 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Many fruit vendors in Nigeria adopt unhealthy practices to induce fruit ripening and increase the availability of ripe fruits in the markets. We investigated the safety of traditional induced ripening techniques on two banana species (Musa acuminata and Musa balbisiana). Unripe mature banana fruits were harvested and subjected to five different local ripening procedures – exposure to sunlight, hot water priming, enclosure in sack bags and nylon bags as well as exposure to calcium carbide (CaC2) in an enclosed container. The study included a control group, which was not exposed to any of the traditional ripening methods. Results showed that banana fruits primed in hot water turned dark throughout the period under review. Although it took control fruits six days to ripen, the fruits exposed to different weights of CaC2 ripened fastest (within 48–hours) irrespective of the mode of application, whether as dried CaC2 or in solution. Increased sugar accumulation was recorded in the CaC2 – ripened fruits, with evidence of arsenic (0.026–0.164 mg/kg) in the endocarp. Arsenic is an impurity in CaC2 and also known to be a harmful heavy metal. Post-harvest spoilage of both Musa species began on the fourth day after exposure to CaC2, whereas spoilage was not reported within nine days for fruits exposed to other ripening procedures. With the accumulation of arsenic and the early post-harvest spoilage of banana fruits due to CaC2 exposure, the local use of CaC2 for fruit ripening should be discouraged. We recommend the use of nylon and sack bags as well as exposure to sunlight because of longer shelf life and minimal effects on fruit quality.
Introduction
Consumption of fruits is promoted for human nourishment and wellbeing as a source of essential vitamins, amino acids, minerals, dietary fiber, antioxidants as well as chemicals that enhance cardiovascular health, liver function, muscle development and metabolism (Wargovich, Citation2000). Naturally, during fruit ripening, fruits release ethylene and increase cellular respiration (Moirangthem and Tucker, Citation2018) and two changes, for example, softness and sweetness, that is flavor, accompany it. The characters of fruits change to make them increasingly appealing to potential shoppers and consumers (Grierson, Citation2013). Natural fruit ripening may take time. Thereby, potentially affecting economic gains of fruit vendors and often motivate artificial ripening strategies, which have existed for centuries. In other to mimic nature, some techniques for induced fruit ripening additionally includes the use of ethylene as a ripening agent. Banana fruits are most desirable when ripe and are often induced to ripen faster. Ethylene is the hormone that triggers ripening but commercial application is relatively expensive and requires an enclosed chamber provided with adequate ventilation to prevent carbon dioxide (CO2) accumulation. The accumulation of CO2 in the process can competitively bind with ethylene receptors through transcriptional regulation and retard the ripening process (Clendennen et al., Citation1997; Xiao et al., Citation2013). Besides the use of ethylene gas, acetylene gas produced from calcium carbide (CaC2), ethephon, propylene, glycol, lamp fuel and ethanol are commercial ripening agents with wide applications, varied effectiveness and effects on fruit quality and safety (Dhembare, Citation2013; Maduwanthi and Marapana, Citation2019; Sazedur et al., Citation2014). However, in other to reduce cost these days calcium carbide is normally utilized for faster ripening. When calcium carbide application is exposed to moisture, acetylene gas is discharged, which has close qualities with ethylene (Ur-Rahman et al., Citation2008). Direct acetylene inhalation diminishes oxygen supply to the brain and causes hypoxia (Fattah and Ali, Citation2010). Calcium carbide distresses the mucosal tissue of the stomach area and reports of stomach issue in the wake of eating carbide-ripened mangoes have been recorded (Siddiqui and Dhua, Citation2010).
In Nigeria, fruit vendors covertly ripen banana fruits in encased vessels where an enormous amount of calcium carbide is added and sprinkled with water before concealing such vessels. This triggers the arrival of acetylene for ripening procedure in fruits, an inflammable gas including the danger of fire perils (Abhishek et al., Citation2016). This method is dangerous given the fact that CaC2 contains arsenic hydride and phosphorus hydride impurities (Abhishek et al., Citation2016; Sazedur et al., Citation2014), and also given the fact that these traders just simply add CaC2 into ripening vessels without recourse to concentrations required for safer outcomes. Other ripening procedures include bagging. Bagging strategy ensures and upgrades the visual nature of fruit by advancing skin coloration and diminishing blemishes, however, changes the miniaturized scale condition for fruit advancement, which can have various valuable impacts on inward fruit quality (Sharma et al., Citation2014). The disadvantage associated with bagging include diminished fruit size and weight due to low light transmittance of the bags (Arakawa et al., Citation1994; Hussein et al., Citation1994; Xu et al., Citation2008), and this discourages several vendors from indulgence in this method. This, therefore, leaves the use of carbide as the most commonly used method for induced fruit ripening. Compared to other common fruit ripening methods adopted by fruit vendors in Nigeria, to what extent is the reliance on CaC2 effective for ripening? Based on the availability of impurities in CaC2, is the accumulation of arsenic in the CaC2 ripened fruit endocarp possible? The outcome of this study will contribute to creating awareness about the implications of traditional fruit ripening methods on fruit quality and value. The objective of this study is to ascertain the extent to which local ripening agents such as the use of calcium carbide, hot water, sunlight, normal ripening, nylon bag and sack bags have on edibility, appearance, and nutritional value of banana fruits. We also aim to determine the amount of calcium carbide impurities (arsenic) in the banana fruits ripened with the compound.
Materials and Methods
Selection of Ripening Methods
Selection of methods adopted for ripening in this study was based on a reconnaissance survey of banana farmers, sellers and venders in Nigeria. These were independently visited and were interviewed via questionnaires on the various methods adopted for ripening banana (Supplementary Table 1). A total of 43 respondents were selected for the survey. The Mean item score was adopted in ranking which methods of ripening were most utilized by the respondents visited. The ranking was based on the premise that the factor with the highest mean item score was ranked as 1st, and then the others follow in descending order.
Where R is the rating per column, Ri = Number of each of the rating scale point. Also given that F is defined in the study as the sample size per rating, Fi = Frequency of each questionnaire item. N is the total sample size or number of respondents. i = 1, 2, 3 … n is the number of rows. A 5-point Likert scaling was adopted for data collection. This was to the extent that the 5 rating point was when the respondent strongly agreed that the suggested ripening method was most commonly adopted and the 1 rating point when the respondent strongly disagreed. The appropriate decision on results based on the mean was achieved through comparison with the theoretical cutoff mean MC. The cutoff mean of the scales (MC) = (1 + 2 + 3 + 4 + 5)/5 = 3. Thus
That is, any item in the instrument which had a mean equal to or higher than 3.0 was accepted while any mean score below 3.0 was rejected.
Collection of Banana
Two species of matured unripe banana (Musa acuminata Colla and M. balbisiana Colla, Musaceae) bunches (approximately 14 kg each) were collected from a plantation in Benin City, Southern Nigeria. As soon as these fruits were removed from parent plants (approximately 85 days after flowering), they were immediately taken to the Laboratory and subjected to experimental ripening procedures. Qualitative assessment of the banana epicarp after harvest suggest some of the peels had few spots or no spots (Supplementary Table 2).
Exposure of Banana to Ripening Conditions
Seventeen mature unripe banana fingers weighing 2 kg were exposed to different ripening conditions in the study. Calcium Carbide (CaC2) was prepared in two different forms (wet and dry). For dry CaC2, 2, 5 and 10 g CaC2 per 2 kg fresh weight (FW) of banana was weighed and placed in the middle of a plastic container (58 cm long and in diameter 42 cm) containing the banana fruits. For wet CaC2, the same quantity of CaC2 was measured and transferred into 100 ml of water and placed in a container of similar dimension as the dry CaC2. The banana was exposed to each quantity of the dry and wet CaC2 for 48 hours and then kept at room temperature (23–25 °C) on a sterile laboratory bench. During ripening by exposure to CaC2, care was taken not to touch the banana and the containers were immediately covered. Hot water ripening method involved the use of boiled 3.5 liters of water, which was allowed to cool to a temperature of 65 °C. Then 2 kg FW of banana was placed in the hot water for 30 seconds and then kept under ambient room temperature on a sterile laboratory bench. The use of sack bag was done at room temperature. The bananas were placed in the bags, covered properly to limit the entry of oxygen and then maintained under room temperature (24.5–27.5 °C) and relative humidity (48%) for nine days with 2 hours interval for observation and data collection. The same was done for bananas bagged in polyethylene materials. The respondents believed that nylon materials hardly allowed the inflow of oxygen. According to locals, this was the basis for ripening. For ripening in the sun, banana fruits were placed in an open field under the direct scorching heat of sunlight from dawn to dusk and then returned to the laboratory dry storage facility at dusk, which is maintained at room temperature and humidity. Benin City (6.33 °N and 5.62 °E) receives an average of 12 hours of sunlight every day and a global average daily solar radiation of 17.44–12.50 MJ/m2 and average daily temperature of 26.5 °C (Njoku et al., Citation2018). The banana fruits exposed to the different ripening conditions were examined daily. The control group was not subjected to any form of treatment during the study period. Fruit firmness (softness), fruit color codes and spoilage were determined according to methods reported in Vilcarromero (Citation2005) and Soltani et al. (Citation2010) where they investigated the quality of banana during ripening and the characteristics of surface color.
Determination of Total Phenol Content
Total phenol content was measured using the Folin Ciocalteu reagent method adopted from McDonald et al. (Citation2001) and Kopjar et al. (Citation2009). About 1 ml of the banana endocarp sample extract was mixed in 3 ml of distilled water and 0.5 ml of Fe and C reagent (Folic cicatol reagent) was added. This was allowed to stand for 3 minutes and then a 2 ml 20% of Sodium Carbonate was added before mixing every part properly and allowed to stand. This resulted in a blue colored solution and the absorbance values were determined at 650 nm using a UV visible spectrophotometer. Catecholcol or gallic acid was used in running the standard and preparing the standard curve. The total phenol content was calculated from the standard curve.
Determination of Ascorbic Acid Content
Total ascorbic acid content was determined using the 2,4-dinitrophenylhydrazine method (Kapur et al., Citation2012). The method relies on the oxidation of ascorbic acid to hydroascorbic acid by bromine water in the presence of acetic acid. About 1.5 ml of 4% trichloroacetic acid was added to 0.5ml of the sample extract. This was followed by adding 0.5 ml of DNPH, a drop of 10% Thiourea and boiling at 37ºc of the sample extract for 3hrs was carried out. It was allowed to cool and 2.5 ml of 85% H2SO4 was added, and thereafter allowed to stand for 30 minutes, before taking absorbance readings at 540 nm using a UV visible spectrophotometer.
Determination of Total Sugar Content
Total sugar content in the banana samples was measured following the phenol-sulfuric method (Dubois et al., Citation1956). About 0.1 ml of sample extract was pipetted and then to 1 ml with water was added to it, to make it up. Thereafter, 1 ml of phenol solution and 5 ml concentrated H2SO4 was added to the sample extract before boiling for 20 minutes in a water bath and taking absorbance readings at 490 nm using the spectrophotometer.
Determination of Arsenate in Banana Samples
Arsenate content of the calcium carbide treated banana fruits were determined using the atomic absorption spectrophotometric (AAS) Model-Solaar 969 Unicam Series and air acetylene flame. The digestion process was carried out by following the method of Radojevic and Bashkin (Citation1999). Aqua regia was used by measuring 75 ml of concentrated HCl and 25 ml concentrated HNO3 into 100 ml (3:1) volumetric flask. Then 1 g each of the banana endocarps from samples that were ripened with calcium carbide samples was placed in a beaker and 10 ml aqua regia added before leaving it to stay for a day to dissolve completely after which 90 ml of distilled water was added before filtering with Whatman filter paper, which was then taken to the AAS for the determination of As (Arsenic).
Data Analysis
The study adopted a completely randomized design with three replicates per treatment. The experimental data were subjected to analysis of variance and mean comparison using SPSS statistical software version 21 for Windows.
Results
We examined the effects of calcium carbide, hot water priming, sacking, nylon bagging and exposure to sunlight on fruit quality, safety and spoilage of banana. The methods were selected through a recognizance survey aimed at documenting the diverse traditional fruit ripening practices associated banana (Supplementary Table 1). Results of the survey showed that the most prominently used method of ripening was bagging with sack bag (Supplementary Table 1). However, the use of calcium carbide was also prominent. Most of the respondents were afraid to give information on the use of carbide; probably they already knew that it is unhealthy. The information provided was given on the grounds of anonymity.
shows the measurement of ripening period based on epicarp (peel) colors and number of days after exposure to different local induced ripening conditions for M. acuminata () and M. balbisiana (). The fruits of M. balbisiana were majorly forest green at harvest. At the second day, the banana exposed to hot water acted a khaki green color, which was prominent for the entire 9 days. However, in CD2, CD5, CD10, CW2, CW5 and CW10, ripening had begun in the second day, wherein the banana epicarp showed lime green color. Ripening was fully achieved by the 3rd day after harvest wherein the color of the banana was completely yellow. However, when compared with the control, the color of the banana did not turn yellow until the 6th day in which case, one would say the fruit ripening physically may have been achieved in control at the 6th day after harvest as compared to the 3rd day after harvest in the fruits exposed to CaC2. For M. acuminata, results showed that ripening (complete yellowness) was achieved earlier than in M. Balbisiana on the 3rd day for fruits exposed to CaC2. However, for the control, complete yellowness was achieved on the 6th day. Supplementary Table 2 shows the physical observation on the banana skin immediately after harvest and after exposure to experimental treatments. On the day of harvest, the skin was entire, which meant that there were no spots or blemish on the banana skin. Whereas, whenever it is reported that the skin is spotted, it meant that they were dark legions on the skin. On the very day of harvest, the skin was entire, however from the 3rd day, some parts of the skin showed spottiness, however for those banana fruits that were exposed to CaC2, the skin was entire and they were no sign of dark legions on the banana epicarp. However, for those banana fruits that were either placed in the sun or placed in a sack bag or nylon bag after 3 days, there were signs of dark necrotic legions on the skin. This may be as a result of injury particularly whenever the banana was been carried to the field to be placed under the sun or the injury may have occurred inside the bag where they were kept. The same observation regarding the entireness of the skin as observed in M. acuminata was also observed in M. balbisiana.
Figure 1. Measurement of the ripening period of banana using the epicarp color and number of days after exposure to different local induced ripening techniques of (a) Musa acuminata and (b) Musa balbisiana.
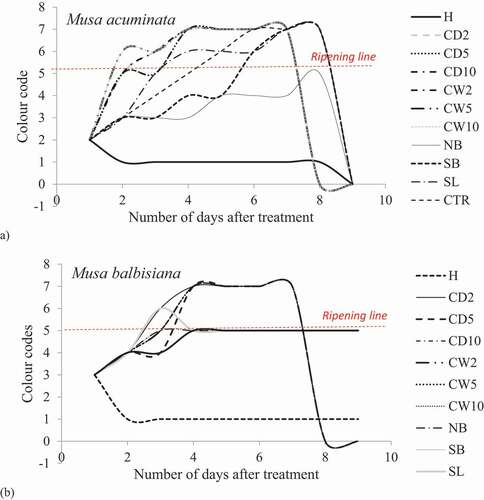
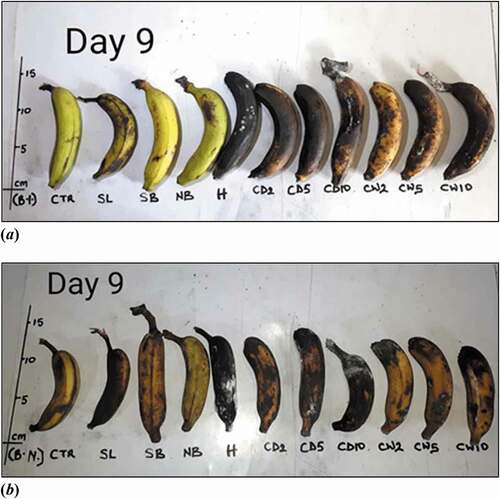
show M. acuminata and M. balbisiana fruits at 3 days after exposure to the different local induced ripening treatment conditions. The banana exposed to individual treatments was placed side by side against the control for proper comparison. The same has been presented for M. acuminata and M. balbisiana fruits at 6 days after exposure to the different local induced ripening treatment conditions (Supplementary Figures 1 and 2). M. acuminata and M. balbisiana fruits at 7 days (). At the 9th day after exposure to the various experimental treatments, it could be observed that all bananas exposed to CaC2 treatments as well as hot water treatments had significantly decayed whereas M. acuminata still appeared yellow-green although ripe. Those exposed to CaC2 treatment were already rotten (). The same was presented for M. balbisiana ().
Figure 2. Musa acuminata at 3 days after exposure to different local induced ripening treatments
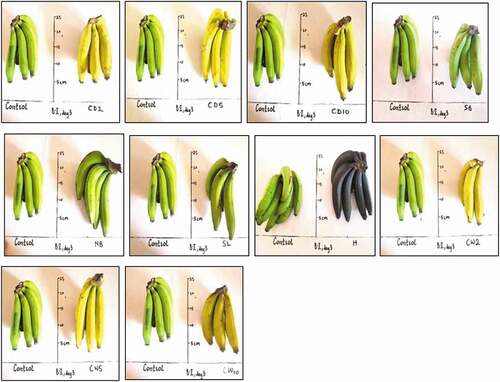
Figure 3. Musa balbisiana at 3 days after exposure to different local induced ripening treatments
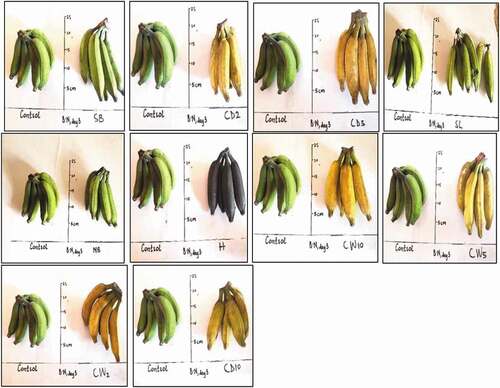
Figure 4. Musa acuminata at 7 days after exposure to different local induced ripening treatments
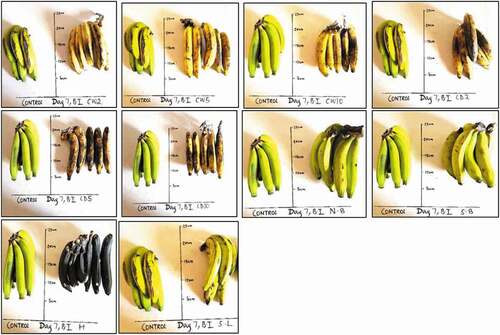
Figure 5. Musa balbisiana at 7 days after exposure to different induced local ripening treatments

Figure 6. (a) Musa acuminata and (b) Musa balbisiana at 9 days after exposure to different local induced ripening treatments
Further, the study attempted to investigate the degree of softness of each of the banana species exposed to the various ripening regimes (). Endocarp firmness is a useful indicator of banana ripeness. For M. acuminata, softness was achieved on the 5th day after harvest for banana species that were exposed to hot water treatment. Softness was achieved one day after exposure to experimental treatment for all M. acuminata species exposed to CaC2 either dry or wet. The degree of softness increased up till the 3rd day and then by the 6th day, it attained a 3+ softness. Whereas, in M. balbisiana, the degree of softness was 1+ for species exposed to CaC2 one day after treatment but this worsened and increased to 3+ degree of softness at 7 days after exposure. The banana that was ripened by placement in nylon bags were only 1+ soft 5 days after exposure to this method and could only attain a 2+ degree of softness at both 8th and 9th day after exposure to ripening methods. The control M. acuminata and M. balbisiana showed a 1+ degree of softness at 4th day after exposure thus increased to 2+ degree of softness on the 9th day for Musa acuminata and the 6th day for M. balbisiana. shows the degree of spoilage due to the ripening of the banana fruits. Results showed that for banana species exposed to hot water treatment, the fruits got bad as from the 2nd day upon exposure. However, for the controlled fruit as well as fruit exposed to nylon bag treatment, sack bag treatment and ripening through sunlight there was no report of spoilage up till 9 days after exposure to ripening measures for both M. acuminata and M. balbisiana. However, as from the 4th day after exposure to ripening measures using CaC2 either wet or dry, fruit spoilage was reported. The degree of spoilage was worsened on the 6th day for M. acuminata and the 7th day for M. balbisiana.
Table 1. Number of days and degree of softness of the fruits after exposure to the experimental treatments
Table 2. Number of days and the degree of spoilage observed in the fruits after treatment with different local induced ripening conditions
Total phenols content in the banana after exposure to different local induced ripening conditions is for both the second and fifth day (). Results showed that for M. acuminata, the total phenols were 115.65 mg/100 g fresh weight (FW) in the second day after exposure to hot water ripening measure and this significantly increased to 413.55 mg/100 g FW on the 5th day. Results showed that on the 2nd day after exposure to ripening measures by either using dry or wet carbide, total phenols ranged from 278.74 to 332.94 mg/100 g FW at the 2nd day after exposure to treatment or this rose on the 5th day from 380 to 911.21 mg/100 g FW. Observably, total phenols were significantly higher in the banana fruits that were ripened using carbide for both the second and fifth day. For M. balbisiana, total phenols were also higher in the CaC2 exposed banana fruits compared to banana fruits ripened by other measures. Overall, the results for total phenol content suggest that as ripening progress the phenol content increases.
Table 3. Total phenols content of banana after exposure to different local induced ripening conditions
The ascorbic acid content of the ripened banana were significantly higher in the fruits ripened by CaC2 (). At 2 days after exposure to CaC2, the ascorbic acid content of M. acuminata ranged from 113.79 to 331.03 mg/100 g FW, whereas ascorbic acid content in M. balbisiana ranged from 154–363.80 mg/100 g FW. By comparing ascorbic acid levels for 2nd and 5th days after exposure to ripening measures, the result showed significant increases for CaC2 exposed fruits from the 2nd to the 5th day after exposure in both banana species. However, there were no increases in ascorbic acid levels for 2nd and 5th days after exposure into ripening measures for those fruits that were ripened using a hot water treatment, coverage in nylon bags and sack bags, as well as banana exposure to sunlight.
Table 4. The ascorbic acid content of banana after exposure to different local induced ripening conditions
Total sugar content in the ripened banana was 4.84 g/100 g in the control for M. acuminata. This significantly rose to 12.45 g/100 g 5 days after treatment (). For M. balbisiana, there were no significant differences in total sugar content of the ripened fruits as total sugar ranged from 15.85 to 17.45 g/100 g in 2nd and 5th days after harvest. Similarly, total sugar in M. acuminata exposed to hot water treatments increased from 6.18 g/100 g on the second day after harvest to 29.70 g/100 g 5 days after harvest. Results generally showed that significant increases in total sugar content were reported for fruits exposed to CaC2 whether dry or wet for M. acuminata. However, in M. balbisiana results showed that there were no significant differences in total sugar content between the second and fifth day after exposure to CaC2 whether wet or dry, as total sugar content values ranged from 41.41 to 47.91 g/100 g FW in the 2nd day after harvest to between 59.87 and 69.13 g/100 g FW 5 days after harvest of M. balbisiana.
Table 5. Total sugar concentration in banana after exposure to the experimental conditions
shows the arsenic concentration in the fruit endocarp, whereas the concentration of arsenate in raw calcium carbide was 10.12 mg/kg. Arsenic concentration in ripe M. acuminata ranged from 0.026 to 0.083 mg/kg in the carbide exposed plants compared to non-detectable values in the control. Similarly, in M. balbisiana, arsenic concentration was also non-detectable. However, in those fruits exposed to CaC2 wet or dry, arsenate concentration ranged from 0.084 to 0.164 mg/kg.
Table 6. Arsenic concentration in fruit endocarp after exposure to different calcium carbide concentration to induce ripening
Discussion
We have studied the rate of spoilage, fruit quality and safety of local induced fruit ripening techniques for banana and found varying effects associated with the different methods. The experiment of Hartshorn (Citation1931), recognized the impacts of acetylene on the ripening procedure of banana through a consistent yellow fruit color, great flavor, medium starch content, and a soft texture. During ripening, textural changes and fruit softening are brought about by depolymerization and solubilization of cell wall segments and loss of cell structure (Maduwanthi and Marapana, Citation2019). This in line with the observations made in this study albeit with different textural quality determined by the ripening techniques. Changes in turgor pressure, a decrease of cell wall polysaccharides and enzymatic reduction of starch are determinant systems of fruit softening. Cell-wall polysaccharides experience solubilization, de-esterification, and depolymerization during ripening (Clendennen et al., Citation1997; Maduwanthi and Marapana, Citation2019). In the present study, the rate of fruit softening was enhanced with the application of calcium carbide, a substance that mimics ethylene-based ripening by releasing acetylene, which reacted with moisture. In climacteric fruits like bananas, which contain a high measure of starch in its flesh, enzymatic hydrolysis of starch is a central factor in fruit yellowing. From this examination, softness was accomplished one day after introduction to experimental treatment for all M. acuminata species presented to CaC2 either dry or wet. The fruits attained the highest level of softness (3+) presented for the study. However, in M. balbisiana, the level of softness was 1+ for species presented to CaC2 one day after treatment. Notwithstanding, softness levels increased to 3+ at 7 days after exposure. During ripening, there is an expansion in the breakdown of starch inside the fruit, and a comparing increment in the measure of basic sugars, which taste sweet, for example, sucrose, glucose, and fructose and a reduction in acidity as the fruit ripen and a lessening in bitter plant substances, for example, alkaloids (Moirangthem and Tucker, Citation2018). This is in line with findings from this study as total sugar content in the ripened banana, was 4.84 g/100 g in the control for M. acuminata, which ascended significantly to 12.45 g/100 g 5 days after treatment (). Again, results from this present examination uncovered that absolute sugar in M. acuminata presented to hot water medications expanded from 6.18 g/100 g at day 2 after harvest to 29.7 g/100 g 5 days after harvest as well as for results showing significant increases in total sugar content reported for fruits exposed to CaC2 whether dry or wet for M. acuminata. Ascorbic acid values of both ripened banana species in this study where fundamentally higher in the fruits ripened by CaC2. Thompson et al. (Citation2019) revealed that ascorbic acid content of ‘Dwarf Cavendish’, ‘Rasabale’ and ‘Rajabale’ increased during ripening at 20°C for 21 days and diminished marginally up to 35 days.
The use of calcium carbide imposes health implications on both the users and the consumers of the CaC2-ripened bananas. Acetylene gas exposure by the fruit vendors brings about migraine, vertigo, discombobulation, wooziness, seizure, and even unconsciousness (Maduwanthi and Marapana, Citation2019). Moreover, with calcium carbide ripening, arsenate impurities were found present in banana fruits. Studies from this examination uncovered that arsenic concentration in ripe M. acuminata ran from 0.026 to 0.083 mg/kg in the Carbide-exposed plants contrasted with non-perceptible values in the control. Thus, in M. balbisiana, arsenic concentrations were likewise non-perceptible. Arsenic impurities stored in the banana samples are in all probability because of the fume discharges radiated when the calcium carbide is applied on the fruit, giving rise to its non-detectable values as obtained from this study with M. Balbisiana. However, for the fact that the banana fruit skin has an extraordinary capacity for moisture retention of these disintegrated impurities during the release of acetylene gas from Calcium Carbide, arsenic impurities were imbibed by both banana species. Calcium carbide ripened fruits are soft, have a decent peel color development but with lesser flavor (Surbhi et al., Citation2016). Moreover, these heavy metals are non-biodegradable and have long natural half-lives just as the capacity for aggregation in various organs of the body prompting undesirable issues (Ogunkunle et al., Citation2014). Importantly, the possible adverse effects associated with some of the heavy metals, including arsenic, have been widely reported in anemia, hepatotoxicity, renal failure and gastrointestinal disorders, gastrointestinal disturbances such as nausea, abdominal cramp, vomiting and diarrhea, central nervous system disorder leading to permanent disability (Tchounwou et al., Citation2012; Thirulogachandar et al., Citation2014). Most importantly is the fact that arsenic exposure has been found to exert cytotoxic, mutagenic and carcinogenic effects to major tissues and organs of the body (Tchounwou et al., Citation2012).
In this study, the use of hot water discolored the bananas. It induced a khaki green color, which was prominent for the whole 9 days and never encouraged induced banana fruit ripening but rather accelerated spoilage effects of both banana species at day 2 and day 3 for M. balbisiana and M. acuminata respectively. The use of hot water treatment raised glutathione, ascorbic acid, free phenolic and flavonoids values during storage and quality parameters including peel color and pulp firmness demonstrated that boiling water treatment postponed banana fruit ripening (Ummarata et al., Citation2011). This is in line with our observation as hot water treatments delayed ripening of both banana samples. We also observed that as ripening progresses due to induced ripening conditions, it significantly increases total phenol and ascorbic acid contents (). This is in agreement with the report of Fernando et al. (Citation2014) that the chemical composition of banana including total phenol and ascorbic acid contents increases during ripening. In another study, Vu et al. (Citation2019) noted that the behavior of phytochemicals during ripening of banana subjected to induced ripening condition could be used as a basis of classification for the ripening stage. Although the mechanism for phenol accumulation during ripening in banana is not well understood, it is suggested that the process is regulated by dopamine and senescence cues (Teeranud et al., Citation2005). Brown colored spots on the skins of bananas is a typical phase of ripening that generally happens when the skin has changed color from green to yellow or completely yellow contingent upon the genotype (Thompson et al., Citation2019). This concurs with the result on spottiness from this examination as all samples of both banana species developed spots at different days during the investigation.
In conclusion, this study ascertained the extent to which each of the ripening agents used in this study affects banana fruits and found that hot water priming and calcium carbide treatments had more irreversible consequences on the fruits taking cognizance of edibility, appearance, and nutritional value. The presence of arsenic can be used as a tool to detect banana fruits ripened with calcium carbide. In line with Chandel et al. (Citation2018) we recommend the soaking fruits in 2% sodium carbonate solution for 12 hours to remove arsenic from calcium carbide ripened fruit. However, consumers are often unaware of the ripening method used by vendors and vendors are often unaware of the risk associated with using calcium carbide to ripen fruits or uneducated about the safe application of sodium carbonate to reduce the risk of arsenic poisoning from consuming the fruits. Hence, the government through agriculture extension officers and approved task force as well as non-governmental organizations should embark on a public campaign to discourage the use of calcium carbide as a fruit-ripening agent for the protection of human health, sustainable environmental development. From our results, we recommend the use of nylon and sack bags as well as exposure to sunlight because of the longer shelf life, cheaper cost and minimal effects on fruit quality.
Authors’ Contributions
BI and OO conceived the research questions. BI, OO and MO developed the research plan. OO carried out the experiments. BI and OO analysed the data and interpreted the results. OO wrote the first manuscript draft. BI and MO revised the manuscript. All the authors approved the final version and publication of the work.
Acknowledgments
The authors are grateful to Mrs. Ruth Atoe-Ebiye, postgraduate student of the Department of Plant Biology and Biotechnology, University of Benin, Nigeria and Mr. Pieter-Jan Loveniers of the Faculty of Bioscience Engineering, University of Ghent, Belgium for their technical assistance during the study.
Disclosure Statement
The authors declare there is no competing interest.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Literature Cited
- Abhishek, R.U., H.N. Venkatesh, K. Manjunath, and D.C. Mohana. 2016. Artificial ripening of fruits—misleading ripe and health risk. Everyman’s Sci. 6:1–7.
- Arakawa, O., N. Uematsu, and H. Nakajima. 1994. Effect of bagging on fruit quality in apples. Bull. Faculty Agric. Hirosaki Univ. 57:25–32.
- Chandel, R., P.C. Sharma, and A. Gupta. 2018. Methods for detection and removal of arsenic residues in calcium carbide ripened mangoes. J. Food Process. Preserv. 42(2):e13420. doi: https://doi.org/10.1111/jfpp.13420.
- Clendennen, S.K., P.B. Kipp, and G.D. May. 1997. The role of ethylene in banana fruit ripening. In: A.K. Kanellis, C. Chang, H. Kende, and D. Grierson (eds..). Biology and biotechnology of the plant hormone ethylene. NATO ASI Series (3 high technology). Vol. 34. p. 141–148. Germany: Springer Dordrecht.
- Dhembare, A.J. 2013. Bitter truth about fruit with reference to artificial ripener. Arch. Appl. Sci. Res. 5:45–54.
- Dubois, M., K.A. Gilles, J.K. Hamilton, P.A. Rebers, and P. Smith. 1956. Colorimetric method for determination of sugars and related substances. J. Analyzing Chem. 28:350–356. doi: https://doi.org/10.1021/ac60111a017.
- Fattah, S.A., and M.Y. Ali. 2010. Carbide ripened fruits - a recent health hazard. Faridpur Med. Coll. J. 5(2):37. doi: https://doi.org/10.3329/fmcj.v5i2.6816.
- Fernando, H.R.P., V. Srilaong, N. Pongprasert, P. Boonyarithongchai, and P. Jitareerat. 2014. Changes in antioxidant properties and chemical composition during ripening in banana variety ‘Hom Thong’ (AAA group) and ‘Khai’ (AA group). Int. Food Res. J. 21(2):749–754.
- Grierson, D. 2013. Ethylene and the control of fruit ripening, p. 43–73. In: S. Grahman, P. Mervin, G. James, and T. Gregory (eds.). The molecular biology and biochemistry of fruit ripening. Blackwell Publishing Ltd, Boston.
- Hartshorn, R. 1931. Some effects of acetylene on the ripening processes of bananas. Plant Physiol. 6(3):467. doi: https://doi.org/10.1104/pp.6.3.467.
- Hussein, A.A., A.G. Abdel-Rahman, and R.B. Ahmed. 1994. Effectiveness of fruit bagging on yield and fruit quality of pomegranate (Punica granatum L.). Ann. Agric. Sci. 32:949–957.
- Kapur, A., A. Haskovic, A. Copra-Janicijevic, L. Klepo, A. Topcagic, I. Tahirovic, and E. Sofic. 2012. Spectrophotometric analysis of total ascorbic acid content in various fruits and vegetables. Bull. Chem. Technol. Bosnia Herzegovina 15:40–43.
- Kopjar, M., V. Pilizota, J. Hribar, and M. Simcic. 2009. Total phenol content and antioxidant activity of water solutions of plant extracts. Croatia J. Food Sci. Technol. 1(1):1–7.
- Maduwanthi, S.D.T., and R.A.U.J. Marapana. 2019. Induced ripening agents and their effect on fruit quality of banana. Int. J. Food Sci. 2019: 8. Article ID 2520179. doi:https://doi.org/10.1155/2019/2520179.
- McDonald, S., P.D. Prenzler, M. Autolovich, and K. Robards. 2001. Phenolic content and antioxidant activity of olive extracts. Food Chem. 73:73–84. doi: https://doi.org/10.1016/S0308-8146(00)00288-0.
- Moirangthem, K., and G. Tucker. 2018. How do fruits ripen? Front. Young Minds. 6:16. doi: https://doi.org/10.3389/frym.2018.00016.
- Njoku, M.C., I. Ofong, N.V. Ogueke, and E.E. Anyanwu. 2018. Characterization of sky conditions at Benin city and Owerri in Nigeria. J. Fundam. Renewable Energy Appl. 8(5):1000270. doi: https://doi.org/10.4172/2090-4541.1000270.
- Ogunkunle, A.T.J., O.S. Bello, and O.S. Ojofeitimi. 2014. Determination of heavy metal contamination of street-vended fruits and vegetables in Lagos state, Nigeria. Int. Food Res. J. 21(5):1725–1730.
- Radojevic, M., and V.N. Bashkin. 1999. Practical environmental analysis, p. 342–466. Royal Society of Chemistry, Cambridge, UK.
- Sazedur, R.A.H.M., M.N. Islam, Y.I. Mollik, F.P. Abdullah, M. Mehnaz, S.A. Sabrina, and S.K. Mohidus. 2014. Nutrition value analysis of artificially ripened banana (bari-1 hybrid banana, Musa spp.), p. 172–176. Proceedings of the International Conference on Chemical Engineering, ICChE2014, Dhaka, Bangladesh, 29–30 Dec.
- Sharma, R.R., S.V.R. Reddy, and M.J. Jhalegar. 2014. Pre-harvest fruit bagging: A useful approach for plant protection and improved post-harvest fruit quality – A review. J. Hortic. Sci. Biotechnol. 89:101–113. doi: https://doi.org/10.1080/14620316.2014.11513055.
- Siddiqui, M.W., and R.S. Dhua. 2010. Eating artificial ripened fruits is harmful. Curr. Sci. 99(12):1664–1668.
- Soltani, M., R. Alimardani, and M. Omid. 2010. Prediction of banana quality during ripening stage using capacitance system. Aust. J. Crop Sci. 4(6):443–447.
- Surbhi, G., S. Mahak, and B. Barkha. 2016. Comparative study on the ripening ability of artificial ripening agent (Calcium Carbide) and natural ripening agents. Global J. Biol. Agric. Health Sci. 5(2):106–110.
- Tchounwou, P.B., C.G. Yedjou, A.K. Patlolla, and D.J. Sutton. 2012. Heavy metals toxicity and the environment. EXS 101:133–164.
- Teeranud, R., S. Jingtair, U. Yoshinori, A. Kazuhiro, and C. Kazoo. 2005. Changes in concentration of phenolic compounds and polyphenol oxidase activity in banana peel during storage. Food Preserv. Sci. 31(3):111–115. doi: https://doi.org/10.5891/jafps.31.111.
- Thirulogachandar, M.E., M. Rajeswari, and S. Ramya. 2014. Assessment of heavy metals in Gallus and their impacts on human. Int. J. Sci. Res. Publ. 4(6):1–8.
- Thompson, A.K., S. Supapvanich, and J. Sirison. 2019. Banana ripening, science and technology, p. 150, Springer Briefs in Food, Health and Nutrition.
- Ummarata, N., T.K. Matsumoto, M.M. Wall, and K. Seraypheap. 2011. Changes in antioxidants and fruit quality in hot water-treated ‘Hom Thong’ banana fruit during storage. Sci. Hortic. 130(4):801–807. doi: https://doi.org/10.1016/j.scienta.2011.09.006.
- Ur-Rahman, A., F.R. Chowdhury, and M.B. Alam. 2008. Artificial ripening: What we are eating. J. Med. Chem. 9(1):42–44.
- Vilcarromero, F.A.M. 2005. Characterization of surface appearance and color in some fruits and vegetables by image analysis, p. 123. Ponticifia Universidad Catolica de Chile, Escuela de Ingenieria.
- Vu, H.T., C.J. Scarlett, and Q.V. Vuong. 2019. Changes in phytochemicals and antioxidant capacity of banana peel during the ripening process; with and without ethylene treatment. Sci. Hortic. 253:252–262. doi: https://doi.org/10.1016/j.scienta.2019.04.043.
- Wargovich, M.J. 2000. Anticancer properties of fruits and vegetables. HortScience. 35:573–575. doi: https://doi.org/10.21273/HORTSCI.35.4.573.
- Xiao, -Y.-Y., J.-Y. Chen, J.-F. Kuang, W. Shan, H. Xie, Y.-M. Jiang, and W.-J. Lu. 2013. Banana ethylene response factors are involved in fruit ripening through their interactions with ethylene biosynthesis gene. J. Exp. Bot. 64(8):2499–2510. doi: https://doi.org/10.1093/jxb/ert108.
- Xu, C.X., H.B. Chen, R.Y. Huang, and Y.J. He. 2008. Effects of bagging on fruit growth and quality of carambola. Acta Hortic. 773:195–200. doi: https://doi.org/10.17660/ActaHortic.2008.773.28.
