 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In the present study, total phenolic and flavonoid contents in the methanol extracts of kernels obtained from the selected fruits such as Mangifera indica, Citrus sinensis, Citrus limetta, Punica granatum, and Carica papaya commonly marketed in India and their antioxidant properties were studied. Total phenolic and flavonoid contents in kernel extracts (measures in mg gallic acid equivalent (GAE)/g fresh weight and mg quercetin equivalent (QE)/g fresh weight, respectively) varied significantly with species (p <0.05) and ranged between 2.35–7.79 and 0.16–1.17, respectively. Antioxidant activities in kernel extract in terms of 2, 2-Diphenyl-1-picrylhydrazyl, 2, 2ʹ-Azino-bis 3-ethylbenzothiazoline-6-sulfonic acid and ferric reducing antioxidant power (expressed in µM ascorbic acid equivalent (AAE)/g fresh weight) was found maximum in M. indica (102.8, 182.3 and 48.8, respectively) among the tested fruits. Cluster analysis based on biochemical attributes indicated that the kernel of M. indica is biochemically different from other kernels. Thus the present study concludes that the kernel of M. indica should be scaled up for industrial utilization as it possesses significantly higher antioxidant activities as compared to other kernels.
Introduction
Free radicals are generated in the human body during the normal metabolic processes as well as during their exposure to adverse pathophysiological conditions (Lakkab et al., Citation2019; Pereira et al., Citation2013). Free radicals are important in the signaling system when present in low amounts but induced cellular damage in several ways due to their unstable nature. Cell damage due to free radicals results in different types of degenerative diseases such as aging, DNA damage, which are associated with the process of carcinogenesis, cardiovascular diseases, cataracts, immune system decline, peroxidation of lipid, brain dysfunction, etc. (Nagala et al., Citation2013). Free radicals are also major causes for food quality deterioration and generation of off-odors and off-flavors, decreasing shelf life, altering texture and color, and decreasing the nutritional value of food by the oxidation of lipid and other materials (Alamed et al., Citation2009; Contini et al., Citation2014; Oudjedi et al., Citation2019).
Antioxidants are the compounds that have the ability to delay or stop the oxidation of other compounds. Therefore, antioxidants have great importance in terms of reducing oxidative stress, which could cause damage to biological molecules. Nowadays many synthetic antioxidants e.g. tertiary butylatedhydroxytoluene, butylatedhydroxyanisole, gallic acid esters, and tertiary-butylhydroquinone, etc., are used which have the potential to neutralize free radicals. Since synthetic antioxidants are less soluble and toxic to the human body, therefore discovery of new potential sources of natural antioxidants is urgently required (Parejo et al., Citation2002; Piconi et al., Citation2003).
Now a day, good health and nutrition is generally achieved by the consumption of vegetables, fruits, macro-algae, macro-fungi, etc. The residual parts of fruits and vegetables, especially peels, seeds, etc., are generally discarded after the use. These residual parts contain large amounts of nutritive compounds and have higher antioxidant potential (Sharma et al., Citation2017, Citation2015). Seed kernels of fruits have been less explored as a source of natural antioxidants, and this could be due to a lack of their popularity and commercial applications (Soong and Barlow Citation2014; Lachowicz et al., Citation2019). Currently, millions of tons of fruit seeds, pulps, and peels are wasted every year.
At present, most researches are based on the discovery of natural sources of antioxidants with pharmacological interests to use them in the prevention and therapy of several diseases (Benabderrahim et al., Citation2019; Lakkab et al., Citation2019; Saoudi et al., Citation2020). Several studies have shown that kernels of selected fruits possess more potent antioxidant activity than common fruits and vegetables. Antioxidant activities in kernel extract of Citrus (Bocco et al., Citation1998), Vitis vinifera (Jayaprakasha et al., Citation2001), Mangifera indica (Puravankara et al., Citation2000), and Lupinus spp. (Tsaliki et al., Citation1999) Glycine max, (Prakash et al., Citation2007), Nelumbo nucifera (Rai et al., Citation2006) and Carica papaya (Salla et al., Citation2016) have been studied recently. Yawadio et al. (Citation2008) reported the antioxidant activity in various extracts and fractions of Chenopodium quinoa and Amaranthus spp. seeds. Xu et al. (Citation2016) studied the variations in phenolic compounds and antioxidant activity in seed extracts of seven different cultivars of apple from China. Sharma et al. (Citation2017) reported the total phenolic contents and antioxidant activities in seed extracts of Olea ferrugenia Royle at two stages of maturity. Antioxidant activity and phytochemical profiling of seed and peel extracts of Ceratonia siliqua have been reported by Lakkab et al. (Citation2019). Alonso-Esteban et al. (Citation2019) have studied the phenolic composition, antioxidant and antimicrobial properties of hop (Humulus lupulus) seeds.
Keeping the above in view, the present study was conducted to assess the total phenolic and total flavonoid contents as well as antioxidant potential in the methanol extracts obtained from kernels of fruit such as C. papaya, M. indica, P. granatum, C. limetta and C. sinensis, frequently consumed by local people for their health benefits. The results of the present study will help promote the industrial uses of kernels of tested fruits as a source of natural antioxidants and reduced the solid waste load in the environment up to some extent.
Materials and Methods
Seed Materials
Fruits, approximately 2 Kg each of C. papaya, M. indica, C. sinensis, P. granatum and C. limetta were collected separately in a distilled water rinsed polyethylene bag during July/August 2017 from the fruit shop corner located in the campus of Banaras Hindu University, Varanasi, India and were brought back to the laboratory. All the fruit samples were fresh, ripe, and healthy. The kernels were manually separated from the fruits and washed under running tap water followed by double distilled water. The kernels were dried using blotting paper and stored at 4OC in a refrigerator to prepare the methanol extract.
Chemicals
2, 2-Diphenyl-1-picrylhydrazyl (DPPH), 2, 2ʹ-Azino-bis 3-ethylbenzothiazoline-6-sulfonic acid (ABTS), 2,4,6-tripyridyl-S-triazine (TPTZ) were purchased from Sigma-Aldrich Pvt. Ltd., India. Folin-Ciocaltue Phenol Reagent, gallic acid, quercetin, ascorbic acid, sodium carbonate, methanol, aluminum chloride, etc., were purchased from Merck, Pvt. Ltd, India. All the chemicals used in the present study were of analytical grade.
Preparation of Methanol Extracts
For the preparation of methanol extracts of the kernels, a mixture of water and methanol (2:8 v/v) was used as an extractor. Fresh kernels (2 g) of each fruit was weighed and crushed in 25 ml aqueous methanol by using mortar and pestle. The mixture was then kept at 25 °C for 72 hours in an orbital shaking incubator (PID-B-702, Shivam, India). Then, the extract was filtered by using Whatman No. 1 filter paper and centrifuged at 5000 rpm for 10 minutes. The supernatant was collected in a conical flask and pellets were further extracted using the same procedure. The extraction procedure was repeated twice and all the supernatants were combined. The volume of supernatant/extract was reduced to 20 ml using a rotator evaporator (Shivam, India) at a constant temperature. The methanol extract was then stored at 4°C in a refrigerator till further analysis.
Determination of Total Phenolics Content
Total phenolics content in the methanol extracts of kernels obtained from the selected fruits was quantified by using the method of Wolfe et al. (Citation2003). Briefly, 0.5 ml of double-distilled water was added to 125 µl of methanol extract and was mixed well after adding 150 µl of Folin-Ciocalteu Phenol Reagent. The reaction mixture was then kept at room temperature for 6 minutes. After that 1.25 mL of aqueous sodium carbonate solution (7%, w/v) was added to the reaction mixture. The final volume of the samples was maintained to 3 ml by adding double-distilled water. Reaction mixtures were then allowed to stand for 90 minutes at room temperature and the absorbance was read at 760 nm using a spectrophotometer (Model no.166, Systronics, India). Different concentrations (0–0.5 mg/ml) of gallic acid were used for the preparation of the standard curve. Total phenolics content was expressed as mg GAE/g fresh weight.
Determination of Total Flavonoids Content
Total flavonoids content in the methanol extracts of kernels was quantified by using the aluminum chloride colorimetric method as described by Ordon-Ez et al. (Citation2006). Briefly, 1.0 ml of methanol extract was mixed with 1 ml of 2% (w/v) AlCl3 and shaken thoroughly. The sample mixture was allowed to stand for 60 minutes at room temperature. The absorbance of the golden yellow color was measured at 420 nm using a spectrophotometer (Model no.166, Systronics, India). Different concentrations of quercetin ranging between 0–0.05 mg/ml were used for the preparation of the standard curve. Total flavonoids content in methanol extracts was expressed as mg QE/g fresh weight.
Antioxidant Assays
DPPH Assay
The effect of methanol extracts of kernels of tested fruits on scavenging of DPPH radical was estimated using the method of Liyana-Pathiranan and Shahidi (Citation2005). Briefly, 1.0 ml of methanol extracts were mixed properly with 5.0 ml of DPPH (0.135 mM) and the reaction mixture was left for 30 minutes in dark at room temperature. The absorbance of the reaction mixture was measured at 517 nm using a spectrophotometer (Model no.166, Systronics, India). The DPPH radical scavenging activity was calculated by using the equation.
Where,
Ab = Absorbance of blank
As = Absorbance of sample
ABTS Assay
ABTS assay for methanol extracts of seed kernels was performed by using the method as described by Re et al. (Citation1999) and spectrophotometer (Model No.166, Systronics, India). The stock solution of 7 mM 2, 2-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid), and 2.4 mM potassium per sulfate were prepared in methanol. The working solution was prepared by mixing an equal volume of 7 mM ABTS and 2.45 mM potassium per sulfate and allowing them for 12–16 hours in dark at room temperature for the ABTS radical (ABTS.) production. The solution was then diluted using 80% methanol (v/v) to obtain an absorbance of 0.706 ± 0.001 at 734 nm. 1.0 ml of methanol extract was mixed with 4.0 ml of ABTS radical (ABTS.) and shaken properly. The absorbance of the reaction mixture was measured at 734 nm using a spectrophotometer (Model no.166, Systronics, India). ABTS radical scavenging activity was calculated using following equation.
Where,
Ab = Absorbance of blank
As = Absorbance of sample
FRAP Assay
FRAP assay was performed according to the method of Benzine and Strain (Citation1996). The stock solution of 300 mM acetate buffer (pH 3.6), 10 mM TPTZ (2, 4, 6-tripyridyl-s-triazine) in 40 mM HCl, 20 mM FeCl3.6H2O were prepared. The fresh working solution was prepared by mixing 25 ml of acetate buffer, 2.5 ml of TPTZ, and 2.5 ml of FeCl3.6H2O, and then the temperature of the working solution was raised up to 37°C before use. 150 µl of methanol seed extract was added to 2850 µl of FRAP working solution and the reaction mixture was kept in dark for 30 minutes. The absorbance of the reaction mixture was measured at 593 nm using a spectrophotometer (Systronics, model no. 166, India). The standard curve was prepared by using ferrous sulfate solution at concentrations ranging between 0–0.6 mM using the above-said procedure. The result for ferric-reducing antioxidant power was expressed as µM Fe (II)/g fresh weight of seed kernel.
The results obtained as % inhibition of DPPH and ABTS radicals, and FRAP activity in µM Fe (II)/g were converted to mM AAE/g FW by using a simple line equation derived from the relationship between DPPH (%) and AA (y = mx; R2 = 0.97; P = 0.002), ABTS and AA (y = mx; R2 = 0.99; P = 0.001) FRAP and AA concentrations (y = mx; R2 = 0.96; P = 0.001).
Estimation of Inhibition Concentration (IC50) Values
An IC50 value is the concentration of methanol extract required to inhibit 50% of DPPH radical activity. The IC50 value for kernel extract was calculated using a simple line equation derived from the relationship between the increasing concentrations of kernel extracts and their DPPH inhibition potential and expressed in mg/ml.
Statistical Analysis
All the experimental measurements were carried out in triplicates and results were expressed as a mean ± standard error of three analyses. The statistical analyses were performed using statistical software (SPSS, Version 16). Significant differences between the means were achieved by Duncan’s multiple range tests at p ≤ 0.05. Cluster and Principle component analysis was also performed to access the relationships between the tested parameters/plants.
Results and Discussion
Several studies have shown that the seed kernels of plants are rich in polyphenols and antioxidants which promote health benefits in human beings (Benabderrahim et al., Citation2019; Derakhshan et al., Citation2018; Saoudi et al., Citation2020; Sharma et al., Citation2017). Pieces of evidence from literature have shown that phenolic compounds inhibit the chain reaction of oxidation by acting as hydrogen donors or free radical acceptors and generation of more stable radicals (Oscar et al., Citation2020)The results of the present study are shown in and 3 and and 3. The total phenolic contents expressed as mg GAE/g FW was found maximum in seed extract of M.indica followed by C. sinensis, C. limetta, C. papaya, and least in P. granatum and also varied significantly at p ≤ 0.05 (). The variations in total phenolic contents may be ascribed to variation in plant species, habitat, age of plants, local agricultural practices, harvesting stages, etc. (Derakhshan et al., Citation2018; Sharma et al., Citation2017).
Table 1. Total phenolic and flavonoid contents in methanol extracts of kernels from selected fruits commonly consumed by urban population in India
Table 2. In-vitro antioxidant activities in methanol extracts of kernels from selected fruits commonly consumed by urban population in India
Figure 1. Cluster analysis of kernelsfrom selected fruits on the basis of their biochemical attributes
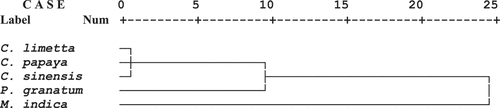
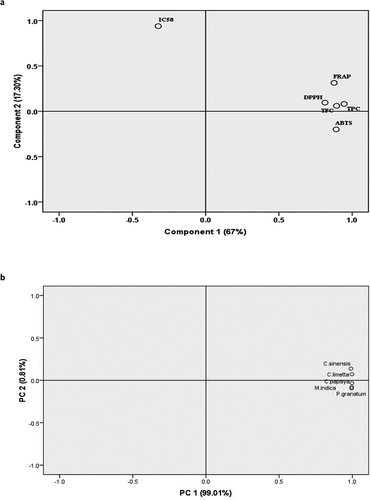
Figure 2. Principal component analysis of biochemical attributes of kernels from tested fruits (a) and tested fruits based on biochemical characterization of their kernels (b)
In the present study, the total phenolic contents in P. granatum kernels (2.35 mg GAE/g FW) was found slightly higher than those (1.29–2.17 mg GAE/g) reported by Jing et al. (Citation2012). Total phenolic contents in C. papaya kernels (3.15 mg GAE/g FW) were found greater than C. papaya seed (0.107–3.179 mg GAE/g) reported by Kothari and Seshadri (Citation2010). Kernels of M. indica showed higher content of total phenolics (7.79 mg GAE/g FW) as compared to the value (3.5–7.4 mg GAE/g) reported by Dorta et al. (Citation2012). Significant variations in total phenolics content in kernels extract, reported in the present study and literature may be ascribed to the solvent used for extraction of bioactive compounds from the plants (Ambigaipalan et al., Citation2016).
The Health benefits of flavonoids and their potent antioxidant effects are very well documented in the literature (Ghahroudi et al., Citation2017; Sharma et al., Citation2017). In the present study, the seed extract of M. indica contained the highest level of total flavonoids among the tested kernels (). Total flavonoids content in the tested kernels varied significantly (p ≤ 0.05) and ranged between 0.16 mg QE/g FW −1.17 mg QE/g FW. In the present study, total flavonoids content in M. indica seed was found higher than the ripe seeds (1.11 mg QE/g FW) of O. ferruginea, Royal (Sharma et al., Citation2017). However, total flavonoids content in the seed extracts of C. limetta, P. granatum and C. papaya were found lower than the raw seeds (0.93 mg QE/g FW) of O. ferruginea. The present study showed higher flavonoids content in C. sinensis seeds (1.05 mg QE/g FW) as compared to C. sinensis peel (0.62 mg QE/g FW-0.68 mg QE/g FW) as reported by Ghahroudi et al. (Citation2017).
The results of in-vitro antioxidant activities in methanol extracts of seed kernels of tested fruits measured in terms of DPPH, ABTS, and FRAP assays are given in . The results showed that DPPH, ABTS, FRAP activities in methanol extract of kernel varied significantly (p ≤ 0.05) and ranged from 94.14 µM AAE/g FW to 102.77 µM AAE/g FW, 159.46 µM AAE/g FW to 182.32 µM AAE/g FW and 26.5 µM AAE/g FW to 48.8 µM AAE/g FW, respectively (). The DPPH, ABTS, and FRAP activities were found maximum in methanol extract of seed kernel of M. indica and minimum in P. granatum seed extract (). In the present study, the DPPH antioxidant activity of methanol extract of P. granatum kernels was found higher (78.4%) than those (26%-54%) reported by Derakhshan et al. (Citation2018). The DPPH activity in seed extracts of C. sinensis (81.51%) was also found higher as compared to those of its peel (68%) (Ghahroudi et al., Citation2017). Seed extracts of M. indica also showed higher DPPH activity (85.64%) than those (73%) reported by Kaur and Brar (Citation2015). Variation in antioxidant activities in the seed kernels of tested plants in the present study and those reported in the literature may be ascribed to the solvents used in extraction, extraction time, temperature, stage of seeds, biochemical constituents and their particular amount present in seeds, etc.
The effect of increasing concentrations of methanol extracts of tested seed kernels on inhibition of DPPH radical is shown in . The result showed that IC50 (mg/ml) of methanol extract of tested kernels varied from a minimum of 0.68 mg/ml (C. sinensis) to a maximum of 1.70 mg/ml (P. granatum). IC50 value for the tested kernels varied significantly (p ≤ 0.05). In the present study, the IC50 value of C. sinensis is the lowest but phenolics content and antioxidant activity were found maximum in M. indica. It may be ascribed to the presence of particular chemical constituents in the kernels of C. sinensis which showed higher antioxidant activity at a lower concentration as compared to other tested kernels which had high antioxidant activity at higher concentration. In the present study, the IC50 value of C. sinensis was found lower as compared to the value reported by Ghahroudi et al. (Citation2017) in its peel (48–106 mg/ml). The IC50 value of C. papaya seed extracts (1.0 mg/ml) was found lower than those (3.11 mg/ml) reported by Kothari and Seshadri (Citation2010).
Table 3. Regression equations (Y = m x + c) and IC50 of methanol extracts of kernels from selected fruits commonly consumed by urban population in India
Total phenolics content in kernels also showed a positive correlation with antioxidant activities (Aryal et al., Citation2019; Oscar et al., Citation2020; Piluzza and Bullitta, Citation2011). In the present study, total phenolics content had positive and significant correlation with total flavonoids content (R = 0.99, p˂0.01), DPPH (R = 0.80, p˂0.05) and ABTS (R = 0.80, p˂0.05). DPPH is significantly and positively correlated with FRAP (R = 0.91, p˂0.01). Correlation study further showed a significant and positive correlation between FRAP and ABTS (R = 0.82, p < 0.05). The results indicated that the antioxidant activities in methanolic extract are associated with total phenolics and flavonoids contents.
Cluster and Principal Component Analysis
The cluster analysis was performed to identify the kernels of plants as a rich source of natural antioxidants based on tested biochemical attributes such as total phenolics, total flavonoids, DPPH, ABTS, and FRAP. Two representative clusters (cluster 1 and cluster 2) were obtained, the first cluster was represented by M. indica, whereas second cluster was represented by C. sinensis, C. limetta, C. papaya and P. granatum. The cluster analysis indicated that M. indica showed the longest arm in the cluster which indicates that the kernels of M. indica are rich in natural antioxidants as compare to other tested kernels of plants ().
The principal component analysis was performed to know the relationship between different biochemical parameters and PC 1 (67%) and PC 2 (17%) were obtained. The PC analyses showed that the maximum biochemical attributes such as total phenolics contents, total flavonoids, DPPH, ABTS, and FRAP activities were exhibited in component 1. However, IC50 was recorded in component 2. The results showed that the total phenolics and flavonoid contents, and antioxidant activities were negatively correlated with IC50 (). Further PCA was also obtained between tested plants in which PC 1 and PC 1 represents 99.01% and 0.81%, respectively. The PC analysis showed that Citrus species is slightly different than of M. indica, C. papaya and P. granatum. These variations may be ascribed to its phytochemical compositions.
Conclusion
The present study revealed that kernels, generally discarded as waste could be a potential source of natural antioxidants for pharmaceutical and food industries and could also be promoted for human health benefits. From the data, it can be recommended that the kernels of M. indica should be scaled up for industrial utilization as it contains significantly higher amounts of total phenolics and flavonoids contents as well as possess antioxidant activities. Detailed research study on the phytochemical composition of kernels of tested plants especially M. indica under different agro climatic-regions is suggested to strengthen the present findings.
Highlights
Kernels of five fruits frequently consumed by the local population were analysed for antioxidant activities.
Kernels of M. indica are rich in total phenolics and possess antioxidant activities.
M. indica is biochemically different from other tested kernels.
Abbreviations
Acknowledgments
The authors are thankful to the Head, Department of Botany, Institute of Science, Banaras Hindu University, Varanasi, and DST-FIST for providing the necessary research facilities. University Grant Commission, Government of India, New Delhi is also acknowledged for providing Junior Research Fellowship to Indrajeet Kumar and financial support to the present work.
Disclosure statement
The authors declared that there is no conflict of interest.
Additional information
Funding
References
- Alamed, J., W. Chaiyasit, D.J. McClements, and E.A. Decker. 2009. Relationships between free radical scavenging and antioxidant activity in foods. J. Agric. Food Chem. 57(7):2969–2976.
- Alonso-Esteban, J.I., J. Pinela, L. Barros, A. Ćirić, M. Soković, R.C. Calhelha,and, and I.C.F.R. Ferreira. 2019. Phenolic composition and antioxidant, antimicrobial and cytotoxic properties of hop (Humulus lupulus L.) seeds. Ind. Crops Prod 134:154–159.
- Ambigaipalan, P., A.C. De Camargo, and F. Shahidi. 2016. Phenolic compounds of pomegranate by-products (outer skin, mesocarp, divider membrane) and their antioxidant activities. J. Agric. Food Chem. 64:6584–6604.
- Aryal, S., M.K. Baniya, K. Danekhu, P. Kunwar, R. Gurung, and N. Koirala. 2019. Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from Western Nepal. Plants 8(4):96.
- Benabderrahim, M.A., Y. Yahia, I. Bettaieb, W. Elfalleh, and K. Nagaz. 2019. Antioxidant activity and phenolic profile of a collection of medicinal plants from Tunisian arid and Saharan regions. Ind. Crops Prod. 138:111427. doi: https://doi.org/10.1016/j.indcrop.2019.05.076.
- Benzine, I.F.F., and J.J. Strain. 1996. Ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: The FRAP assay. Anal. Biochem. 239:70–76.
- Bocco, A., M. Cuvelier, H. Richard, and C. Berset. 1998. Antioxidant activity and phenolic composition of citrus peel and seed extracts. J. Agric. Food Chem. 46(6):2123–2129.
- Contini, C., R.O. Álvarez, M. Sullivan, D.P. Dowling, S.O. Gargan, and F.J. Monahan. 2014. Effect of an active packaging with citrus extract on lipid oxidation and sensory quality of cooked turkey meat. Meat Sci. 96(3):1171–1176.
- Derakhshan, Z., M. Ferrante, M. Tadi, F. Ansari, A. Heydari, M.S. Hosseini, G.O. Conti, and E.K. Sadarabad. 2018. Antioxidant activity and total phenolic content of ethanolic extract of pomegranate peels, juice and seeds. Food Chem. Toxicol. 14:108–111.
- Dorta, E., M.G. Lobo, and M. González. 2012. Using drying treatments to stabilise mango peel and seed: Effect on antioxidant activity. LWT Food Sci. Technol. 45:261–268.
- Ghahroudi, F.R., M. Mizani, K. Rezaei, and B.M. Moghadam. 2017. Mixed extracts of green tea and orange peel encapsulated and impregnated on black tea bag paper to be used as a functional drink. Int. J. Food Sci. Technol. 52:1543.
- Jayaprakasha, G.K., R.P. Singh, and K.K. Sakariah. 2001. Antioxidant activity of grape seed (Vitis vinifera) extracts on peroxidation models in vitro. Food Chem. 73(3):285–290.
- Jing, P., T. Ye, H. Shi, Y. Sheng, M. Slavin, B. Gao, L. Liu, and L.L. Yu. 2012. Antioxidant properties and phytochemical composition of China-grown pomegranate seeds. Food Chem. 132:1457–1464.
- Kaur, A., and J.K. Brar. 2015. Use of mango seed kernels for the development of antioxidant rich biscuits. Int. J. Sci. Res. 78(96):535–538.
- Kothari, V., and S. Seshadri. 2010. Antioxidant activity of seed extracts of Annona squamosa and Carica papaya. Nutr. Food Sci. 40:403–408.
- Lachowicz, S., L. Seliga, and S. Pluta. 2019. Distribution of phytochemicals and antioxidative potency in fruit peel, flesh, and seeds of saskatoon berry. Food Chem. 125430. doi: https://doi.org/10.1016/j.foodchem.2019.125430.
- Lakkab, I., H. El Hajaji, N. Lachkar, R. Lefter, A. Ciobica, B. El Bali, and M. Lachkar. 2019. Ceratonia siliquac L. seed peels: phytochemical profile, antioxidant activity, and effect on mood disorders. J Funct Foods 54:457–465.
- Liyana-Pathiranan, C.M., and F. Shahidi. 2005. Antioxidant activity of commercial soft and hard wheat (Triticum aestivum L.) as affected by gastric pH conditions. J. Agric. Food Chem. 53:2433–2440.
- Nagala, S., M. Yekula, and R.R. Tamanam. 2013. Antioxidant and gas chromatographic analysis of five varieties of jackfruit (Artocarpus) seed oils. Drug Invent. Today 5(4):315–320.
- Ordon-Ez, A.A.L., J.D. Gomez, M.A. Vattuone, and M.I. Isla. 2006. Antioxidant activities of Sechiumedule (Jacq.) swart extracts. Food Chem. 97:452–458.
- Oscar, S.A., C.N. Antonio, G.V. Marina, R.S. Elsa, and V.A. Gabriel. 2020. Phytochemical screening, antioxidant activity and in vitro biological evaluation of leave extracts of Hyptis suaveolens (L.) poit. S. Afr. J. Bot. 128:62–66.
- Oudjedi, K., S. Manso, C. Nerin, N. Hassissen, and F. Zaidi. 2019. New active antioxidant multilayer food packaging films containing Algerian Sage and Bay leaves extracts and their application for oxidative stability of fried potatoes. Food Control 98:216–226.
- Parejo, I., F. Viladomat, J. Bastida, A. Rosas-Romero, N. Flerlage, J. Burillo, and C. Codina. 2002. Comparison between the radical scavenging activity and antioxidant activity of six distilled and non-distilled mediterranean herbs and aromatic plants. J. Agric. Food Chem. 50(23):6882–6890.
- Pereira, C., R.C. Calhelha, and L. Barros. 2013. Ferreira. Antioxidant properties, anti-hepatocellular carcinoma activity and hepatotoxicity of artichoke, milk thistle and borututu. Ind. Crops Prod 49:61–65.
- Piconi, L., L. Quagliaro, and A. Ceriello. 2003. Oxidative stress in diabetes. Clin. Chem. Lab. Med. 41(9):1144–1149.
- Piluzza, G., and S. Bullitta. 2011. Correlations between phenolic content and antioxidant properties in twenty-four plant species of traditional ethnoveterinary use in the mediterranean area. Pharm. Biol. 49(3):240–247.
- Prakash, D., G. Upadhyay, B.N. Singh, and H.B. Singh. 2007. Antioxidant and free radical-scavenging activities of seeds and agri-wastes of some varieties of soybean (Glycine max). Food Chem. 104:783–790.
- Puravankara, D., V. Boghra, and R.S. Sharma. 2000. Effect of antioxidant principles isolated from mango (Mangifera indica L) seed kernels on oxidative stability of buffalo ghee (butterfat). J. Sci. Food Agric. 80(4):522–526.
- Rai, S., A. Wahile, K. Mukherjee, B.P. Saha, and P.K. Mukherjee. 2006. Antioxidant activity of Nelumbo nucifera (sacred lotus) seeds. J .Ethnopharmacol. 104:322–327.
- Re, R., N. Pellegrini, A. Proteggente, A. Pannala, M. Yang, and E.C. Rice. 1999. Antioxidant activity applying an improved ABTS radical cation decolourization assay. Free Radic. Biol. Med. 26:1231–1237.
- Salla, S., R. Sunkara, S. Ogutu, L.T. Walker, and M. Verghese. 2016. Antioxidant activity of papaya seed extracts against H2O2 induced oxidative stress in HepG2 cells. LWT Food Sci. Technol. 66:293–297.
- Saoudi, S., S. Khennouf, N. Mayouf, S. Amira, S. Dahamna, and K. Hosni. 2020. Phytochemical screening and in vivo and in vitro evaluation antioxidant capacity of Fargaria ananassa, Prunus armeniaca and Prunus persica fruits growing in Algeria. Progress in Nutrition. 22 (1): 236-252.
- Sharma, R.K., R. Kundra, S.S. Samant, and S.K. Nandi. 2017. Antioxidant properties of methanol extracts from Olea ferruginea royle seeds. National Acad. Sci. Lett. 40(5):379–382.
- Sharma, R.K., N. Sharma, S. Khatri, and R. Kundra. 2015. Antioxidant properties of fruit pulp and peel of eight apple cultivars grown in Himachal Pradesh. Int. J. Food Nutr. Sci. 4(4):102–108.
- Soong, Y.Y., and P.J. Barlow. 2004. Antioxidant activity and phenolic content of selected fruit seeds. Food Chem. 88(3): 411–417.
- Tsaliki, E., V. Lagouri, and G. Doxastakis. 1999. Evaluation of the antioxidant activity of lupin seed flour and derivatives (Lupinus albusssp. Graecus). Food Chem. 65(1):71–75.
- Wolfe, K., X. Wu, and R.H. Liu. 2003. Antioxidant activity of apple peels. J. Agric. Food Chem. 51:609–614.
- Xu, Y., M. Fan, J. Ran, T. Zhang, H. Sun, M. Dong, and H. Zheng. 2016. Variation in phenolic compounds and antioxidant activity in apple seeds of seven cultivars. Saudi J. Biol. Sci. 23(3):379–388.
- Yawadio, N.R., H. Kikuzaki, and Y. Konishi. 2008. Antioxidant activity of various extracts and fractions of Chenopodium quinoa and Amaranthus spp. seeds. Food Chem. 106(2):760–766.
