ABSTRACT
The rising demand for guava fruit worldwide is due to its richness in vitamin C, high yielding and two bearing seasons. Given the salt stress threat to guavas and the necessity of protective strategies, in the present research guava seedlings were subjected to sodium chloride (0 and 50 mM) and salicylic acid (0 and 1 mM). To ascertain a logical conclusion, the activity of antioxidant enzymes, antioxidant capacity of DPPH, ferric reducing antioxidant power, total flavonoid and phenol contents were monitored in salt-exposed guava seedlings. Based on our findings, the most superoxide dismutase (87.46 µmol min−1g−1DW) activity, ferric reducing antioxidant power (408.82 mmol g−1 DW), DPPH scavenging activity (80.65%), flavonoid (14.15 mg QE−1 g DW) and phenol (34.02 mg GAE g−1 DW) contents were found in 50 mM NaCl-exposed plants, which were enriched by 1 mM salicylic acid. A high correlation was observed among non-enzymatic antioxidant parameters (DPPH scavenging activity, ferric reducing antioxidant power, total flavonoid content and total phenol content). In conclusion, salicylic acid improved guavas salt tolerance by increasing both enzymatic (peroxidase and superoxide dismutase) and non-enzymatic (ferric reducing antioxidant power, DPPH scavenging activity, total phenol and flavonoid contents) pathways.
Introduction
Salinity due to human-induced practices, changes in irrigation systems and rising temperature is growing in the most developing countries and becoming a serious concern which declines the productivity of sensitive crops (Munns and Tester, Citation2008). The ionic homeostasis, under high salt concentrations, leads to osmotic stress and consequently extra generation of reactive oxygen species (ROS) (Khan and Panda, Citation2007) that causes destructive oxidative stress, chlorophyll breakdown, lipid peroxidation, protein oxidation and finally DNA impairment (Terzi and Kadioglu, Citation2006).
Salinity threatens plant production over at least 20% of irrigated lands, thus introducing of capable approaches to mitigate stress injury is of a great priority. Over recent decades, some efforts have been introduced as efficient strategies to lessen the destructive impacts of salt stress on plant metabolism, i.e. the exogenous applications of putrescine (Esfandiari Ghalati et al., Citation2020), nitric oxide (Kaya et al., Citation2019), proline (Freitas et al., Citation2019) and salicylic acid (Naser Alavi et al., Citation2014).
Salicylic acid (SA), an endogenous growth regulator, belongs to a group of phenolic compounds (Knörzer et al., Citation1999). This compound influences metabolism, biosynthesis, photosynthesis, respiration, ion transport and enzymes activities (Borsani et al., Citation2001), and enhances plant tolerance under both biotic and abiotic stresses (Knörzer et al., Citation1999).
In recent years, an extensive attention has attracted to the salicylic acid application in plants subjected to environmental stresses. The exogenous usage of SA has decreased the chilling injury in stored peach fruits (Cao et al., Citation2010). SA has also augmented heat-adaptation in tobacco (Dat et al., Citation2000). In addition, it has improved plants tolerance to salt stress (Al-Hakimi and Hamada, Citation2001; Gunes et al., Citation2007).
The proposed mechanisms of SA in abiotic stresses are mainly changes in catalase, ascorbic peroxidase and peroxidase activities (Ahjib-Ul-Arif et al., Citation2018; Fujita et al., Citation2006; Horvath et al., Citation2007). Exogenous salicylic acid reduces membrane damage under salinity stress, reflects by decreased malondialdehyde level. Furthermore, the activity of antioxidant enzymes considerably changes following SA application (Xiaohua et al., 2017; Ahjib-Ul-Arif et al., Citation2018; Farhangi-Abriz and Ghassemi-Golezani, Citation2018). SA enhances photosynthesis-related parameters (including photosynthetic rate, carboxylation efficiency, water usage efficiency and chlorophyll content) (Ahjib-Ul-Arif et al., Citation2018; Pirasteh-Anosheh and Emam, Citation2018). Also, SA enhances salinity tolerance by a reduction in Na+ and increases in K+ and Ca+ contents (Farhangi-Abriz and Ghassemi-Golezani, Citation2018; Jini and Joseph, Citation2017).
Guava (Psidium guajava L.), belongs to the Myrtaceae family, is native to tropical areas such as America, Mexico, and Peru. The fruit is rich in vitamin C and edible fiber (Jiménez-Escrig et al., Citation2001). In addition, the guava leaves contain phenolic compounds such as Isoflavonoids, Gallic Acid, Catechin, Epi-Catechin, Rutin, Naringenin and Kaempferol (Barbalho et al., Citation2012) with anti-inflammatory and antioxidant effects (Chiari et al., Citation2012).
According to FAO report (2018), world guava production reached 7.25 million tons (FAO statistics. Production. Crops, Citation2018). Guava cultivation in the tropical areas of Iran (Hormozgan and Sistan-Baluchistan provinces) has a 400-year-old history. According to the Iranian Ministry of Agriculture statistics, from 14,000 hectares of tropical fruits plantation, 1750 ha dedicated to guava and the yearly production of guava in Iran is about 4119 tons (The center of information and technology, Citation2019). The impacts of NaCl on the seed germination (Hooda and Yamdagni, Citation1991), young (Cavalcante et al., Citation2007; Esfandiari Ghalati et al., Citation2020) and mature plants (Ali-Dinar et al., Citation1999) of guava have demonstrated its destructive effects.
Since no information is available on the salicylic acid involvement in salt tolerance of guava, the responses of guava seedlings irrigated with saline water (contains NaCl) and foliar sprayed with salicylic acid were the concerns of the present research. To find out a logical conclusion, the activity of antioxidant enzymes, antioxidant capacity of DPPH, ferric reducing antioxidant power, total flavonoid and phenol contents were monitored in salt-exposed guava seedlings.
Material and Methods
Plant Materials and Experimental Design
The present research was carried out at the nursery of the Horticulture Department, Faculty of Agriculture, University of Hormozgan, Iran (57° 4 E 27° 9 N, Elevation: 10 m, Relative humidity: 70.32%, Mean temperature of 24 ± 1.2°C) during 2018–2019.
The purpose was to examine the impact of salicylic acid on guava plants which were irrigated with saline water. After the literature review on plant crops (Gunes et al., Citation2007; Safari et al., Citation2017), treatments were included saline water (0 and 50 mM NaCl) and foliar application of salicylic acid (0 and 1 mM). The seedlings of guava (one-year-old) were alike in height (80 ± 2 cm). The average leaf Na content of seedlings was 2.5 mg−1 g−1 DW±0.054. The media was a sandy-clay-loam soil (37% sand, 38.5% silt, 25% clay, and 25% lime) which had an electrical conductivity of 0.93 ds−1 m, CEC of 10.84 meq−1100 g and pH of 8.4. Based on soil characteristics, urea, potassium sulfate and simple superphosphate were mixed with the media (0.13 g, 0.13 g and 1.10 g in 5 kg of soil, respectively). Both Sodium chloride (CAS number: 7647–14-5, Molar Mass: 58.44 g−1mol) and Salicylic acid (CAS number: 69–72-7, Molar Mass: 138.12 g−1 mol) were Merck products.
Foliar salicylic acid (pH of 3, the volume of 250 ml for every plant) was applied (for Plants related to the mentioned treatment) at the fourth and sixth week after the establishment of the seedlings. Salt (NaCl) application, through irrigation, was started from the 5th week of plants establishment until the 7th week (Cavalcante et al., Citation2007; Esfandiari Ghalati et al., Citation2020). NaCl application was started with 17.094 mM and slowly reached the final level (50 mM). Afterward, all experimental units were irrigated with distilled water for 8 weeks. Finally, the leaf blades of upper branches were collected for the following measurements.
The Antioxidant Enzymes Assay
About 0.5 g of the fresh leaf was powdered by liquid nitrogen and mixed with the extraction buffer (100 ml of 50 mM phosphate buffer with pH = 7, 1 g of Polyvinyl pyrrolidone (PVP) and 0.0372 g of EDTA). The mixture was centrifuged (16128 G force, 15 min, 4°C) (Dhindsa et al., Citation1981). The supernatant was used as the enzyme extract for following assays.
The peroxidase (POD) activity was assessed by Chance and Maehly (Citation1995) protocol. Briefly, 33 µl of the enzyme extract was added to one ml of the peroxidase reaction solution (13 mM Guaiacol, 5 mM hydrogen peroxide and 50 mM phosphate buffer with pH = 7). Then the absorbance was recorded at 470 nm using a Cecil CE2501 spectrophotometer (Chance and Maehly, Citation1995).
The superoxide dismutase (SOD) activity was assayed via the method was described by Beauchamp and Fridovich (Citation1971). Briefly, 50 μl of the enzyme extract was added to one ml of the SOD reaction solution (50 mM phosphate potassium buffer with pH = 7.8, 75 mM NBT, 13 mM L-methionine, 0.1 mM EDTA and 2 mM riboflavin) and incubated for 15 minutes (under fluorescence light). Then the absorbance was recorded at 560 nm (Beauchamp and Fridovich, Citation1971).
Assays of Total Flavonoid Content, Total Phenol Content, Ferric Reducing Antioxidant Power and DPPH Scavenging Activity
The leaf samples were powdered, then 0.5 g of the powder was soaked in 80% methanol and centrifuged (112000 G force, 10 min, 4°C). The supernatant was used as the leaf extract for the following assays.
The total flavonoid content (TFC) was measured by Beketov et al. (Citation2005) procedure. The leaf extract (0.2 ml) and quercetin (25 µg ml −1) were mixed with 0.2 ml of 10% AlCl3 in 0.1 ml of methanol, 33% acetic acid and 90% ethanol. The samples were incubated at room temperature for 30 minutes. The procedure was terminated by recording the absorbance at 414 nm. The total flavonoid content was expressed as mg g−1 of quercetin (QE) equivalent in milligram g−1 dry weight (mg QE−1 g DW) (Beketov et al., Citation2005).
The total phenol content (TPC) was assayed using the spectrophotometric technique (Spanos and Wrolstad, Citation1990). The reaction mixture was 10 µl of the methanol leaf extract, 500 µl of 10% Folin-Ciocalteu’s reagent in water, 500 µl of 1% NaHCO3 and 490 µl of distilled water. The samples were vortexed and incubated at 24°C for 12 hrs. Then, the absorbance was determined at 765 nm. Gallic acid (100–200 mg l−1) was used to prepare the standard curve. The total phenolic content was expressed in mg Gallic acid equivalent g−1 DW (mg of GAE g−1 DW).
The Benzie and Strain (Citation1999) technique was used for the ferric reducing antioxidant power (FRAP) assay. The first step was the preparation of 300 mM acetate buffer (3.1 g of CH3COONa and 16 ml of CH3OOH, pH = 3.6), 20 mM FeCl3 · 6H2O and 10 mM TPTZ (2, 4, 6-tripyridyl-s-triazine) solutions in 40 mM HCl. The second step was the preparation of FRAP solution by mixing 25 ml of the acetate buffer, 2.5 ml of FeCl3 · 6H2O and 2.5 ml of TPTZ. The next step involved mixing 150 μL of the leaf extract with 2850 μl of the FRAP solution and allow to react under dark conditions at 37°C for 30 min. The final step was recording the optical density of the extracts at 593 nm. The standard curve was prepared using 50–1000 μM FeSO4. The results were expressed in mmol g−1 dry weight (Benzie and Strain, Citation1999).
For the DPPH scavenging activity assay, the leaf extract (50 μl) was mixed with 950 μl of 0.1 N DPPH (1,1-diphenyl-2-picryl- hydroxyl). The samples were kept under dark conditions (24°C, 30 min). Then, the absorbance was recorded at 517 nm. The free radical scavenging activity was calculated using the following equation (Singelton et al., Citation1999):
DPPH scavenging activity (%) = ((Acont – Asamp) ×100)/Acont
Where Acont and Asamp are the absorbance values of the standard and the sample.
Data Analysis
The SAS version 9.1.3 (“SAS® Procedures, Citation1990) was used for data analysis. The research was a factorial experiment in a completely randomized block design with 6 replications. The factors were salicylic acid and salinity levels. The data normality was tested with Shapiro-Wilks test. The homogeneity of variance was confirmed with the Pillai’s trace test. The mean comparisons were computed by Tukey’s test (P < .01). The correlation analysis was used to determine parameters relations (Pearson coefficient). The graphs were figured using Sigma Plot (n.d, 10.0).
Results
The analysis of variance showed that the studied parameters had a significant behavior, due to NaCl treatment, salicylic acid application and their interaction ().
Table 1. The analysis of variance of salinity and salicylic acid on the measured parameters in guava
Interaction of Salt and Salicylic Acid on POD and SOD Activities
According to our findings, the POD activity was reduced by salt treatment, compared to control (salt-free and no salicylic acid usage). Maximum POD activity (0.42 µmol min−1g−1DW) occurred in the control plants which were received SA treatment. Besides, the application of 1 mM salicylic acid significantly caused an increase in POD activity. The lowest POD activity (0.25 µmol min−1g−1DW) was followed by 50 mM NaCl under SA-free conditions ().
Figure 1. The interaction of salicylic acid and NaCl on POD (A) and SOD (B) activities in guava seedlings. Means ± SD of six replicates are given. The same letters indicate no statistically significant difference (Tukey, p < .01)
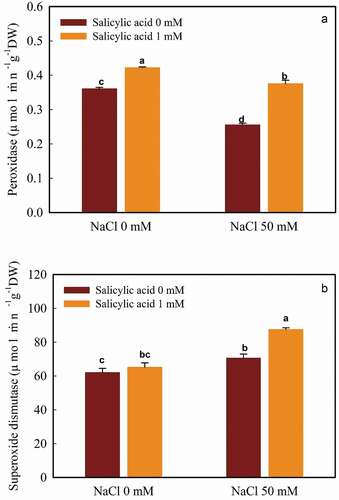
Treating with 50 mM NaCl increased the SOD activity (12.06% increment compared to control) and reached 70.58 µmol min−1g−1DW. Salicylic acid, in salt-free plants, caused a rise in SOD activity (4.88% increase). The highest level of SOD activity (87.46 µmol min−1g−1DW) was found in 50 mM NaCl with 1 mM salicylic acid ().
Interaction of Salt and Salicylic Acid on FRAP and DPPH Capacity
NaCl significantly increased the ferric reducing antioxidant power (FRAP) in guava leaves (5.3% increase); supplementary salicylic acid also caused an increase in FRAP (0.25% increase). As illustrated in , the most FRAP value was obtained in the plants which had received both NaCl and salicylic acid (408.82 mmol g−1 DW). The minimum FRAP value (327.89 mmol g−1 DW) was observed in the control plants ().
Figure 2. The interaction of salicylic acid and NaCl on the FRAP (A) and DPPH (B) capacity in guava seedlings. Means ± SD of six replicates are given. The same letters indicate no statistically significant difference (Tukey, p < .01)
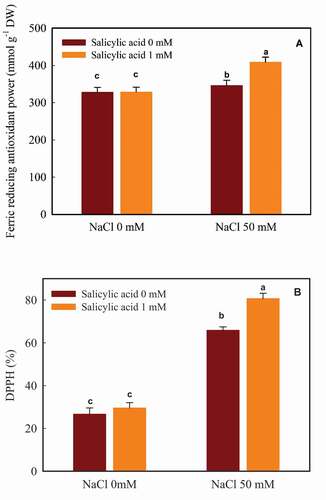
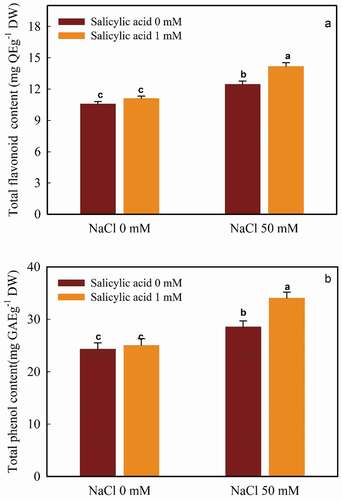
Figure 3. The interaction of salicylic acid and NaCl on TFC (A) and TPC (B) in guava seedlings. Means ± SD of six replicates are given. The same letters indicate no statistically significant difference (Tukey, p < .01)
Both NaCl and salicylic acid made an increase in DPPH capacity (59.50% and 10.76% upturn, respectively). The most DPPH value (80.65%) was found with 50 mM NaCl enriched by salicylic acid (1 mM). The minimum value (26.67%) was observed in the control plants ().
Interaction of Salt and Salicylic Acid on TFC and TPC
The application of both NaCl and salicylic acid resulted in an increase in TFC. The minimum flavonoid content (10.56 mg QE g−1 DW) was detected in the control plants, which was not significantly different from those received SA (11.09 mg QEg−1 DW). The most value (14.15 mg QE g−1 DW) was related to 50 mM NaCl and SA treated plants ().
According to our data, salt made an 14.85% increase in TPC in comparison with the control plants (no salt, SA-free). However, the application of 1 mM salicylic acid non-significantly increased TPC in the control plants (3.95% raise). The most TPC value was related to the combination of both 50 mM NaCl and 1 mM salicylic acid (34.02 mg GAE g−1 DW). The minimum value occurred in the control plants (24.28 mg GAE g−1 DW) ().
Correlation Analysis
The results of bivariate correlations (Pearson coefficient) are provided in . The analysis indicated several significant positive or negative correlations. Total phenol content was positively correlated with total flavonoid, SOD, FRAP and DPPH (0.99**, 0.99**, 0.97** and 0.95**, respectively). Total flavonoid content was positively correlated with SOD, DPPH and FRAP (0.98**,0.97** and 0.95**, respectively). FRAP had significant correlations with SOD and DPPH (0.99** and 0.87**, respectively).
Table 2. The correlation analysis of guava physiological parameters
Discussion
Abiotic stresses cause the interruption of electron transport chains in mitochondria and chloroplasts of plants. Under such conditions, O2 donates an electron and rises the ROS accumulation (Grob et al., Citation2013). The plant defense response is an extra-activation of some antioxidant enzymes and deactivation of some others to protect the macro-molecules (Ahmed et al., Citation2009). SOD is the primary enzyme in the detoxification mechanism, which converts superoxide FRs to H2O2 (Sairam et al., Citation2002). The over-production of H2O2 reduces the membrane permeability and leads to lipid peroxidation and oxidative stress. Thus, the generated H2O2 is scavenged by catalase, ascorbate peroxidase and glutathione peroxidase (Hamanaka and Chandel, Citation2009). Our results about the increased activity of SOD under salt stress, are in line with those in mulberry (Sudhakar et al., Citation2001) and pistachio (Lotfi et al., Citation2015). Also, we identified a decrease in the POD activity in salt-exposed plants. This decline is related to the distracted scavenging activity of this enzyme (Shahbazi et al., Citation2011). This finding was in line with those of Ahmad Sajid et al. (Citation2016), Yordanova and Popova (Citation2007) and Esfandiari Ghalati et al. (Citation2020).
SA adjusts the antioxidant capacity of the salt exposed-plants via increasing or inhibiting the activity of antioxidant enzymes including catalase, superoxide dismutase and ascorbate peroxidase (Ahmad et al., Citation2018; El-Esawi et al., Citation2017; Li et al., Citation2014; Pirasteh-Anosheh and Emam, Citation2018). Also, exogenous application of salicylic acid encourages the expression of the genes encoding the antioxidant enzymes (Farooq et al., Citation2009; Horvath et al., Citation2007). Exogenous SA has improved the activity of POD (War et al., Citation2011) and PPO (Schneider and Ullrich, Citation1994) enzymes. Foliar applied SA boosted the activity of superoxide dismutase and peroxidase in tomato (Hayat et al., Citation2008) and mustard plants (Yusuf et al., Citation2008).
Salinity prompts the generation and accumulation of reactive oxygen species (Khan and Panda, Citation2007). The changes in antioxidant levels modulate ROS scavenging mechanisms (Mittler, Citation2002). Hence, plants have equipped with several protection mechanisms, including the biosynthesis of phenolic and flavonoids compounds, against harmful ROS (Koyro et al., Citation2012). Phenolic compounds, the plant’s secondary metabolites, are synthesized via both shikimic acid and phenylpropanoid metabolic pathways. In plants, these compounds might act as signaling molecules, motivate disease resistance and protect against reactive oxygen species, produced by abiotic stresses such as salinity (Crozier et al., Citation2006). The role of phenolic compounds as an anti-oxidative agent and their increment due to salt stress has already reported (Chaparzadeh and Hosseinzad-Behboud, Citation2015; El-Esawi et al., Citation2017).
The accumulation of ROS under unfavorable environmental conditions, shifts to increase the plant hormones, such as salicylic acid (Ahlfors et al., Citation2004). Induced salicylic acid, as well as the exogenously applied form, provokes the synthesis of secondary metabolites (Idrees et al., Citation2010; Ghasemzadeh et al., Citation2012; Pirasteh-Anosheh and Emam, Citation2018). Actually, when applied in low concentrations, salicylic acid might cause a temporary and non-serious oxidative stress, which turns the hardening process in plants, improves the anti-oxidative ability of the plants (Knörzer et al., Citation1999) and hence assists in the biosynthesis induction of phenolic compounds (Németh et al., Citation2002). There are documents of increased plant antioxidant capacity in saline conditions after SA treatment (Chaparzadeh and Hosseinzad-Behboud, Citation2015; Farhangi-Abriz and Ghassemi-Golezani, Citation2018; Gorni et al., Citation2019; Grzeszczuk et al., Citation2018; Li et al., Citation2014). Also, exogenous SA is found to boost the production of plant flavonoids through increasing the activity of core flavonoid biosynthetic genes (Xu et al., Citation2009), which are in line with our results.
Correlation analysis examines the relationships between variables (Yadav, Citation2018). As previously reported by Rodriguez-Dominguez et al. (Citation2016), salt stress modifies cell wall integrity and leaf turgor, lead to a decrease in photosynthesis rate and leaf number. Such changes alter physiological processes and the relationships between the growth parameters under different conditions. According to correlation analysis, a high correlation was observed among non-enzymatic antioxidant parameters (DPPH, FRAP, TPC, TFC).
Conclusion
Based on our findings, NaCl made a reduction in POD activity, while an increment in SOD activity, TPC, TFC, FRAP and DPPH capacities in guava plants. Salicylic acid improved the antioxidant ability of salt-exposed guavas by increasing the SOD, POD, FRAP, DPPH, TPC and TFC. The effects of salicylic acid in mitigating oxidative stress suggest the SA application as a potential, simple and efficient approach to diminish the injury of oxidative stress in salt-exposed guavas.
Competing interest
The authors declare that they have no competing interests.
Acknowledgments
The authors thank the Head of Research and Technology, University of Hormozgan for their financial supports.
References
- Ahjib-Ul-Arif, M., M.N. Siddiqui, A.A.M. Sohag, M.D. Arif Sakil, M. Rahman, M.A. Sadik Polash, M.G. Mostofa, and L.S. Tren Phan. 2018. Salicylic acid-mediated enhancement of photosynthesis attributes and antioxidant capacity contributes to yield improvement of maize plants under salt stress. J. Plant Growth Regul. 37(4):1318–1330. doi: https://doi.org/10.1007/s00344-018-9867-y.
- Ahlfors, R., V. Macioszek, J. Rudd, M. Brosche, R. Schlichting, D. Scheel, and J. Kangasjarvi. 2004. Stress hormone-independent activation and nuclear translocation of mitogen-activated protein kinases in Arabidopsis thaliana during ozone exposure. Plant J. 40(4):512–522. doi: https://doi.org/10.1111/j.1365-313X.2004.02229.x.
- Ahmad, P., M.N. Alyemeni, M.A. Ahanger, D. Egamberdieva, L. Wijaya, and P. Alam. 2018. Salicylic Acid (SA) Induced Alterations in Growth, Biochemical Attributes and Antioxidant Enzyme Activity in Faba Bean (Vicia faba L.) Seedlings under NaCl Toxicity. Russian Journal of Plant Physiology 65(1):104–114. doi: https://doi.org/10.1134/S1021443718010132.
- Ahmad Sajid, Z., M. Safdar, and S.A. khilji. 2016. Amelioration of salinity stress tolerance in pea (pisum sativum L.) by exogenous application of salicylic acid. Biologia 62(1):69–78.
- Ahmed, P., C. Jaleel, M. Azooz, and G. Nabi. 2009. Generation of ROS and non-enzymatic antioxidants during abiotic stress in plants. Botany Research International 2:11–20.
- Al-Hakimi, A.M.A., and A.M. Hamada. 2001. Counteraction of salinity stress on wheat plants by grain soaking in ascorbic acid, thiamin or sodium salicylate. Biologia Plantarum 44(2):253–261. doi: https://doi.org/10.1023/A:1010255526903.
- Ali-Dinar, H.M., G. Ebert, and P. Ludders. 1999. Growth, chlorophyll content, photosynthesis and water relations in guava (Psidium guajava L.) under salinity and different nitrogen supply. Gartenbauwissenschaft 64:54–59.
- Barbalho, S.M., F.M.V. Farinazz-Machado, R.D.A. Goulart, A.C.S. Brunnati, A.M.M.B. Ottoboni, and C.C.T. Nicolau. 2012. Psidium guajava (Guava): A plant of multipurpose medicinal applications. Med Aromat Plants 11:104–108.
- Beauchamp, C., and I. Fridovich. 1971. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Analytical Biochemistry 44(1):276–287. doi: https://doi.org/10.1016/0003-2697(71)90370-8.
- Beketov, E.V., V.P. Pakhomov, and O.V. Nesterova. 2005. Improved method of flavonoid extraction from bird cherry fruits. Pharmaceutical Chemistry Journal 39(6):316–318. doi: https://doi.org/10.1007/s11094-005-0143-7.
- Benzie, I.F.F., and J.J. Strain. 1999. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Meth. Enzymol. 299:15–27.
- Borsani, O., V. Valpuesta, and M.A. Botella. 2001. Evidence for a role of salicylic acid in the oxidative damage generated by NaCl and osmotic stress in Arabidopsis seedlings. Plant Physiol. 126(3):1024–1030. doi: https://doi.org/10.1104/pp.126.3.1024.
- Cao, S., Z. Hu, Y. Zheng, and B. Lu. 2010. Synergistic effect of heat treatment and salicylic acid on alleviating internal browning in cold-stored peach fruit. Postharvest Biol. Technol. 58(2):93–97. doi: https://doi.org/10.1016/j.postharvbio.2010.05.010.
- Cavalcante, I.H.L., L.F. Cavalcante, Y. Hu, and M.Z.B. Cavalcante. 2007. Water salinity and initial development of four Guava (Psidium guajava L.) cultivars in North-Eastern Brazil. J. Fruit Ornam. Plant Res 15:71–80.
- Chance, B., and A.C. Maehly. 1995. Assay of catalase and peroxidases. Methods Enzymology 11:755–764.
- Chaparzadeh, N., and E. Hosseinzad-Behboud. 2015. Evidence for Enhancement of Salinity Induced Oxidative Damages by Salicylic Acid in Radish (Raphanus sativus L.). J. Plant Physiol. Breed 5:23–33.
- Chiari, B.G., J.A. Severi, P. Credendio, C.M. De Sylos, W. Vilegas, M.A. Corrêa, and V.L. Borges Isaac. 2012. Assessment of the chemical profile, polyphenol content and antioxidant activity in extracts of Psidium Guajava L fruits. Int J Pharm Sci Res. 4:331–336.
- Crozier, A., I.B. Jaganath, and M.N. Clifford. 2006. Phenols, polyphenols and tannins: An overview, p. 1–24. In: A. Crozier and H. Ashihara (eds.). Plant secondary metabolites: Occurrence, structure and role in the human diet. Blackwell, Oxford, UK.
- Dat, J.F., H. Lopez-Delgado, C.H. Foyer, and I.M. Scott. 2000. Effects of Salicylic Acid on Oxidative Stress and Thermotolerance in Tobacco. Journal of Plant Physiology 156(5–6):659–665. doi: https://doi.org/10.1016/S0176-1617(00)80228-X.
- Dhindsa, R.S., P. Plumb-Dhindsa, and T. Thorpe. 1981. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 32(1):93–101. doi: https://doi.org/10.1093/jxb/32.1.93.
- El-Esawi, M.A., H.O. Elansary, N.A. El-Shanhorey, A.M.E. Abdel-Hamid, H.M. Ali, and M.S. Elshikh. 2017. Salicylic Acid-Regulated Antioxidant Mechanisms and Gene Expression Enhance Rosemary Performance under Saline Conditions. Front. Physiol. 8:1–14. doi: https://doi.org/10.3389/fphys.2017.00716.
- Esfandiari Ghalati, R., M. Shamili, and A. Homaei. 2020. Effect of putrescine on biochemical and physiological characteristics of guava (Psidium guajava L.) seedlings under salt stress. Sci. Hortic. 261:108961. doi: https://doi.org/10.1016/j.scienta.2019.108961.
- FAO statistics. Production. Crops, 2018. FAO. Available online at: http://www.fao.org/ag/agl/agll/spush/. Accessed 18 December 2019.
- Farhangi-Abriz, S., and K. Ghassemi-Golezani. 2018. How can salicylic acid and jasmonic acid mitigate salt toxicity in soybean plants? Ecotoxicol. Environ. Saf. 147:1010–1016. doi: https://doi.org/10.1016/j.ecoenv.2017.09.070.
- Farooq, M., S. Basra, A. Wahid, N. Ahmad, and B. Saleem. 2009. Improving the Drought Tolerance in Rice (Oryza sativa L.) by Exogenous Application of Salicylic Acid. . Journal of Agronomy and Crop Science 195(4):237–246. doi: https://doi.org/10.1111/j.1439-037X.2009.00365.x.
- Freitas, P.A.F., H.H. Carvalho, J.H. de Costa, R. Miranda, K.D.D.C. De, S., Saraiva, F.D.B. deOliveira, D.G. Coelho, J.T. Prisco, and E. Gomes-Filho, E. 2019. Salt acclimation in sorghum plants by exogenous proline: Physiological and biochemical changes and regulation of proline metabolism. Plant Cell Reports 38(3):403–416. doi: https://doi.org/10.1007/s00299-019-02382-5.
- Fujita, M., Y. Fujita, Y. Noutoshi, F. Takahashi, Y. Narusaka, K. Yamaguchi-Shinozaki, and K. Shinozaki. 2006. Crosstalk between abiotic and biotic stress responses: A current view from the points of convergence in the stress signaling networks.. Current Opinion in Plant Biology 9(4):436–442. doi: https://doi.org/10.1016/j.pbi.2006.05.014.
- Ghasemzadeh, A., H. Jaafar, and E. Karimi. 2012. Involvement of salicylic acid on antioxidant and anticancer properties, anthocyanin production and chalcone synthase activity in ginger (Zingiber officinale roscoe) varieties. International Journal of Molecular Sciences 13(12):14828–14844. doi: https://doi.org/10.3390/ijms131114828.
- Gorni, P.H., A.C. Pacheco, J.F.A. Silva, R.R. Moreli, K.D. Spera, and R.M.G. Silva. 2019. Plant elicitation with salicylic acid increases bioactive compounds content and antioxidant activity in the infusion of Achillea millefolium L.. Biosci. J. 35:289–295. doi: https://doi.org/10.14393/BJ-v35n1a2019-41788.
- Grob, F., Durner, J., Gaupels, F., 2013. Nitric oxide, antioxidants and prooxidants in plant defence responses. Frontiers in Plant Science, 4, 1–15.
- Grzeszczuk, M., P. Salachna, and E. Meller. 2018. Changes in Photosynthetic Pigments, Total Phenolic Content, and Antioxidant Activity of Salvia coccinea Buc’hoz Ex Etl. Induced by Exogenous Salicylic Acid and Soil Salinity. Molecules 23(6):1–11. doi: https://doi.org/10.3390/molecules23061296.
- Gunes, A., A. Inal, M. Alpaslan, F. Eraslan, E.G. Bagci, and N. Cicek. 2007. Salicylic acid induced changes on some physiological parameters symptomatic for oxidative stress and mineral nutrition in maize (Zea mays L.) grown under salinity. Journal of Plant Physiology 164(6):728–736. doi: https://doi.org/10.1016/j.jplph.2005.12.009.
- Hamanaka, R.B., and N.S. Chandel. 2009. Mitochondrial reactive oxygen species regulate hypoxic signaling. Current Opinion in Cell Biology 21(6):894–899. doi: https://doi.org/10.1016/j.ceb.2009.08.005.
- Hayat, S., S.A. Hasan, Q. Fariduddin, and A. Ahmad. 2008. Growth of tomato (Lycopersicon esculentum) in response to salicylic acid under water stress. Journal of Plant Interactions 3(4):297–304. doi: https://doi.org/10.1080/17429140802320797.
- Hooda, P.S., and R. Yamdagni. 1991. Salt tolerance of guava (Psidium guajava L.) and amla (Emblica officinalis) at germination stage. Res. Dev. Tech. Rep 8:36–38.
- Horvath, E., G. Szalai, and T. Janda. 2007. Induction of abiotic stress tolerance by salicylic acid signaling. Journal of Plant Growth Regulation 26(3):290–300. doi: https://doi.org/10.1007/s00344-007-9017-4.
- Idrees, M., M.M.A. Khan, T. Aftab, M. Naeem, and N. Hashmi. 2010. Salicylic acid-induced physiological and biochemical changes in lemongrass varieties under water stress. Journal of Plant Interactions 5(4):293303. doi: https://doi.org/10.1080/17429145.2010.508566.
- Jiménez-Escrig, A., M. Rincón, R. Pulido, and F. Saura-Calixto. 2001. Guava Fruit (Psidium guajava L.) as a New Source of Antioxidant Dietary Fiber. . Journal of Agricultural and Food Chemistry 49(11):5489–5493. doi: https://doi.org/10.1021/jf010147p.
- Jini, D., and B. Joseph. 2017. Physiological Mechanism of Salicylic Acid for Alleviation of Salt Stress in Rice. Rice Science 24(2):97–108. doi: https://doi.org/10.1016/j.rsci.2016.07.007.
- Kaya, C., N.A. Akram, and M. Ashraf. 2019. Influence of exogenously applied nitric oxide on strawberry (Fragaria × ananassa) plants grown under iron deficiency and/or saline stress. Physiologia Plantarum 165(2):247–263. doi: https://doi.org/10.1111/ppl.12818.
- Khan, M.H., and S.K. Panda. 2007. Alterations in root lipid peroxidation and antioxidative responses in two rice cultivars under NaCl-salinity stress. Acta Physiologiae Plantarum 30(1):81–89. doi: https://doi.org/10.1007/s11738-007-0093-7.
- Knörzer, O.C., B. Lederer, J. Durner, and P. Böger. 1999. Antioxidative defense activation in soybean cells. Physiol. Plant. 40(3):294–302. doi: https://doi.org/10.1034/j.1399-3054.1999.100306.x.
- Koyro, H.-W., P. Ahmad, and N. Geissler. 2012. Abiotic stress responses in plants: An overview, p. 1–28. In: P. Ahmad and M.N.V. Prasad (eds.). Environmental adaptations and stress tolerance 1 of plants in the era of climate change. Springer, New York.
- Li, T., Y. Hu, X. Du, H. Tang, C. Shen, and J. Wu. 2014. Salicylic acid alleviates the adverse effects of salt stress in Torreya grandis cv. merrillii seedlings by activating photosynthesis and enhancing antioxidant systems. PLoS One 9(10):1–10.
- Lotfi, A., Z. Jahanbakhshian, F. Faghihi, and S.M. Seyedi. 2015. The effect of salinity stress on survival percentage and physiological characteristics in three varieties of pistachio (Pistacia vera). Biologia 70(9):1185–1192. doi: https://doi.org/10.1515/biolog-2015-0135.
- Mittler, R. 2002. Oxidative stress, antioxidants and stress tolerance. Trends in Plant Science 7(9):405–410. doi: https://doi.org/10.1016/S1360-1385(02)02312-9.
- Munns, R., and M. Tester. 2008. Mechanisms of salinity tolerance. Annu Rev Plant Biol 59(1):651–681. doi: https://doi.org/10.1146/annurev.arplant.59.032607.092911.
- Naser Alavi, S.M., M. Arvin, and M.K. Kalantari. 2014. Salicylic acid and nitric oxide alleviate osmotic stress in wheat (Triticum aestivum L.) seedlings. . Journal of Plant Interactions 9(1):683–688. doi: https://doi.org/10.1080/17429145.2014.900120.
- Németh, M., T. Janda, E. Horváth, E. Páldi, and G. Szalai. 2002. Exogenous salicylic acid increases polyamine content but may decrease drought tolerance in maize. Plant Science 162(4):569–574. doi: https://doi.org/10.1016/S0168-9452(01)00593-3.
- Pirasteh-Anosheh, H., and Y. Emam. 2018. Modulation of oxidative damage due to salt stress using salicylic acid in Hordeum vulgare. Archives of Agronomy and Soil Science 64(9):1268–1277. doi: https://doi.org/10.1080/03650340.2018.1423556.
- Rodriguez-Dominguez, C.M., T. Buckley, G. Egea, A. de Cires, V. Hernandez-Santana, S. Martorell, and A. Diaz-Espejo. 2016. Most stomatal closure in woody species under moderate drought can be explained by stomatal responses to leaf turgor. Plant, Cell & Environment 39(9):2014–2026. doi: https://doi.org/10.1111/pce.12774.
- Safari, H., S. MadahHosseini, A. Azari, and M. Heshmati Rafsanjani. 2017. Effects of pretreatment with salicylic acid on growth and nutrient uptake of sesame seedlings under salt stress. J. Field Crops Res. 15(4):734–746.
- Sairam, R.K., K.V. Rao, and G. Srivastava. 2002. Differential response of wheat genotypes to long term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant Science 163(5):1037–1046. doi: https://doi.org/10.1016/S0168-9452(02)00278-9.
- SAS® Procedures. 1990. Version 9.1.3. SAS Institute, Cary, NC.
- Schneider, S., and W.R. Ullrich. 1994. Differential induction of resistance and enhanced enzyme activities in cucumber and tobacco caused by treatment with various abiotic and biotic inducers. Physiological and Molecular Plant Pathology 45(4):291–304. doi: https://doi.org/10.1016/S0885-5765(05)80060-8.
- Shahbazi, E.S., A. Arzani, and G. Saeidi. 2011. Effects of NaCl treatments on seed germination and antioxidant activity of canola (Brassica napus L.) cultivars. Bangladesh J. Bot. 41(1):67–73.
- Sigma Plot, Inc., San Jose California USA. (n.d.). Retrieved from http://www.sigmaplot.co.uk/products/sigmaplot/sigmaplot-details.php.
- Singelton, V.L., R. Orthofer, and R.M. Lamuela-Raventos. 1999. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Meth. Enzymol. 299:152–178.
- Spanos, G.A., and R.E. Wrolstad. 1990. Influence of processing and storage on the phenolic composition of Thompson seedless grape juice. J. Agric. Food Chem. 38(7):1565–1571. doi: https://doi.org/10.1021/jf00097a030.
- Sudhakar, C., A. Lakshmi, and S. Giridarakumar. 2001. Changes in the antioxidant enzyme efficacy in two high yielding genotypes of mulberry (Morus alba L.) under NaCl salinity. Plant Science 161(3):613–619. doi: https://doi.org/10.1016/S0168-9452(01)00450-2.
- Terzi, R., and A. Kadioglu. 2006. Drought stress tolerance and the antioxidant enzyme system in Ctenanthe setosa. Acta Biol. Cracov. Bot. 48:89–96.
- The center of information and technology. 2019. National agriculture data. The iraian ministry of agriculture, Iran, Tehran.
- War, A.R., M.G. Paulraj, M.Y. War, and S. Ignacimuthu. 2011. Role of salicylic acid in induction of plant defense system in chickpea (Cicer arietinum L.). Plant Signal Behav 6(11):1787–1792. doi: https://doi.org/10.4161/psb.6.11.17685.
- Xu, M., J. Dong, H. Wang, and L.Q. Huang. 2009. Complementary action of jasmonic acid on salicylic acid in mediating fungal elicitor-induced flavonol glycoside accumulation of Ginkgo biloba cells. Plant Cell Environ. 32(8):960–967. doi: https://doi.org/10.1111/j.1365-3040.2009.01976.x.
- Yadav, S. 2018. Correlation analysis in biological studies. J Pract Cardiovascular Sci 4(2):116–121. doi: https://doi.org/10.4103/jpcs.jpcs_31_18.
- Yordanova, R., and L. Popova. 2007. Effect of exogenous treatment with salicylic acid on photosynthetic activity and antioxidant capacity of chilled wheat plants. Gen. Appl. Plant Physiol. 33:155–170.
- Yusuf, M., S.A. Hasan, B. Ali, S. Hayat, Q. Fariduddin, and A. Ahmad. 2008. Effect of Salicylic Acid on Salinity-induced Changes in Brassica juncea. J Integr Plant Biol 50(9):1096–1102. doi: https://doi.org/10.1111/j.1744-7909.2008.00697.x.
