ABSTRACT
The clonal propagation of T. cacao by somatic embryogenesis (SE) is a promising approach to multiply elite genotypes. Assessing clonal fidelity in plants regenerated from somatic embryos is the first step toward ensuring genetic uniformity in the mass production of planting material. This study assessed the genetic stability of cacao plantlets propagated by SE and conventional grafting for genotypes CCN51 and TSH565 using 13 SSR. The leaves of in vitro plantlets (IVL) were collected from 6-month-old plants and leaves of field plants (FPL) were selected from 3-year-old trees. The 13 analyzed loci revealed 25 alleles in genotype CCN51 and 24 alleles in genotype TSH565. The highest PIC value was observed for all SSR, only mTcCIR8 and mTcUNICAMP09 were intermediate, with PIC values of less than 0.250. IVL and FPL populations were genetically equal. According to the results, no differences in allelic composition were observed between FPL and IVL in each genotype. This indicates that plants propagated by SE did not show perceptible detriment to their genome with the used SSR. In addition, Jaccard’s coefficient showed more than a 91% similarity for TSH565 and 92% for CCN51. The UPGMA and PCA showed that the populations tended to group within two genotypes. The SSR results obtained do not exclude the occurrence of other changes in the nuclear genome. However, considering the morphological stability of in vitro propagated plants, the results indicate that the protocol used is suitable and efficient for large scale, true-to-type propagation of genotypes CCN51 and TSH565 for commercial purposes.
Introduction
Cacao (Theobroma cacao L.) is a neotropical tree fruit species distributed in tropical regions as cocoa crops, mainly in Africa and South America, where its beans are used to produce chocolate. Cacao is currently a commodity in global markets, and its most recent attractive capacity for public awareness is the health benefits acquired through consumption (Ajijah et al., Citation2016).
Despite cacao’s high growth in demand and use, there are currently no highly efficient systems to produce plant material for planting. When cacao is propagated clonally by cuttings or grafts, the result in terms of volume and production rate is low, in addition to an undesirable bushy growth pattern. In this sense, according to Thondaiman et al. (Citation2013), using plant tissue culture technology and molecular biology is required to accelerate cacao breeding programs.
Seeking to produce sufficient plant material for planting, in the last three to four decades, using large-scale propagation tools has become a serious approach to many economically important plant species like cocoa. For this reason, in vitro micro-propagation techniques using somatic embryogenesis have shown several potential advantages over conventional propagation methods (Nandhakumar et al., Citation2017). However, that is not always the case. There is a risk of producing so many mutant regenerants that the approach’s economic feasibility is compromised (Etienne and Bertrand, Citation2016). Maintaining the true-to-type nature of in vitro propagated plants in commercial and marketing processes is crucial for upholding certain agronomic and horticultural traits when using elite genotypes (Alizadeh et al., Citation2015).
Nevertheless, over long-term periods, plant micropropagation technology has a phenotypic and genetic variation of propagated plants known as somaclonal variation (Larkin and Scowcroft, Citation1981). This epigenetic phenomenon can remain quite stable for many generations (Kaeppler et al., Citation2000). Nowadays, the phenomenon of somaclonal variation still threatens the commercial viability of somatic embryogenesis technology (Butiuc-Keul et al., Citation2016). This variation implies genomic changes (large-scale deletions and gross changes in chromosome structure/number and directed and undirected point mutations) and epigenetic changes (histone acetylation, DNA methylation, chromatin remodeling, etc.) (Wang and Wang, Citation2012).
As reported by Rodríguez et al. (Citation2010), the scarcity and stochastic nature of genetic mutations represent a significant challenge for scientists seeking to characterize de novo mutation frequency at specific loci. Therefore, evaluating the genetic fidelity of plants multiplied in vitro has been a central theme in research. Molecular markers, an important tool to assess the genetic homogeneity of micro-propagated plants (Chittora et al., Citation2015), have been used to elucidate punctual changes and establish relationships through phylogenetic analysis. Something similar occurs with DNA methylation level, which is strongly related to somatic embryogenesis. Locus-specific modulations can alter genes, and their use mainly depends on the objective of research and type of tissue (Fehér, Citation2015). Molecular techniques like Restriction fragment length polymorphism (RFLP) (Devarumath et al., Citation2002), Methylation sensitive amplification polymorphism (MSAP) (Ghosh et al., Citation2017), Random Amplified Polymorphic DNA (RAPD) (Devi et al., Citation2017; El-Mahrouk et al., Citation2016; Moharana et al., Citation2018; Patil and Bhalsing, Citation2015; Singh et al., Citation2016), Amplified fragment length polymorphism (AFLP) (Coste et al., Citation2015; Maki et al., Citation2015; Onay et al., Citation2016; Scherer et al., Citation2015) and Inter simple sequence repeat (ISSR) (Alansi et al., Citation2017; Lata et al., Citation2016; Teeluck et al., Citation2016; Viehmannova et al., Citation2016), have been used to assess the genetic fidelity of many micro-propagated plants species
However, microsatellites, also known as simple sequence repeats (SSR) short tandem repeats (STR), or simple sequence length polymorphisms (SSLP), have shown to be very important in genetic fidelity assessment due to some desirable characteristics, such as high reproducibility, co-dominant inheritance nature, the enormous extent of allelic diversity, high abundance in organisms, and strong discriminatory power (Mithra et al., Citation2017).
SSR markers have previously been used to detect somaclonal variation chromosomes, and locus loss represents an additional source of genetic change – another advantage of this technique (Bandupriya et al., Citation2017; Bradaï et al., Citation2019; Chhajer and Kalia, Citation2016; Goyali et al., Citation2015). During PCR, reproducibility in the relative amplification efficiencies of alternate alleles may allow detecting allele loss or the presence of a new allele within a mutation chimera, even if they are present in relatively few cells (Mithra et al., Citation2017). Since parental and mutant allele amplifications are performed in the same reaction and with the same primers, directly comparing the parental genotypes’ allele peak profiles with that of the chimeric mutant may even allow a semi-quantitative assessment of mutant abundance.
Somaclonal variation continues to be presented as a phenomenon that threatens the commercial viability of somatic embryogenesis reproduction technology for cacao. Therefore, it is necessary to validate if the reproduction method allows maintaining the genetic characteristics of cacao populations to apply it as a mass reproduction method and overcome these uncertainties – either through molecular markers or markers sensitive to epigenetic methylation changes.
The main purpose of this study was to determine the genetic stability of two important cacao genotypes, CCN51 and TSH565, regenerated via somatic embryogenesis through SSR. The former, CCN51, is planted extensively and exhibits many attractive agronomic traits, such as high productivity and disease resistance. This cacao is particularly rich in fats, which defines it for its niche market. For example, CCN51 constitutes 36% of national cacao production in Ecuador (Vega and Beillard, Citation2016). Furthermore, genotype TSH565 is planted widely in several countries, mainly Brazil, due to its productivity, disease resistance, and traditional, fine-flavored bean quality (Bastos et al., Citation2019).
The research considers two populations: a population of in vitro plantlets (IVL) derived from somatic embryogenesis and a population of field plants (FPL) derived from grafting, which is the primary conventional multiplication method for cacao plants in Colombia. This work is the first report on genetic uniformity for the studied genotypes. The advances in somatic embryo production provide the basis for continuing breeding programs for the widely used CCN51 and TSH565 genotypes and developing their genetic transformation.
Materials and Methods
Plant Material Propagated by Grafting in the Field
Plants were collected between 2019–2020 in the field for cacao genotypes TSH565 and CCN51 from a 20-year-old clonal garden in Yariguíes farm (nursery and clonal garden certified by the Instituto Colombiano Agropecuario – ICA) owned by Compañía Nacional de Chocolates (CNCH) (Barrancabermeja, Santander, Colombia). Each plant from the clonal garden was identified and labeled with tree marking tapes (individual number and project information). After a careful visual inspection, at least 10 young leaves in perfect phytosanitary conditions were taken from a total of 20 cacao trees propagated by grafting for each genotype.
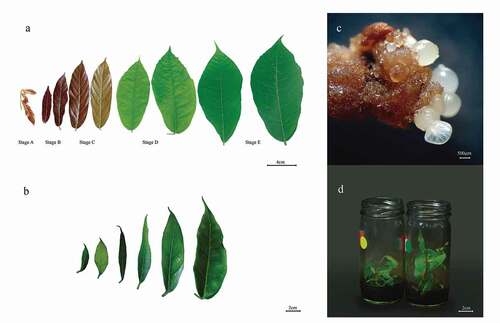
The young leaves were packed in tissue wipers, grouped in resealable plastic bags, and placed in coolers with dry ice to ensure the lowest possible temperature for transportation back to the Plant Physiology lab at the Universidad de Antioquia (Medellín, Antioquia, Colombia) for long-term storage at −80°C until processing (Thermo Scientific). To ensure analysis, cacao leaves were collected in the D stage (). This developmental leaf stage is identified 25 days after emergence, when elongation ceases and leaves accumulate chlorophyll, displaying a light green color (). Henao et al. (Citation2018a) suggest that the D stage of development in field leaves was efficient for extracting high-quality genomic DNA.
Plant Material Propagated by Somatic Embryogenesis
The CCN51 and TSH565 genotypes were propagated in vitro through somatic embryogenesis between 2019–2020. From previously marked clonal garden trees, floral buds were collected and stored in sterile basal DKW salts on the ice during transportation to the Plant Physiology and Plant Tissue Culture Laboratory, always maintaining the traceability of the plant material. The collection of flower buds was carried out several times since only 50% of the in vitro introductions respond to the embryogenic process. Flower buds were sterilized following the protocol reported by Urrea et al. (Citation2011). Staminodes and petals were extracted from the basal portion of the flower bud, and different phases of SE (induction, expression, maturation, conversion) were induced according to Henao et al. (Citation2018b) protocol (b-c). A pool of at least 40 cacao plantlets propagated by somatic embryogenesis was selected from each genotype for DNA extraction.
DNA Extraction
Total DNA extraction was achieved from in vitro grown plantlets (IVL) with three months in a MM6 medium, showing leaves from 2–5 cm length (). Leaves were selected and removed from the plant under sterile conditions in a laminar flow chamber. Likewise, for field plant leaves (FPL), 1 cm x 1 cm portions were cut with a scalpel, avoiding the removal of midrib tissue. This was performed in equally sterile conditions. The leaves were cut and immediately transferred to 2 ml tubes in liquid nitrogen and stored at −80°C until processing.
The protocol reported by Henao et al. (Citation2018a) was used to extract DNA from the FPL and IVL. The Power Plant® Pro DNA Isolation Kit MoBio (Qiagen) modified with an additional sorbitol buffer wash was successfully used to avoid mucilage, typical of cacao leaves. Mucilage is an abundance of polyphenols and polysaccharides. In summary, the sorbitol buffer was added to 0.1 g of macerated leaf tissue in Eppendorf tubes. The tubes were then placed in a cellular disruptor device (BeadBug™) for 5 minutes at 3,000 rpm. They were then heated to 65°C. Finally, samples were centrifuged for 10 minutes at 5,000 rpm, and the supernatant was ultimately discarded. This procedure was repeated twice, continuing with the other steps of the Power Plant® Pro DNA Isolation Kit MoBio protocol (Cat. no. 13400–50). DNA extraction experiments were performed using twenty biological replicates of FPL and IVL for each genotype. At least ten technical replicates were performed.
The integrity and concentration of obtained DNA were determined using NanoDrop™ (Thermo Scientific) spectrophotometer and later verified on 0.8% agarose gel stained with ethidium bromide (EB) (0.5 mg/ml) and visualized under a UV lamp. DNA quantity and purity were evaluated by measuring the A260/A280 and A260/A230 absorbance ratios.
SSR Analysis
From previous T. cacao genetic diversity analyses such as (Irish et al., Citation2010; Lanaud et al., Citation1999; Santos et al., Citation2012) were chosen thirteen SSR according to their high polymorphic information content (PIC): “mTcCIR6, mTcCIR8, mTcCIR11, mTcCIR15, mTcCIR25, mTcCIR26, mTcCIR33, mTcUNICAMP01, mTcUNICAMP02, mTcUNICAMP05, mTcUNICAMP09, mTcUNICAMP16, and mTcUNICAMP17” (). The primers were synthesized by Macrogen (Macrogen Humanizing Genomics, Seoul, Korea). For each SSR marker, the forward oligonucleotide (F) was labeled with a different fluorescent dye (6-FAM, HEX, and TAMRA): mTcCIR15, mTcCIR25, mTcUNICAMP05, mTcUNICAMP09 were labeled with 6-FAM (blue); mTcCIR6, mTcCIR26, mTcCIR33, mTcUNICAMP01, mTcUNICAMP16 were labeled with HEX (green); mTcCIR8, mTcCIR11, mTcUNICAMP02, mTcUNICAMP17 used TAMRA (Red).
Table 1. Theoretical characteristics of the 13 microsatellite markers (SSR) were used to assess genetic fidelity experiments
Each SSR was tested and amplified with the previously reported annealing temperature until its standardization (). As explained by Don et al. (Citation1991), a thermal touchdown amplification profile was used to set the exact banding temperature, reducing unspecific artifacts during replication. First was 5 min at 94°C, melting 40s at 94°C and annealing 40s at primer temperature. Second were ten cycles with a decrease of 1°C per cycle until it reached melting temperature and 40s at 72°C for extending. This was followed by using the standard annealing temperature of each primer 20 more times. Lastly, there was a final 10 min extension at 72°C, reaching 30 replication cycles. The enzyme Taq DNA Polymerase of Thermo Scientific (1 U/μl) was used. The PCR stock solution (20 μl) included 1.35 µl of DNA template (10 ng/μl), 1X PCR Thermo buffer, 2 mM dNTPs, 2.5 pmol each of forward (labeled) and reverse primers, 2 mM MgCl2, 0.5 ng/ml BSA (Bovine Serum Albumin), 0.1 μl Taq DNA polymerase and sterile water to complete the total volume of the reaction. Amplifications were made in a thermal cycler (LTCG Labocon 48–101). The amplified products were checked by 3% agarose gel electrophoresis after staining with ethidium bromide (0.5 mg/ml). Allele sizes were estimated by comparison with a DNA standard length marker (Gene ruler 1kb DNA Ladder® Bioline). Control samples were included to provide the correct approach to allele size SSR, and the PCR was repeated to ensure PCR reproducibility (6–10 replicates for SSR). Fragment analyses were performed in an automated ABI3730XL sequencer by the company, Macrogen (Macrogen Humanizing Genomics, Seoul, Korea).
Data Analysis
Allelic peak sizes were identified in Geneious Prime software 2018.2.1 (https://www.geneious.com) using the microsatellite plugin. The polymorphism information content (PIC) was calculated using the formula established by Botstein et al. (Citation1980). Genetic diversity parameters like the number of alleles (Na) and observed heterozygosity (Ho) were computed for each marker using GenAlEx (Peakall and Smouse, Citation2006, Citation2012). Pairwise similarity matrices between the FPL and IVL plants were generated by Jaccard’s coefficient of similarity using the R Project Software (Team, Citation2013). Likewise, based on the genetic similarity matrix, a cluster analysis was performed using Poppr library of R Project Software (Kamvar et al., Citation2014) with the Nei distances (Nei, Citation1972) by the unweighted pair group method with arithmetic average (UPGMA). In parallel, a principal component analysis (PCA) was performed.
Results
DNA Extraction
The total extractions were obtained using Power Plant® Pro DNA Isolation Kit MoBio modified with an additional double Sorbitol buffer wash. Enough high-quality DNA was obtained (). A higher concentration of DNA was obtained from field plant leaves than from in vitro plant leaves. For every 0.1 g of tissue, an average of 121.96–129.43 ng/µL for FPL and 58.46–60.75 ng/µL for IVL was obtained for both genotypes (). The absorbance ratio values at 260/230 were below 2 (≤2), indicating some DNA contamination by carbohydrates, salts, or organic solvents; however, it was sufficient and useful for using SSR markers.
Table 2. Quantity and quality (OD260/A280-OD260/A230) of DNA isolated from field plants leaves (FPL) and in vitro plantlet leaves (IVL) of TSH565 and CCN51.
Figure 2. a) 0.8% agarose gel stained with ethidium bromide (EB) with DNA samples of CCN51. Lanes 1 − 5 in vitro somatic embryogenesis (IVL) and 6–10 ex vitro field graft plants (FPL). M: 1 kb molecular marker (HyperLadder® Bioline). Amplification of different SSR in agarose gel (3%) separation PCR products for FPL and IVL from CCN51: lanes 1–6 IVL, 8–14 FPL, 7, and 15 negative controls (NC). b) mTcCIR25. c) mTcCIR15. d) mTcUniCamp9. M: 50bp molecular marker (HyperLadder® Bioline)
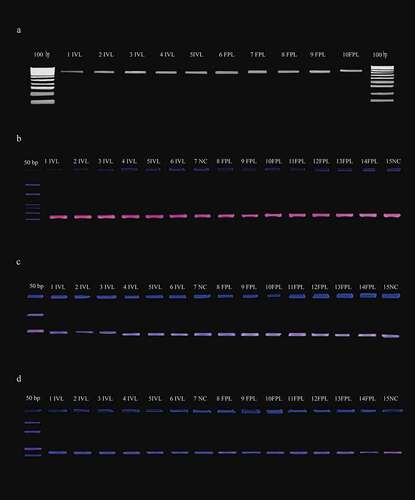
The annealing temperatures of previous studies were used for SSR amplification, but they were not successful. Therefore, each SSR annealing temperature was standardized individually: mTcCIR6 and mTcCIR8 (46º); mTcCIR11 (38º); mTcCIR25 and mTcCIR26 (40º); mTcCIR33 (45º); mTcUNICAMP01 (47º); mTcUNICAMP02 (62º); mTcUNICAMP05 (55º); mTcUNICAMP09 (61º); mTcCIR15 (54º); mTcUNICAMP16 (59º); mTcUNICAMP17 (53º) (Data not shown). Product fragments of SSR amplification were verified using agarose gel (3%) separation electrophoresis for both FPL/IVL of TSH565 and CCN51 ().
Genetic Structure
The use of microsatellites mTcCIR6, mTcCIR8, mTcCIR11, mTcCIR15, mTcCIR26, mTcCIR33, mTcUNICAMP02, mTcUNICAMP05, mTcUNICAMP09, and mTcUNICAMP16 was presented in 10 individuals for both IVL/FLP of TSH565 and IVL/FLP of CCN51. Microsatellites mTcCIR25, mTcUNICAMP17, and mTcUNICAMP01 were obtained for 6 individuals of each treatment by genotype ().
Table 3. Description and polymorphism information content (PIC) values and allele sizes established of 13 SSR of T. cacao derived of in vitro somatic embryogenesis (IVL) and ex vitro field grafts plants (FPL) from genotype TSH565 and CCN51.
Among the 13 analyzed loci, 25 alleles were found in CCN51 and 24 in genotype TSH565. According to the detected loci, no differences were observed between FPL and IVL populations in each genotype, indicating genetic fidelity between the individuals resulting from propagation by somatic embryogenesis. Through the approach used with the SSR, no fixed alleles, or perceptible detriment were observed (). The mTcUNICAMP01 locus of TSH565 had a 193/193 and 193/209 allelic composition in FLP/IVL, respectively. The allelic variation of parental field plants remains in plants derived from somatic embryogenesis (). A particular change was found at the mTcUNICAMP02 locus for the two genotypes studied in the allelic composition in the FLP population. In the IVL population of TSH565, the allelic composition was 277/295 for all individuals. For CCN51, the allelic composition was 269/295 for all individuals. However, the 290/306 loci were only found in the FPL populations in both genotypes and were not found in the IVL population. It should be noted that this change only occurred in 2 individuals.
Figure 3. Allele peak profiles of mTcUniCamp1 locus, TSH565 genotype has an allelic composition of 193/193 and 193/209 for both FLP and IVL. CCN51 genotype has an allelic composition of 193/211 for both FLP and IVL
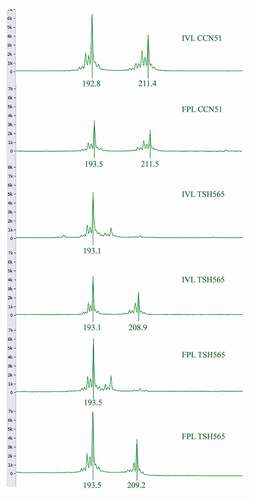
Figure 4. Allele frequency comparison. Field plant leaves (FPL) and in vitro plantlet leaves (IVL) treatments in T. cacao CCN51 and TSH565 genotypes for loci: mTcCIR6, mTcCIR8, mTcCIR11, mTcCIR15, mTcCIR25, mTcCIR26, mTcCIR33, mTcUNICAMP01, mTcUNICAMP02, mTcUNICAMP05, mTcUNICAMP09, mTcUNICAMP16 and mTcUNICAMP17

The polymorphic information content (PIC) of the 13 SSR ranged from 0.305–0.997. Botstein et al. (Citation1980) reported that the PIC index can evaluate the level of gene variation. When PIC>0.5, the locus is of high diversity when PIC<0.25, the locus is of low diversity, and the locus is of intermediate diversity when the PIC is between 0.25 and 0.5. Among these 13 SSR, 11 of them (mTcCIR6, mTcUNICAMP05, mTcCIR33, mTcUNICAMP16, mTcUNICAMP02, mTcCIR25, mTcUNICAMP01, mTcUNICAMP17, mTcCIR15, mTcCIR26, mTcCIR11) were high polymorphic loci with PIC values of over 0.500. Only mTcCIR8 and mTcUNICAMP09 were intermediate; none were low polymorphic loci with PIC values less than 0.250 ().
Genetic parameters (Na) and (Ho) were also determined for the applied SSR (). It was concluded that 10 and 9 polymorphic loci were presented for the CCN51 and TSH565 genotypes, respectively. Likewise, high heterozygosis was observed for both genotypes. A low homozygosis was observed for CCN51, present in loci mTcCIR25, mTcCIR26 and mTcUNICAMP09, and TSH565 in markers mTcCIR6, mTcCIR8, mTcCIR25, and mTcUNICAMP17 ().
Table 4. Details and genetic parameters of the 13 SSR used for evaluating the genetic stability of CCN51 and TSH565 genotypes of T. cacao. Allelic parameters: (Na) number of alleles and (Ho) observed heterozygosity
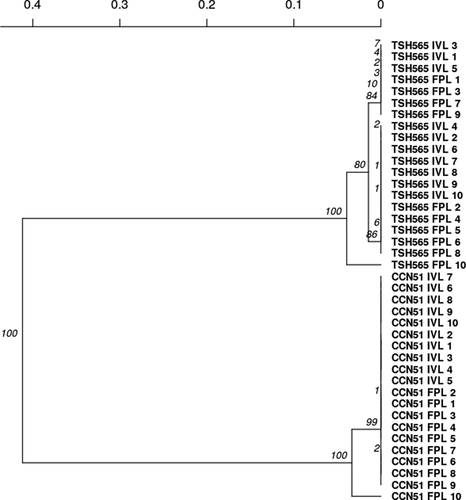
Jaccard’s similarity coefficient (Jaccard, Citation1908) generated from SSR marker profiles indicated that all the IVL plants and FPL plants were genetically similar and could be grouped under one major cluster, showing more than 91% similarity for TSH565 and 93% for CCN51. The cluster analysis using the similarity dendrogram () shows the differentiation pattern between individuals based on the 13 loci studied in the genotypes. This result coincides with the results obtained for allelic composition. This explains the short genetic distance between individuals when comparing the same genotypes and treatments. The clusters that occur within TSH565 are determined by the alleles or polymorphisms of mTcUNICAMP01 and mTcUNICAMP02. Likewise, this clade drastically separates the two analyzed genotypes, CCN51, and TSH565. A Principal Component Analysis (PCA) was performed to confirm and better understand relationships between FPL and IVL populations, showing a similar cluster structure to the UPGMA method (). Principal component 1 explained 95.49% of the variation, and principal component 2 explained 2.71%. The PCA plots reveals two groups that correspond to genotypes CCN51 and TSH565. Since most individuals per genotype have the same genetic profile, all samples overlap in only 5 individuals (Supplemental Figure 1). The PCA grouping pattern was very similar to the cluster revealed by UPGMA, with some FPL population individuals being different in the two studied genotypes, an additional result that corroborates the genetic uniformity of IVL plants. The genetic relationship between the individuals in each genotype and each population is probably due to their gene pool variation’s stability. This similarity demonstrates how useful plant regeneration through somatic embryogenesis is for carrying out T. cacao multiplication processes from the field’s parental material.
Figure 5. Hierarchical grouping between populations of CCN51 and TSH565 genotypes using the Nei distances (Nei, Citation1972) by UPGMA method
Discussion
Producing true-to-type plants through in vitro propagation is one of the most important criteria for any successful protocol for economically important crop plants. Plant tissue culture techniques cause genetic instability due to variations in ploidy level, point mutations, or translocations, leading to somaclonal variation, which occurs by using various growth regulators in the medium (Largia et al., Citation2015). Plants regenerated as explants using axillary buds, and meristematic tissue has shown the lowest genetic variation between 0–10% (Sherif et al., Citation2018; Vinoth and Ravindhran, Citation2016). However, many authors reported that plants regenerated through somatic embryogenesis exhibit genetic uniformity and integrity (Rai et al., Citation2012), some reports substantiate the presence of genetic modification in plants derived from somatic embryos (Viehmannova et al., Citation2016). The callus phase during indirect somatic embryogenesis can be an important source of somaclonal variation. In this study, we have regenerated plants through somatic embryogenesis with intermittent callus phases, which increases the chance of genetic change among regenerants. A disorganized growth phase in tissue culture, using growth regulators, the number and duration of a subculture, stress, and genotype are all factors that enhance somaclonal variation (Bairu et al., Citation2011). Due to the above, evaluating the genetic stability of in vitro-raised plants is a requirement for applying biotechnology to micro-propagate true-to-type clones. For this purpose, molecular markers could be effectively engaged to assess the genetic homogeneity and true-to-type nature of in vitro-regenerated plants.
This study reported a remarkable genetic uniformity in vitro plantlets when compared to the genetic composition of plants propagated by the conventional grafting method in genotypes CCN51 and TSH565. Among the IVL and FLP populations for each genotype, no differences were found in the genetic composition of the 13 analyzed loci, indicating that there were no stochastic variations caused by the propagation method. The conservation of allelic composition between the two populations shows that no somaclonal variations appeared. This result coincides with other studies on genetic polymorphism in soma-clones by SSR in T. cacao (Ajijah et al., Citation2016; Fang et al., Citation2009; Rodríguez et al., Citation2004).
The high genetic fidelity displayed by the regenerated plants regarding the source of explants shows no significant variation induced by the applied regeneration protocol. The minor changes observed in the genetic similarity of 91% for TSH565 and 92% for CCN51 using 13 SSR alleles are from the presence, in the allelic composition changes, of the FPL population of the mTcUNICAMP02 locus in both genotypes and the change in allelic composition in the mTcUNICAMP01 locus in TSH565 in both the FLP and IVL populations. Therefore, it cannot be concluded that somaclonal changes have occurred in regenerants, which is in line with previous studies’ results. Rodríguez et al. (Citation2004) used fifteen SSR alleles at heterozygous loci in T. cacao and somatic embryogenesis cacao regenerants. They observed 31% putative chimeric mutants for slippage mutation or allele loss across two loci. In addition, Fang et al. (Citation2009) used eighteen SSR to screen a population of primary somatic embryos and secondary somatic embryos. They observed 38.1% polymorphic profiles in the primary somatic embryos, while the frequency was 23.3% in secondary embryos. Ajijah et al. (Citation2016) observed a 97–100% level of similarity among regenerated plantlets, using nineteen SSR markers. The differences with the previous studies may be the result of the genotypes used in each study. For example, Rodríguez et al. (Citation2004) and Rodríguez et al. (Citation2010) used LCTEEN 37/1, LCTEEN 162/S-1010, SC3, and SIAL93, and Ajijah et al. (Citation2016) used Cimanggu 2.
Likewise, the frequency of somaclonal variation differences may have occurred because of genotype differences or the PGR’s used for inducing somatic embryogenesis in cacao. Fang et al. (Citation2009) and Rodríguez et al. (Citation2004) used TDZ in primary embryo induction media, while Ajijah et al. (Citation2016) uses 2,4-D and KIN, but only for inducing primary embryogenesis. This study 2,4-D was used during the induction of primary embryogenesis with 2,4,5-T in the induction of successive embryogenesis. Some studies suggest that different PGR’s can induce morphological abnormalities during T. cacao somatic embryogenesis because of its multi-dimensional function and chemical nature, leading to genetic modification (Garcia et al., Citation2019). More significant abnormalities have been observed using TDZ (Rodríguez et al., Citation2004, Citation2010) compared to 2,4-D and 2,4,5-T (Henao et al., Citation2018b). In T. cacao, the somaclonal variation could result from genetic changes (mutation) (Ajijah et al., Citation2016; Fang et al., Citation2009; Rodríguez et al., Citation2004) or epigenetic modifications (Adu-Gyamfi et al., Citation2016; Pila et al., Citation2017; Rodríguez et al., Citation2010). These reports claim that morphological, cytological, and molecular variations may be generated in vitro for several factors, such as the genotypes and protocols used for in vitro culture. Consequently, more studies are required to elucidate the genetic and epigenetic changes according to each stressful stimulus that leads to the embryogenic process, such as the type of PGR and culture conditions.
Therefore, the percentage of similarity for the regenerant pool was higher using SSR (>90%). According to Ajijah et al. (Citation2016), the genetic stability obtained from the somatic embryogenesis method used in this work presents adequate values to be used as a method for the clonal propagation of cacao. This result supports the conclusion that the regenerated CCN51 and TSH565 plants produced via somatic embryogenesis had no variations with respect to field plants. Similar results, where a genetic homogeneity of close to 100%, have been obtained from other species derived from somatic embryogenesis, both directly and indirectly, from Hibiscus sabdariffa L (Konar et al., Citation2018), Abutilon indicum L. (Seth et al., Citation2017), Cucumis melo L. (Raji et al., Citation2018), Anoectochilus elatus Lindl (Sherif et al., Citation2018), Citrullus lanatus (Vinoth and Ravindhran, Citation2016) and Bacopa monnieri (Largia et al., Citation2015).
In conclusion, this study validates the propagation protocol via somatic embryogenesis to produce plants from genotypes of interest, such as CCN51 and TSH565, for commercial cultivation purposes. Furthermore, it confirms that molecular markers like SSR are reliable, robust, and quick tools that require very little plant material and relatively low-cost inputs with good results in terms of information for analyzing somaclonal variation in somatic embryogenesis cacao regenerants.
Supplemental Material
Download MS Word (28.8 KB)Acknowledgments
We would like to thank Laboratory of Plant Physiology and Plant Tissue Culture of the Universidad de Antioquia. A special acknowledgement to Universidad de Antioquia’s Research Development Committee (CODI) and Granja Yariguíes – Compañia Nacional de Chocolates.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Adu-Gyamfi, R., A. Wetten, and C.M. Rodríguez. 2016. Effect of cryopreservation and post-cryopreservation somatic embryogenesis on the epigenetic fidelity of cocoa (Theobroma cacao L.). PLoS One 11(7):1–13. doi: https://doi.org/10.1371/journal.pone.0158857.
- Ajijah, N., R.S. Hartati, R. Rubiyo, D. Sukma, and D. Sudarsono. 2016. Effective cacao somatic embryo regeneration on kinetin supplemented DKW medium and somaclonal variation assessment using SSRs markers. Agrivita 38(1):80–92. doi: https://doi.org/10.17503/agrivita.v38i1.619.
- Alansi, S., F. Al-Qurainy, S. Khan, M. Nadeem, M. Tarroum, A. Alshameri, and A.Z. Gaafar. 2017. Genetic fidelity testing in regenerated plantlets of cryopreserved and non-cryopreserved cultivars of Phoenix dactylifera L. Pakistan J. Bot. 49(6):2313–2320.
- Alizadeh, M., H. Krishna, M. Eftekhari, M. Modareskia, and M. Modareskia. 2015. Assessment of clonal fidelity in micropropagated horticultural plants. J. Chem. Pharm. Res. 7(12):511–514.
- Bairu, M.W., A.O. Aremu, and J. van-Staden. 2011. Somaclonal variation in plants: Causes and detection methods. Plant Growth Regul. 63(2):147–173. doi: https://doi.org/10.1007/s10725-010-9554-x.
- Bandupriya, H.D.D., W.W. Iroshini, S.A. Perera, V.R. Vidhanaarachchi, S.C. Fernando, E.S. Santha, and T.R. Gunathilake. 2017. Genetic fidelity testing using SSR marker assay confirms trueness to type of micropropagated coconut (Cocos nucifera L.) plantlets derived from unfertilised ovaries. Open Plant Sci. J. 10(1):46–54. doi: https://doi.org/10.2174/1874294701710010046.
- Bastos, V., T. Uekane, N. Bello, C. Rezende, and E. Aguila. 2019. Dynamics of volatile compounds in TSH 565 cocoa clone fermentation and their role on chocolate flavor in Southeast Brazil. J. Food Sci. Technol. 56(6):2874–2887. doi: https://doi.org/10.1007/s13197-019-03736-3.
- Botstein, D., R.L. White, M. Skolnick, and R.W. Davis. 1980. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am. J. Hum. Genet. 32(3):314–331. https://pubmed.ncbi.nlm.nih.gov/6247908.
- Bradaï, F., C. Sánchez-Romero, and C. Martín. 2019. Somaclonal variation in olive (Olea europaea L.) plants regenerated via somatic embryogenesis: Influence of genotype and culture age on genetic stability. Sci. Hortic. 251(March):260–266. doi: https://doi.org/10.1016/j.scienta.2019.03.010.
- Butiuc-Keul, A., A. Farkas, and V. Cristea. 2016. Genetic stability assessment of in vitro plants by molecular markers. Stud. Univ. Babes-Bolyai Biol. 61(1):107–114. http://studia.ubbcluj.ro/download/pdf/Biologia_pdf/2016_1/28.pdf.
- Chhajer, S., and R.K. Kalia. 2016. Evaluation of genetic homogeneity of in vitro-raised plants of Tecomella undulata (Sm.) Seem. using molecular markers. Tree Genet. Genomes. 12(5):1–10. doi: https://doi.org/10.1007/s11295-016-1057-0.
- Chittora, M., D. Sharma, C. Veer, and G. Verma. 2015. Molecular markers : An important tool to assess genetic fidelity in tissue culture grown long-term cultures of economically important fruit plants. Asian J. Bio. Sci. 10(1):101–105. doi: https://doi.org/10.15740/HAS/AJBS/10.1/101-105.
- Coste, A., D. Şuteu, I. Băcilă, C. Deliu, S. Vălimăreanu, and A. Halmagyi. 2015. Genetic integrity assessment of cryopreserved tomato (Lycopersicon esculentum Mill .). Genotypes. Turkish J. Biol. 39:638–648. doi: https://doi.org/10.3906/biy-1411-6.
- Devarumath, R.M., S. Nandy, V. Rani, S. Marimuthu, N. Muraleedharan, and S.N. Raina. 2002. RAPD, ISSR and RFLP fingerprints as useful markers to evaluate genetic integrity of micropropagated plants of three diploid and triploid elite tea clones representing Camellia sinensis (China type) and C. assamica ssp. assamica (Assam-India type). Plant Cell Rep. 21(2):166–173. doi: https://doi.org/10.1007/s00299-002-0496-2.
- Devi, K., M.B. Gogoi, S. Singh, B.K. Sarmah, M.K. Modi, and P. Sen. 2017. In vitro Regeneration of Banana and Assessment of Genetic Fidelity in the Regenerated Plantlets through RAPD. Annu. Res. Rev. Biol. 17(6):1–11. doi: https://doi.org/10.9734/ARRB/2017/36339.
- Don, R.H., P.T. Cox, B.J. Wainwright, K. Baker, and J.S. Mattick. 1991. “Touchdown” PCR to circumvent spurious priming during gene amplification. Nucleic Acids Res. 19(14):4008. doi: https://doi.org/10.1093/nar/19.14.4008.
- El-Mahrouk, M., Y. Dewir, and Y. Naidoo. 2016. Micropropagation and genetic fidelity of the regenerants of aglaonema ‘ Valentine ’ using randomly amplified polymorphic DNA. HortScience 51(4):398–402. doi: https://doi.org/10.21273/HORTSCI.51.4.398.
- Etienne, H., and B. Bertrand. 2016. Are genetic and epigenetic instabilities of plant embryogenic cells a fatality? The experience of coffee somatic embryogenesis. Hum. Genet. Embryol. 6(1):1–5. doi: https://doi.org/10.4172/2161-0436.1000136.
- Fang, J.Y., A. Wetten, R. Adu-Gyamfi, M. Wilkinson, and C. Rodriguez-Lopez. 2009. Use of secondary somatic embryos promotes genetic fidelity in cryopreservation of cocoa (Theobroma cacao L.). Agric. Food Sci. 18(2):152–159. doi: https://doi.org/10.2137/145960609789267579.
- Fehér, A. 2015. Somatic embryogenesis - stress-induced remodeling of plant cell fate. Biochim. Biophys. Acta Gene. Regul. Mech. 1849(4):385–402. doi: https://doi.org/10.1016/j.bbagrm.2014.07.005.
- Garcia, C., A. Furtado-de-Almeida, M. Costa, D. Britto, R. Valle, S. Royaert, and J. Marelli. 2019. Abnormalities in somatic embryogenesis caused by 2,4-D: An overview. Plant Cell Tissue Organ. Cult. 137(2):193–212. doi: https://doi.org/10.1007/s11240-019-01569-8.
- Ghosh, A., A.U. Igamberdiev, and S.C. Debnath. 2017. Detection of DNA methylation pattern in thidiazuron-induced blueberry callus using methylation-sensitive amplification polymorphism. Biol. Plant 61(3):511–519. doi: https://doi.org/10.1007/s10535-016-0678-3.
- Goyali, J.C., A.U. Igamberdiev, and S.C. Debnath. 2015. Propagation methods affect fruit morphology and antioxidant properties but maintain clonal fidelity in lowbush blueberry. HortScience 50(6):888–896. doi: https://doi.org/10.21273/hortsci.50.6.888.
- Henao, A., H. Salazar, and A. Urrea. 2018a. Quality of cocoa (Theobroma cacao L.) DNA from foliar tissue at different stages of development. Acta Agron. 67(2):1–10. doi: https://doi.org/10.15446/acag.v67n2.63046.
- Henao, A., T. De-La-Hoz, T. Ospina, L. Garcés, and A. Urrea. 2018b. Evaluation of the potential of regeneration of different Colombian and commercial genotypes of cocoa (Theobroma cacao L.) via somatic embryogenesis. Sci. Hortic. 229:148–156. doi: https://doi.org/10.1016/j.scienta.2017.10.040.
- Irish, B.M., R. Goenaga, D. Zhang, R. Schnell, J.S. Brown, and J.C. Motamayor. 2010. Microsatellite fingerprinting of the USDA-ARS tropical agriculture research station cacao (Theobroma cacao L.) Germplasm collection. Crop Sci. 50(2):656–667. doi: https://doi.org/10.2135/cropsci2009.06.0299.
- Jaccard, P. 1908. Nouvelles recherches sur la distribution florale. Bull. Soc. Vaud. Sci. Nat. 44:223–270.
- Kaeppler, S.M., H.F. Kaeppler, and Y. Rhee. 2000. Epigenetic aspects of somaclonal variation in plants BT - plant gene silencing, p. 59–68. In: M.A. Matzke and A.J.M. Matzke (eds.). Plant Gene Silencing. Springer Netherlands, Dordrecht. doi: https://doi.org/10.1007/978-94-011-4183-3_4
- Kamvar, Z.N., J.F. Tabima, and N.J. Griunwald. 2014. Poppr: An R package for genetic analysis of populations with clonal, partially clonal, and/or sexual reproduction. PeerJ 2014(1):1–14. doi: https://doi.org/10.7717/peerj.281.
- Konar, S., J. Karmakar, A. Ray, S. Adhikari, and T.K. Bandyopadhyay. 2018. Regeneration of plantlets through somatic embryogenesis from root derived calli of Hibiscus sabdariffa L. (Roselle) and assessment of genetic stability by flow cytometry and ISSR analysis. PLoS One 13(8):1–17. doi: https://doi.org/10.1371/journal.pone.0202324.
- Lanaud, C., A. Risterucci, I. Pieretti, M. Falque, A. Bouet, and P. Lagoda. 1999. Isolation and characterization of microsatellites in Rattus rattus. Mol. Ecol. Resour. 8:2141–2152. doi: https://doi.org/10.1111/j.1755-0998.2008.02115.x.
- Largia, M.J.V., J. Shilpha, G. Pothiraj, and M. Ramesh. 2015. Analysis of nuclear DNA content, genetic stability, Bacoside A quantity and antioxidant potential of long term in vitro grown germplasm lines of Bacopa monnieri (L.). Plant Cell Tissue Organ. Cult. 120(1):399–406. doi: https://doi.org/10.1007/s11240-014-0602-5.
- Larkin, P.J., and W.R. Scowcroft. 1981. Somaclonal variation — A novel source of variability from cell cultures for plant improvement. Theor. Appl. Genet. 60(4):197–214. doi: https://doi.org/10.1007/BF02342540.
- Lata, H., S. Chandra, N. Techen, I.A. Khan, and M.A. ElSohly. 2016. In vitro mass propagation of Cannabis sativa L.: A protocol refinement using novel aromatic cytokinin meta-topolin and the assessment of eco-physiological, biochemical and genetic fidelity of micropropagated plants. J. Appl. Res. Med. Aromat. Plants 3(1):18–26. doi: https://doi.org/10.1016/j.jarmap.2015.12.001.
- Maki, S., Y. Hirai, T. Niino, and T. Matsumoto. 2015. Assessment of molecular genetic stability between long-term cryopreserved and tissue cultured wasabi plants. CryoLetters 36(5):318–324. https://www.ingentaconnect.com/content/cryo/cryo/2015/00000036/00000005/art00005.
- Mithra, S., B. Devanna, P. Sharma, P. Shingote, K. Arora and A. Solanke. 2017. Genetic fidelity testing of tissue culture-raised plants, p. 13–20. In: A. Solanke, S. Mithra, B. Devanna, P. Sharma, K. Arora, and P. Shingote (eds.). Genet fidel test tissue cult plants. BCIL-DBT, New Delhi. https://krishi.icar.gov.in/jspui/bitstream/123456789/6235/1/TrainingManualonGeneticFidelityTestingofTissueCultureRasisedPlants2016-17.pdf
- Moharana, A., A. Das, E. Subudhi, and S.K. Naik. 2018. Assessment of genetic fidelity using random amplified polymorphic DNA and inter simple sequence repeats markers of lawsonia inermis L. plants regenerated by axillary shoot proliferation. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 88:133–141. doi: https://doi.org/10.1007/s40011-016-0740-0.
- Nandhakumar, N., K. Soorianathasundaram, D. Sudhakar, and K.K. Kumar. 2017. Genetic fidelity analysis in the micropropagated banana derived from immature primordial male flower bud. Int. J. Curr. Microbiol. Appl. Sci. 6(4):1759–1769. doi: https://doi.org/10.20546/ijcmas.2017.604.211.
- Nei, M. 1972. Genetic distance between populations. Am. Nat. 106(949):283–292.
- Onay, A., E. Tilkat, V. Süzerer, O. Karakas, Y. Ozden, F. Kiling, I. Koc, M. Sakiroglu, H. Yildirin, A. Altinkut, et al. 2016. Rejuvenation of mature lentisk by micrografting and evaluation of genetic stability. Turkish J. Biol. 40:781–796. doi: https://doi.org/10.3906/biy-1510-25.
- Patil, K.S., and S.R. Bhalsing. 2015. Efficient micropropagation and assessment of genetic fidelity of Boerhaavia diffusa L- High trade medicinal plant. Physiol. Mol. Biol. Plants 21:425–432. doi: https://doi.org/10.1007/s12298-015-0301-7.
- Peakall, R., and P. Smouse. 2006. GENALEX 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 6(1):288–295. doi: https://doi.org/10.1111/j.1471-8286.2005.01155.x.
- Peakall, R., and P. Smouse. 2012. GenALEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research-an update. Bioinformatics 28(19):2537–2539. doi: https://doi.org/10.1093/bioinformatics/bts460.
- Pila, L., H. Freitas, L. do-Nascimento, and M. Guerra. 2017. Epigenetics of long-term somatic embryogenesis in Theobroma cacao L.: DNA methylation and recovery of embryogenic potential. Plant Cell Tissue Organ. Cult. 131(2):295–305. doi: https://doi.org/10.1007/s11240-017-1284-6.
- Rai, M.K., M. Phulwaria, A. Harish, A.K. Gupta, N.S. Shekhawat, and U. Jaiswal. 2012. Genetic homogeneity of guava plants derived from somatic embryogenesis using SSR and ISSR markers. Plant Cell Tissue Organ. Cult. 111(2):259–264. doi: https://doi.org/10.1007/s11240-012-0190-1.
- Raji, M.K., M. Phulwaria, A.K. Gupta, N.S. Shekhawat, and U. Jaiswal. 2018. Somatic embryogenesis of muskmelon (Cucumis melo L.) and genetic stability assessment of regenerants using flow cytometry and ISSR markers. Protoplasma 255(3):873–883. doi: https://doi.org/10.1007/s00709-017-1194-9.
- Rodríguez, C., A. Wetten, and M. Wilkinson. 2004. Detection and quantification of in vitro-culture induced chimerism using simple sequence repeat (SSR) analysis in Theobroma cacao (L.). Theor. Appl. Genet. 110(1):157–166. doi: https://doi.org/10.1007/s00122-004-1823-5.
- Rodríguez, C.M., A.C. Wetten, and M.J. Wilkinson. 2010. Progressive erosion of genetic and epigenetic variation in callus-derived cocoa (Theobroma cacao) plants. New Phytol. 186(4):856–868. doi: https://doi.org/10.1111/j.1469-8137.2010.03242.x.
- Santos, E., C. Cerqueira, G. Mori, D. Ahnert, R. Corrêa, and A. Souza. 2012. New polymorphic microsatellite loci for Theobroma cacao: Isolation and characterisation of microsatellites from enriched genomic libraries. Biol. Plant 56(4):789–792. doi: https://doi.org/10.1007/s10535-012-0134-y.
- Scherer, R., H. Freitas, G. Ferrero, D. Almeida, and M. Guerra. 2015. Global DNA methylation levels during the development of nodule cluster cultures and assessment of genetic fidelity of In Vitro-regenerated pineapple plants (Ananas comosus var. comosus). J. Plant Growth Regul. 34(3):677–683. doi: https://doi.org/10.1007/s00344-015-9493-x.
- Seth, S., S.C. Rath, G.R. Rout, and J. Panigrahi. 2017. Somatic embryogenesis in Abutilon indicum (L.) Sweet and assessment of genetic homogeneity using SCoT markers. Plant Biosyst. 151(4):704–714. doi: https://doi.org/10.1080/11263504.2016.1211193.
- Sherif, N.A., J.H. Franklin, T. Senthil, and M.V. Rao. 2018. Somatic embryogenesis, acclimatisation and genetic homogeneity assessment of regenerated plantlets of Anoectochilus elatus Lindl., an endangered terrestrial jewel orchid. Plant Cell Tissue Organ. Cult. 132(2):303–316. doi: https://doi.org/10.1007/s11240-017-1330-4.
- Singh, R., S. Pratap, N. Kumari, and M. Singh. 2016. Regeneration of soapnut tree through somatic embryogenesis and assessment of genetic fidelity through ISSR and RAPD markers. Physiol. Mol. Biol. Plants 22:381–389. doi: https://doi.org/10.1007/s12298-016-0364-0.
- Team, R.C. 2013. R: A language and environment for statistical computing.
- Teeluck, J.M., B.F. Kaudeer, M. Ramful, I. Boodhram, M.R. Sanmukhiya, and J.G. Soulange. 2016. Genetic fidelity of in vitro propagated breadfruit (Artocarpus altilis) using inter simple sequence repeat markers. Int. J. Agric. Biol. 18(5):911–916. doi: https://doi.org/10.17957/IJAB/15.0185.
- Thondaiman, V., K. Rajamani1, N. Senthil, and A.J. Shoba. 2013. Genetic diversity in cocoa (Theobroma cacao L.) plus trees in Tamil Nadu by simple sequence repeat (SSR) markers. Afr. J. Biotechnol. 12(30):4747–4753. doi: https://doi.org/10.5897/ajb2013.12423.
- Urrea, A., L. Atehortúa, and A. Gallego. 2011. Regeneration through somatic embryogenesis of an elite colombian Theobroma cacao L. variety. Rev. Colomb. Biotecnol. 13(2):39–50. http://ref.scielo.org/vj4k59.
- Vega, H., and M. Beillard. 2016. Ecuador cocoa update and outlook. USDA Foreign Agricultural Service. Quito.
- Viehmannova, I., P.H. Cepkova, J. Vitamvas, P. Streblova, and J. Kisilova. 2016. Micropropagation of a giant ornamental bromeliad Puya berteroniana through adventitious shoots and assessment of their genetic stability through ISSR primers and flow cytometry. Plant Cell Tissue Organ. Cult. 125(2):293–302. doi: https://doi.org/10.1007/s11240-016-0949-x.
- Vinoth, A., and R. Ravindhran. 2016. Efficient plant regeneration of watermelon (Citrullus lanatus Thunb.) via somatic embryogenesis and assessment of genetic fidelity using ISSR markers. Vitr. Cell Dev. Biol. Plant 52(1):107–115. doi: https://doi.org/10.1007/s11627-015-9731-8.
- Wang, Q.M., and L. Wang. 2012. An evolutionary view of plant tissue culture: Somaclonal variation and selection. Plant Cell Rep. 31(9):1535–1547. doi: https://doi.org/10.1007/s00299-012-1281-5.