pomological and agronomical characteristics was established, including same that are self-compatible and with medium chilling requirement (Benková et al., Citation2017; Sansavini and Lugli, Citation2008). The selected cultivars show distinctive agronomic characters such as low susceptibility to fruit cracking, high levels of soluble solids, early fruit maturity and great rusticity (Pérez-Sánchez et al., Citation2008).
In Morocco, sweet cherry culture occupies around 2000 hectares, with an annual production of 14.100 tonnes (Ministry of Agriculture, Citation2012). The most popular sweet cherry cultivars grown in Morocco are ‘Bigarreau Van’ and ‘Bigarreau Burlat’, with ‘Napoleon’ as pollinizer (Kodad et al., Citation2016; Oukabli, Citation2004). However, there are other cultivars grown on a small scale such as ‘Cerisette’ and ‘Coeur de pigeon’ (Oukabli, Citation2004). The sweet cherry was introduced before 1920 by the French protectorate and it was propagated by grafting and also by the seeds in middle Atlas Mountains region of Morocco. Generally, the seeds used by the growers were those issued from ‘Burlat’ and ‘Van’ cultivars (Oukabli, Citation2004). These cherry genotypes are grown under conditions where one or more environmental and technical training requirements are limiting. These include water during the growing season, soil depth, and nutrient availability (Kodad et al., Citation2016; Oukabli, Citation2004). Trees (mostly open-pollinated seedlings) are planted on slopes or interplanted with others cherry cultivars, and are given little or no care, at an average density of 100 trees/ha, and are neither pruned nor sprayed. Despite their low productivity, seedling trees represent a good source of pollen for the commercial cultivars in traditional orchards.
In the commercial orchards, the synchronized flowering period among the cultivated commercials varieties (mostly ‘Burlat’ and ‘Van’ association) has not fully overlapped during the last decade, resulting in the lack of pollination and low production (Kodad et al., Citation2016; Oukabli and Mahhou, Citation2007), stressing the growers to introduce new foreign cultivar to ensure a correct pollination. Moreover, one of the most important objectives of the new strategy of the Ministry of Agriculture in Morocco is enhancing the sweet cherry production in the mountain regions in order to improve its marketing and to increase the income of the local growers (Ministry of Agriculture, Citation2012), taking into account the high level of poverty in these regions and the importance of sweet cherry in the economy of the households. In order to follow this strategy, the Moroccan growers have planted new sweet cherry orchards based, mainly on ‘Van’ and ‘Burlat’. However, several problems associated with poor pollination were observed in these new orchards, due to the asynchronous blooming period among these cultivars (Kodad et al., Citation2016). The introduction of local sweet cherry genotypes, well adapted to the local conditions, as pollinators to overcome these problems is a suitable solution. However, no studies have been undertaken to evaluate the genetic diversity and the agronomical behavior of these genotypes in local populations, to select and conserve the most promising genotypes. Autochthonous cultivars are in some cases at a high risk of extinction due to the introduction of foreign varieties, which may have a higher productivity or are better known in foreign markets (Rodrigues et al., Citation2008). Sweet cherry commercial quality refers to all aspects related to the external appearance of the product, including fruit weight, color, firmness, size and shape. Although the concept of «fruit quality» depends on the product itself and the consumer’s preferences, it is widely accepted that the main characteristics related to sweet cherry quality are fruit weight, color, firmness, sweetness, sourness, flavor and aroma with important differences among cultivars (Díaz-Mula et al., Citation2009; Romano et al., Citation2006). Morphological evaluation and characterization are the first steps for the description and classification of germplasm (Badenes et al., Citation2000; Cantini et al., Citation1999). Thus, the main objective of the present work was the evaluation of the morphological and pomological traits of the main local sweet cherry populations in the middle Atlas Mountains of Morocco in order to select the genotypes to be used as a possible pollen donor in the new orchards of sweet cherry in Morocco and to be incorporated in a breeding programme.
Material and Methods
Plant Material
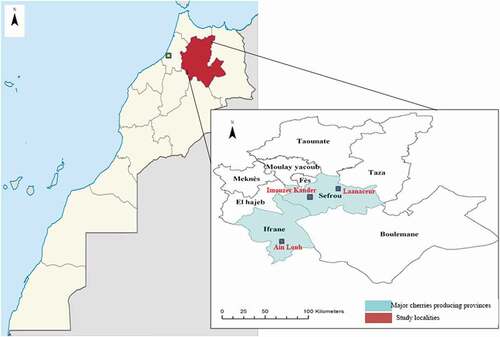
This study was carried out during three consecutive years in three different locations with abundant sweet cherry genetic resources: Imouzer Kander and Laanaceur (Sefrou province) and Toufselt (Ifrane province) situated in the middle Atlas Mountains (central Morocco) (). A total of 47 local genotypes from different sites of each location were selected based on tree characteristics (vigor, ramification, foliar density, and appearance), fruit characteristics and appreciation of their fruit by the local population (). These genotypes were marked and fruits were collected between May and June of each year. The data used in the present work were the mean of the three years of study. The ripening period, corresponding to the commercial maturity of the fruit, was determined on the basis of the color characteristics of each cultivar, taking into account information provided by growers and from personal experience and observation. A sample of 50 fruits was collected randomly around the canopy from the marked plants. The descriptors list for cherry (Schmidt et al., Citation1985) was used for basic description of leaves and fruits. With respect to the trees, only the vigor, habit, flowering and fruit ripen periods were evaluated.
Table 1. Origin and main tree and fruit traits of the studied genotypes
Fruit and Stone Traits
Fruit and stone traits are presented in . The length, thickness and width were measured at the midpoint of the length, perpendicular to each other. Length, width, and thickness were measured with a precision of 0.01 mm in all stone with a digital calliper. Fruit and stone volume was calculated using the formula 4/3πr3, where r = [L + W + T]/6. Geometric mean diameter (Dg) and sphericity (Ø) were calculated using the following equations Dg = (LWT)0.333 and Ø = Dg/L (Aydin, Citation2003; Mohsenin, Citation1986). Following Mohsenin (Citation1986), fruit surface area (S) was expressed as S = π Dg2. Fruit weight was measured on an electronic balance with a sensitivity of ± 0.001 g. The firmness of the fruit was evaluated by maximum compression force in the equatorial region of fruits using electronic firmness tester (AGROSTA®100 Field digital firmness tester).
Table 2. Abbreviation of morphological characteristics of evaluated sweet cherry genotypes
Leaf Traits
Leaves were collected at adult stage, at approximately the second week of July. From each genotype, ten leaves were sampled per year, and the following parameters measured using a digital calliper with a sensitivity of ± 0.01 mm: leaf blade, length and width (cm) and petiole length (cm) ().
Statistical Analysis
All statistical analyses were performed with the SAS program (SAS, Citation2000). The principal component analysis (PCA) was applied to the average data of three years to describe the pattern of sweet cherry diversity. In PCA, intercorrelation among variables (component) was removed (Broschat, Citation1979), thus reducing the number of variables by linear combination of correlated characters into principal orthogonal axes (PC1, PC2 … PCn), which are not correlated (Philippeau, Citation1986). The maximum amount of variance in the data set and its direction are often explained by the first PC. Each PC is defined by a vector known as the eigenvector of the variance-covariance matrix. PCA is used to establish correlations between variables and to visualize the relationships of individuals in two- or three-dimensional graphs. The best model with the minimum number of dimensions explaining the data structure was selected by the exclusion rule, based on the amount of residual variability to be tolerated, retaining a sufficient number of PCs capable of explaining a percentage of variance near to 80%.
Results and Discussion
Tree Traits Variability and Fruit Appreciation
The genotypes evaluated in the present study showed different habits, varying from upright to drooping (). More than 63% of genotypes had an upright habit and only 17% had dropping growing habit. Concerning the vigor, almost genotypes (61%) showed intermediate vigor and only 5% of genotypes showed weak tree vigor. The flowering date of studied genotypes varied from early (second decade of March) to medium blooming period (first decade of April) (). All the genotypes selected in Imouzer Kander showed an earlier flowering date, whereas those selected in Laanaceur site showed medium to late blooming period. Concerning ripen date, most genotypes (76.7%) had an early ripening date, whereas 23% of genotypes, all of them originated from Laanaceur, had medium fruit maturity period.
Concerning the qualitative fruit characteristics, noticeable variability was observed among the genotypes (). Around 15% of genotypes produce fruit with red on yellow ground skin color and cream white flesh color, whereas the others are darker, both externally and internally. Concerning flesh firmness appreciation, only 17% had soft flesh firmness, while the others are intermediate to hard flesh firmness. The eating quality was deemed good in all but nine genotypes.
Fruit and Stone Variability
Fruit weight varied between 3.6 and 7.52 g (). These values are lower than those reported in the literature for wild sweet cherries. Benková et al. (Citation2017) reported that fruit weight from Slovakian sweet cherries varied between 3.7 and 8.9 g (). The Ukrainian cherries had fruit weight with range of 5.10–8.33 g (Hégedüs et al., Citation2013). While the average fruit weight of genotypes from Russia was ranged from 3.45 g to 4.63 g (Bandi et al., Citation2010). Fruit weight is considered to be a very important trait for the fresh-market. Kappel et al. (Citation1996) suggested that for sweet cherry cultivars the optimum fruit weight is preferable to be between 11 and 13 g to be accepted by the consumers. For industrial uses, the fruit weight of 3 to 4 grams is considered the highest fruit mass category (Călin, Citation2007). In the present study, 6 genotypes showed fruit weight between 3 and 5 g, indicating the possibility to destine these fruits to specific industrial uses. Fruit volume varied from 2.42 to 5.95 cm3 (). These values are similar or lower than those reported in other sweet cherry cultivars and local genotypes (Pérez-Sánchez et al., Citation2010). The fruit diameter (thickness) of the studied genotype varied between 15.3 and 22.84 mm (). This parameter is considered as the most important commercial trait defining sweet cherry fruit characteristics, since the larger the fruit, the higher its market quality (Christensen, Citation1996). Bandi et al. (Citation2010) reported that the European Union standard regulates the lower size of the class I at 17 mm diameter in case of the sweet cherry, and for industrial processing the 16–17 mm diameter is appreciated as first class. According to this classification, the fruits of 43 genotypes evaluated in the present study could be destined for fresh market. However, Kappel et al. (Citation1996) suggested that new cultivars would need to be larger, with 25 mm diameter. As a general rule, the local genotypes had lower values of fruit weight than those derived from breeding programmes (Pérez-Sánchez et al., Citation2008). The heaviest and largest fruits of the genotypes selected in the present study may be considered as parents in breeding programmes and for cultivation in their original areas.
Table 3. Fruit weight and dimensions of the Moroccan sweet cherry genotypes
The fruit firmness of the selected genotypes varied between 4.2 and 7.9 N (). These values are lower than thus reported by Pérez-Sánchez et al. (Citation2010) for Spanish cultivars, with values ranged from 7.66 to 15.28 N. These differences are probably due to the genotype, ripening process and climatic factors such as temperature and precipitation. Fruit firmness is a combination of skin and flesh firmness and appears to affect consumer acceptance and fruit shelf life, considering this parameter of high importance by the industry (Kappel et al., Citation1996). With regard to sphericity, almost all genotypes are of flattened form with value superior to 0.95, and only 4 genotypes produced more elongated fruits (Øf < 0.95).
The Moroccan cherries showed fruit with short to medium stalk length, with values varied between 22.24 and 46.44 mm (). In Spanish sweet cherry cultivars, the stalk length was reported to be varied between 30.9 and 69 mm (Pérez-Sánchez et al., Citation2008, Citation2010) and for Portuguese cultivars this parameter fluctuated between 33.4 and 49 mm (Rodrigues et al., Citation2008). In Iranian cultivars, Khadivi-Khub (Citation2014) reported that fruit stalk length ranged from 23.35 to 59.44 mm and in the Latvian cherries this trait varied between 34 and 60 mm with an average of 41.55 mm (Benková et al., Citation2017). For the machine harvesting the longer stalk is better regarding possible damage to the fruit skin as observed in the case of short stalk. However, Pérez-Sánchez et al. (Citation2010) reported that the consumers prefer sweet cherries with short stalk. The shorter peduncle of the Moroccan local cherries could be the results of the seed propagation of this species by the local farmers resulting in new genotypes with short stalks as suggested by Pérez-Sánchez et al. (Citation2010) to explain the presence of the short peduncle in some local Spanish cultivars.
Regarding the stone trait, the stone weight varied between 0.22 and 0.46 g (). Ganopoulos et al. (Citation2015) reported that the stone weight evaluated in 146 sweet cherry cultivars varied from 0.19 to 0.75 g. The stone volume varied from 0.29 to 0.53 cm3 (). According to Pérez-Sánchez et al. (Citation2010) the endocarp volume of Spanish cherries varied from 0.39 to 0.60 cm3. The flesh/stone ratio varied from 0.06 to 0.14. Almost all studied genotypes showed high volume of the flesh, however six genotypes showed low flesh amount, with an endocarp/fruit volume ratio superior to 0.11. Pérez-Sánchez et al. (Citation2010) reported that the local Spanish cultivars “Pical”, “Jarandilla” and “Del Valle”, with an endocarp/fruit volume ratio of about 0.14, had lowest volume of the flesh. These authors reported that the flesh volume is considered an important fruit trait to indicate that consumers generally prefer sweet cherries with large flesh volume. For this reason, one of the most important criteria employed for the main sweet cherry breeding programs is the flesh/stone ratio (Pérez-Sánchez et al., Citation2010).
Table 4. Stone and leaf characteristics of the Moroccan sweet cherry genotypes
Correlation among Traits
All variables were jointly examined and their correlation coefficients are shown in . Fruit weight was highly positively correlated with fruit length (r2 = 0.81), width (r2 = 0.95), thickness (r2 = 0.80), volume (r2 = 0.93), geometric diameter (r2 = 0.94) and fruit surface area (r2 = 0.94), indicating that the fruit weight is influenced by its dimensions. These results are in accordance with those reported in the literature in other sweet cherry cultivars (Ganopoulos et al., Citation2015; Khadivi-Khub, Citation2014; Rakonjac et al., Citation2014; Rodrigues et al., Citation2008). The fruit weight was highly and positively correlated with flesh width (r2 = 0.92) and flesh weight (r2 = 0.99). The fruit linear dimensions are highly and positively correlated ().
Table 5. Correlation matrix among characteristics studied in sweet cherry genotypes
The stone weight was positively correlated with stone width (r2 = 0.63), thickness (r2 = 0.53), geometric diameter (r2 = 0.67), volume (r2 = 0.63) and surface area (r2 = 0.67). There was a positive correlation between the fruit weight and its dimensions with fruit firmness (). These results are in agreement with those reported in other cherries (Christensen, Citation1996; Demirsoy and Demirsoy, Citation2004; Khadivi-Khub, Citation2014). The leaf length was positively correlated with leaf width (r2 = 0.52). These results are in agreement with those reported by Rakonjac et al. (Citation2014) and Chehade et al. (Citation2005). Positive correlation was found between leaf weight and fruit weight, volume and dimensions (). Similar results were found in other cherry cultivars (Ganopoulos et al., Citation2015; Rakonjac et al., Citation2010). These results indicate the role of the leaf in increasing fruit size in sweet cherry (Demirsoy and Demirsoy, Citation2004). Correlation coefficients allow comparing indirect and direct selection, thus computing the correlated response for a second trait when selection pressure is applied to a first one and allowing establishing a selection strategy (Falconer and Mackay, Citation1996). Correlation coefficients greater than 0.71 or smaller than – 0.71 have been suggested to be biologically meaningful (Skinner et al., Citation1999); showing that this correlation is not influenced by climatic and environmental conditions and is genotype-dependent. Thus, the selection for high fruit mass in sweet cherry will improve the flesh weight, considered a most important trait preferred by consumers.
Principal Component Analysis
Statistical methods such as PCA and cluster analysis can be useful tools for screening genotypes in plant collections and natural populations of different species including cherry genotypes (Ganopoulos et al., Citation2015; Lacis et al., Citation2009; Pérez-Sánchez et al., Citation2008; Rodrigues et al., Citation2008; Zamani et al., Citation2012), apricot (Ruiz and Egea, Citation2008) and sour cherry (Krahl et al., Citation1991; Rakonjac et al., Citation2010). To select the best model with the minimum number of dimensions explaining the data structure, the exclusion rule used was based on the amount of residual variability to tolerate, retaining a sufficient number of PCs capable of explaining a percentage of variance near to 80%. Using this rule, the first three axes of the PCA were enough because they explain 76.78% (). The results of the PCA indicate a high genetic diversity within Moroccan local sweet cherry genotypes. The first PC accounted for 39.87% of the variation, whereas the second and third axes accounted for 21.02% and 15.89%, respectively. The fruit traits (weight, length, width, thickness, volume, geometric diameter, surface area and firmness), flesh variables (weight and width), and stalk length were primarily responsible for the separation on the PC1. The stone traits (weight, width, thickness, geometric diameter, volume and surface area), stalk weight and petiole length are highly correlated to PC2. For the PC3, it was associated with fruit and stone sphericity, stone length and leaf length and width. The fruit and leaf traits are considered of highly important criteria in phenotyping and morphologically characterization of the diversity and similarity in sweet cherry vegetative (Antonius et al., Citation2012; Ganopoulos et al., Citation2015; Hjalmarsson and Ortiz, Citation2000). Lacis et al. (Citation2009), (Citation2010) reported that tree architecture and fruit traits are the most useful traits for evaluating sweet and sour cherry accessions. It should be noted that the importance of fruit traits in the characterization and identification of sweet cherry varieties is essentially due to their high heritability (Hjalmarsson and Ortiz, Citation2000) and their expression is primarily depending on the genotype rather than the environmental conditions. The most important traits for grouping wild cherry accessions and sweet cherry cultivars were leaf characters (Farsad and Esna-Ashari, Citation2016; Rakonjac et al., Citation2014). However, it has been reported that leaf traits should be excluded from further evaluation due to high dependence on environmental conditions (Lacis and Rashal, Citation2000). Our results confirm that the fruit and stone traits are the main factors explaining the morphological diversity and similarity among local Moroccan sweet cherries.
Table 6. Eigenvectors of the Three Principal Component Axes from PCA Analysis of the Moroccan sweet cherry Seedlings
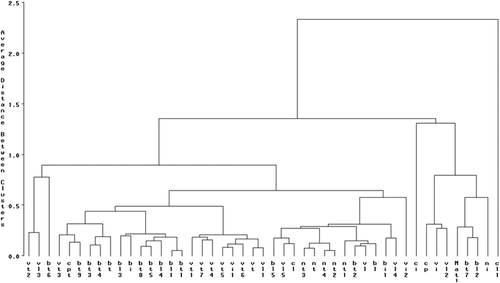
Cluster analysis showed high genetic diversity among studied populations (). In the literature it has been reported that the cluster analysis obtained from morphological traits clearly separated sweet cherries from sour cherries (Rodrigues et al., Citation2008) and also clearly distinguished cultivars of sweet, sour, and duke cherries (Pérez-Sánchez et al., Citation2008). In our study, this algorithm established four groups at a rescaled distance of 1, without the separation of any cluster of genotypes according to their origin (). Our results are in agreement with Rakonjac et al. (Citation2014) for wild cherry genotypes and Farsad et al. (2016) for sweet cherry cultivars, reporting the lack of relationship and similarities among genotypes according to their geographical origin. Ganopoulos et al. (Citation2015) reported that among all 146 cultivars from different world regions, there were no specific clusters based on locality. This result indicates clearly that in Morocco the flux of the vegetative and generative propagation was common practice used by the sweet cherry growers.
Figure 2. Position of the principal component (PC) scores of physical fruit traits for Moroccan sweet cherry genotypes
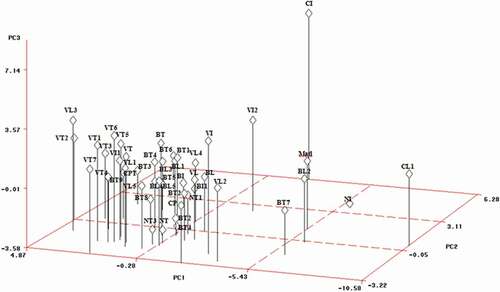
The Cluster 1 includes three genotypes (‘VT2ʹ and ‘BT6ʹ from Toufselt, ‘VL3ʹ from Laanaceur) () with a high positive value of PC1 (). These genotypes are characterized by high values of fruit traits, weight and width, firmest fruits and short stalk (). On the PC2 and PC3, genotypes have medium to low values of the variables explaining these components (). The cluster 2 includes twenty-one genotypes (‘VT’, ‘VT1ʹ, ‘VT3ʹ, ‘VT4ʹ, ‘VT5ʹ, ‘VT6ʹ, ‘VT7ʹ, ‘BT’, ‘BT1ʹ, ‘BT3ʹ, ‘BT4ʹ, ‘BT5ʹ, ‘BT8ʹ, ‘BT9ʹ and ‘CPT’ from Toufselt; ‘BL1ʹ, ‘BL3ʹ, ‘VL1ʹ and ‘BL4ʹ from Laanaceur; and ‘BI’ and ‘VI1ʹ from Imouzer Kander) () with intermediate to low positive PC1 and PC2 values (). They showed medium values for fruit size, weight and width of flesh, stalk length and firmness (). Also they showed intermediate to low values for stone and leaf size, stalk weight, petiole length and sphericity of fruit and stone (; 4). The cluster 3 includes fourteen genotypes (‘BL5ʹ, ‘VL5ʹ, ‘BL’, ‘VL’, ‘NT3ʹ, ‘VL4ʹ, and ‘CL’ from Laanaceur; ‘NT’, ‘NT4ʹ, ‘NT2ʹ, ‘NT1ʹ and ‘BT2ʹ from Toufselt; and ‘BI1ʹ and ‘VI2ʹ from Imozer Kander) with medium to small fruit and low firmness (). Cluster 4 includes eight genotypes (‘CI’, ‘CP’, ‘VI’, ‘NI’, ‘Matl’ from Imouzer Kander; ‘BL2ʹ and ‘VL2ʹ from Laanaceur; ‘BT7ʹ from Toufselt) characterized by the smallest fruit and stones compared to the previous genotypes (, ). The clustering of genotypes using the variability observed does not allow their classification according to their geographical origin, confirming that fruit, stone and leaf traits in sweet cherry depend primarily on the genotype and not on the geographical provenance. In the same way, Ganopoulos et al. (Citation2015), applying multivariate analysis on morpho-physiological traits of 146 sweet cherry cultivars from different world regions, reported that there were no specific clusters based on locality. However, Hjalmarsson and Ortiz (Citation2000), evaluating the morphological and fruit variation of Scandinavian sweet cherry cultivars, reported that the pattern of spatial relationship obtained for Scandinavian populations based on fruit quality and fruit set assessment suggests distinct ecotypes of sweet cherry within Scandinavia. Kolesnikova (Citation1975) (cited by Iezzoni et al., Citation1990) based on differences in winter hardness and fruit quality reported that the sweet cherry cultivars in the former USSR are subdivided in five ecotypes. Lansari et al. (Citation2001) reported that in local walnut genotypes in Morocco, the cluster analysis allowed the separation of genotypes originated from Ketama region (Northern of Morocco) from genotypes of other areas, forming a separate ecotype basing on tree and fruiting habit and physical fruit traits.
Conclusion
The results showed high morphological variability of the fruit and leaf traits among local sweet cherry Moroccan genotypes. All the genotypes are early to medium flowering and ripening period. As a general rule, the fruits produced by Moroccan local cherries are of small to medium weight and size compared to those of other local and sweet cultivars. Also, they are characterized by short to medium stalk length, which is preferred by the consumers.
The combined application of PCA and cluster analysis revealed that fruit and stone traits were suitable for sweet cherry cultivars characterization and provide comprehensive information. Three genotypes (‘VT2ʹ, ‘VL3ʹ and ‘BT6ʹ) were selected because of their high fruit weight and firmness, short stalk and acceptable taste. These genotypes could be planted in their geographical origin in association with commercial varieties. These genotypes could be incorporated as parents in sweet cherry breeding programmes in Morocco in order to create a new generation of cultivars with high fruit quality and more adapted to local conditions. The results may serve as a significant reference for the molecular characterization of local Moroccan sweet cherry genotypes.
Additional information
Funding
References
- Antonius, K., M. Aaltonen, M. Uosukainen, and T. Hurme. 2012. Genotypic and phenotypic diversity in Finnish cultivated sour cherry (Prunus cerasus L.). Genet. Resour. Crop Evol. 59:375–388.
- Aydin, C. 2003. Physical properties of almond nut and kernel. J. Food Eng. 60:315–320.
- Badenes, M.L., J. Martinez-Calvo, and G. Llacer. 2000. Analysis of a germplasm collection of loquat (Eriobotrya japonica Lindl.). Euphytica 114:187–194.
- Bandi, A., R. Thiesz, L. Ferencz, and M.J. Bandi. 2010. Some physical and biochemical compositions of the sweet cherry (Prunus avium L.) fruit. Acta Univ. Sapientiae, Agric. Environ. 2:5–16.
- Benková, M., I. Èièová, D. Benedikova, L. Mendel, and M. Glasa. 2017. Variability of old sweet cherries found in Slovak regions and their preservation. Proc. Latviensis Acad. Sci. 71:184–189.
- Broschat, T.K. 1979. Principal component analysis in horticultural research. HortScience 14:114–117.
- Călin, V. 2007. Fruit tree, small fruits and strawberry cultivars released in Romania. Paralela 45. Piteşti, pp. 210–212.
- Cantini, C., A. Cimato, and G. Sani. 1999. Morphological evaluation of olive germplasm present in Tuscany region. Euphytica 109:173–181.
- Chehade, A., L. Chalak, A. Elbitar, P. Cosson, A. Zanetto, and E. Dirlewanger. 2005. Caractérisation préliminaire morphologique et moléculaire de clones de cerisier cultivés au liban (Prunus Avium L.). Leban. Sci. J. 6:29–40.
- Christensen, J.V. 1996. Rain-induced cracking of sweet cherries: Its causes and prevention, p. 297–327. In: A.D. Webster and N.E. Looney (eds.). Cherries: Crop physiology, production and uses. Oxon, UK.
- de Nettancourt. 2001. Incompatibility and incongruity in wild and cultivated plants. Second ed. Springer-Verlag, Heidelberg.
- Demirsoy, H., and L. Demirsoy. 2004. A study on the relationships between some fruit characteristics in cherries. Fruits 59:219–223.
- Díaz-Mula, H.M., S. Castillo, D. Martínez-Romero, D. Valero, P. Zapata, F. Guillén, and M. Serrano. 2009. Sensory, nutritive and functional properties of sweet cherry as affected by cultivar and ripening stage. Food Sci. Technol. Int. 15:535–543.
- Falconer, D.S., and T.F.C. Mackay. 1996. Introduction to quantitative genetics. 4th ed. Longman Technical, Essex, UK.
- Farsad, A., and M. Esna-Ashari. 2016. Genetic diversity of some iranian sweet cherry (Prunus avium) cultivars using microsatellite markers and morphological traits. Cytol. Gene. 50:8–19.
- Ganopoulos, I., T. Moysiadis, A. Xanthopoulou, M. Ganopoulou, E. Avramidou, F.A. Aravanopoulos, E. Tani, P. Madesis, A. Tsaftaris, and K. Kazantzis. 2015. Diversity of morpho-physiological traits in worldwide sweet cherry cultivars of GeneBank collection using multivariate analysis. Sci. Hortic. 197:381–391.
- Hégedüs, A.F., D. Taller, N. Papp, B. Szikriszt, S. Erçisli, J. Halász, and É. Stefanovits-Bányai. 2013. Fruit antioxidant capacity and self-incompatibility genotype of Ukrainian sweet cherry (Prunus avium L.) cultivars highlight their breeding prospects. Euphytica 191:153–164.
- Hjalmarsson, I., and R. Ortiz. 2000. In-situ and ex-situ assessment of morphological and fruit variation in Scandinavian sweet cherry. Sci. Hortic. 85:37–49.
- Iezzoni, A., H. Schmidt, and A. Albertini. 1990. Cherries (Prunus), p. 111–173. In: J.N. Moore and J.R. Ballington (Eds.), Genetic Resources of Temperate Fruit and Nut Crops 1. Int. Society for Hort. Sci.Wageningen.
- Kappel, F., B. Fisher-Fleming, and E. Hoghe. 1996. Fruit characteristics and sensory attributes of an ideal sweet cherry. HortScience 31(3):443–446.
- Khadivi-Khub, A. 2014. Assessment of cultivated cherry germplasm in Iran by multivariate analysis. Trees 28:669–685.
- Kodad, O., S. En-Nahli, H. Hanine, and M. El Baji. 2016. Année exceptionnelle dans le moyen atlas: Effets des aléas climatiques sur la floraison et sur la qualité du fruit du cerisier. Agric. Maghreb 97:62–63.
- Kolesnikova, A.F. 1975. Breeding and some biological characteristics of sour cherry in central Russia. Priokstoc izdatel'stvo, Orel, Russia
- Krahl, K.H., A. Lansari, and A.F. Iezzoni. 1991. Morphological variation within a sour cherry collection. Euphytica 52:47–55.
- Lacis, G., E. Kaufmane., V. Trajkovski, and I. Rashal. 2009. Morphological variability and genetic diversity within Latvian and Swedish sweet cherry collections. Acta Univ. Latviensis 753:19–32.
- Lacis, G., and I. Rashal. 2000. Evaluation of variability of morphological traits of Latvian local sweet cherry (P. avium) accessions by means of multidimensional analysis. Proceedings of the International Conference “Fruit Production and Fruit Breeding”, Polli, Estonia, p. 147–151.
- Lacis, G., V. Trajkovski, and I. Rashal. 2010. Phenotypical variability and genetic diversity within accessions of the Swedish sour cherry (Prunus cerasus L.) genetic resources collection. Biologija 56:1–8.
- Lansari, A., A. El Hassani, D. Nabil, and E. Germain. 2001. Preliminary results on walnut germplasm evaluation in Morocco. Acta. Hortic. 541:27–35.
- Ministry of Agriculture. 2012. Situation de l’Agriculture Marocaine. N°10. P. 206.
- Mohsenin, N.N. 1986. Physical properties of plant and animal materials: Structure, physical characteristics and mechanical properties. Updated and Revised ed. Gordon and Breach Science Publishers, New York.
- Oukabli, A. 2004. Le cerisier, une culture de zones d’altitude. Transfert technol. Agric. 116:1–4.
- Oukabli, A., and A. Mahhou. 2007. Dormancy in sweet cherry (Prunus avium L.) under Mediterranean climatic conditions. Biotechnol. Agron. Soc. Environ. 11(2):133–139.
- Pérez-Sánchez, R., M.A. Gomez-Sánchez, and Morales-Corts. 2008. Agromorphological characterization of traditional Spanish sweet cherry (Prunus avium L.), sour cherry (Prunus cerasus L.) and duke cherry (Prunus × gondouinii Rehd.) cultivars. Span. J. Agric. Res. 6:42–55.
- Pérez-Sánchez, R., M.A. Gómez-Sánchez, and Morales-Corts. 2010. Description and quality evaluation of sweet cherries cultured in Spain. J. Food Qual. 33:490–506.
- Philippeau, G. 1986. Comment interpréter les résultas d’une analyse en composantes principales, p. 1–28. ITCF Doc. Institut Technique des Céréales et des Fourrages, Paris, France.
- Rakonjac, V., E. Mratinić, R. Jovković, and M.F. Akšić. 2014. Analysis of morphological variability in wild cherry (Prunus avium L.) genetic resources from Central Serbia. J. Agric. Sci. Tech. 16:151–162.
- Rakonjac, V., M.F. Akšić, D. Nikolić, D. Milatović, and S. Čolić. 2010. Morphological characterization of ‘Oblačinska’ sour cherry by multivariate analysis. Sci. Hortic. 125:679–684.
- Rodrigues, L.C., M.R. Morales, A.J.B. Fernandes, and J.M. Ortiz. 2008. Morphological characterization of sweet and sour cherry cultivars in a germplasm bank at Portugal. Genet. Resour. Crop Evol. 55:593–601.
- Romano, G.S., E.D. Cittadini, B. Pugh, and R. Schouten. 2006. Sweet cherry quality in the horticultural production chain. Stewart Postharvest Rev. 6:1–9.
- Ruiz, D., and J. Egea. 2008. Phenotypic diversity and relationships of fruit quality traits in apricot (Prunus armeniaca L.) germplasm. Euphytica 163:143–158.
- Sansavini, S., and S. Lugli. 2008. Sweet cherry breeding programs in Europe and Asia. Acta Hortic. 795:41–57.
- SAS Institute. 2000. SAS/STAT user’s guide. SAS Institute, Carey, NC.
- Schmidt, H., J. Vittrup-Christensen, R. Watkins, and R.A. Smith. 1985. Cherry descriptors. CEC Secretariat, Brussels, IBPGR Secretariat, Rome, p. 1–33.
- Skinner, D.Z., G.R. Bauchan, G. Auricht, and S. Hughes. 1999. A method for the efficient management and utilization of large germplasm collections. Crop Sci. 39:1237–1242.
- Watkins, R. 1976. Cherry, plum, peach, apricot, and almond, p. 242–247. In: N.W. Simmonds (ed.). Evolution of crop plants. Longman, New York.
- Webster, A.D. 1996. The taxonomic classification of sweet and sour cherries and a brief history of their cultivation, p. 3–24. In: A.D. Webster and N.E. Looney (eds.). Cherries: Crop physiology, production and uses. CAB International, Wallingford, Oxon.
- Zamani, Z., A. Shahi-Gharahlar, R. Fatahi, and N. Bouzari. 2012. Genetic relatedness among some wild cherry (Prunus subgenus Cerasus) genotypes native to Iran assayed by morphological traits and random amplified polymorphic DNA analysis. Plant Syst. Evol. 298:499–509.