?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This study aimed to investigate the chemical composition and antifungal activities of essential oil (EO) of Teucrium luteum subsp. flavovirens. The analysis of EO using gas chromatography (GC-FID) and gas chromatography-mass spectroscopy (GC-MS) allowed the identification of 63 compounds, accounting for 89.9% of the total oil. The major constituents were E-nerolidol (31.0%), β-pinene (6.9%), elemol (5.0%), and α-pinene (4.5%). The antifungal activities of EO in liquid and vapor-phase were evaluated in vitro against three apple pathogenic fungi using poisoned food (PF) and volatile activity (VA) techniques. The results indicated that the EO inhibited significantly the mycelial growth of all strains tested (p < .05). The minimum inhibitory concentration (MIC) against Alternaria sp. was 2 µL/mL in VA assay, whereas >2 µL/mL in PF technique for all strains. Fungal spore production, in vapor phase, was completely inhibited at 2 µL/mL for three phytopathogens. The antifungal activities of T. luteum subsp. flavovirens from the Southeast of Morocco are reported for the first time.
Introduction
Fruits and vegetables are the most important foods for human health thanks to their varied and balanced compositions. They offer important nutritional elements to the human organism and thus contribute to the prevention of chronic degenerative diseases (Nartvaranant, Citation2019; Saravanan and Parimelazhaga, Citation2014). Therefore, more or less long storage of these fruits is crucial to maintain their nutritional qualities and preserve their organoleptic properties (Fallahi et al., Citation2013). However, even if this step is essential, it is also delicate and sensitive because the fruits can, unfortunately, be subjected to deterioration and economic loss caused mainly by phytopathogenic fungi (Tripathi et al., Citation2008; Zain, Citation2011). To overcome these post-harvest diseases, many techniques have been implemented. Indeed, chemical control by the use of fungicides is the most widespread method currently for controlling postharvest fungal decay. However, excessive application of these molecules has harmful effects both on the environment and on human health, and also leads to the appearance of fungal resistance. These problems lead scientists to develop eco-friendly alternatives that reduce the use of these synthetic molecules to preserve the postharvest quality of fruits (Aminifard and Bayat, Citation2018).
Recently, biologically active essential oils offer a very promising alternative for disease management in food and agriculture industries. This orientation has its origin in the fact that the production of these molecules by plants is mainly a defense mechanism against pathogens and pests (Suhr and Nielsen, Citation2003). Then, they have been exhibited antimicrobial and antifungal properties and thus could be served as natural antifungal agents in the field of fruit protection in post-harvest (Laghchimi et al., Citation2014a, Citation2014b, Citation2013b; Znini et al., Citation2011, Citation2013a). Several bioassays are well known and commonly used to evaluate or screen the in vitro antifungal activities of essential oil or a pure compound. However, two methods are used especially for liquid and vapor-phase testing, such as contact direct or poisoned food (PF) and volatile activity (VA) assays (Laghchimi et al., Citation2014a, Citation2014b, Citation2013b; Znini et al., Citation2011; Znini et al. Citation2013a).
Botanically, Teucrium luteum subsp. flavovirens (Batt.) Greuter & Burdet is a synonym to T. polium subsp. flavovirens (Marzouk and El-Badan, Citation2018; Navarro and El Oualidi, Citation1999). It is an aromatic and endemic plant growing wild in Middle and High Atlas of Morocco. It is a very ligneous perennial plant, height 10–35 cm. The opposite leaves are grayish-green, oval-elongated, with a coiled edge and deeply crenate. The flowers are grouped in oval-rounded terminal heads with a yellow corolla in a globular inflorescence. The calyx with narrow triangular teeth and a stiff, branching yellow indiment (Navarro and El Oualidi, Citation1999). T. luteum subsp. flavovirens usually develops in regions belonging to the arid and perhumid bioclimates with hot and extremely cold variants. It grows in rocks and rock, in low and medium mountains, on calcareous and siliceous substrates (El Oualidi et al., Citation2002). This plant, known locally by the vernacular name of “Jaadah or Tayrart”, is considered to have medicinal properties ().
In the literature, there is only one paper report the composition of the essential oil (EO) of T. luteum subsp. flavovirens from Morocco (Ouknin et al., Citation2019), whereas, the phytochemical screening revealed the existence of certain classes of secondary metabolites such as caffeic acid esters and flavonoids (El Oualidi et al., Citation2002) and a family of neo-clerodane diterpenoids (Castro et al., Citation2010). However, there is no previous study on antimicrobial activities of this species, and therefore, the present study was made, for the first time, to determine the chemical composition of T. luteum subsp. flavovirens EO and to evaluate its antifungal activities in liquid and vapor-phase against three phytopathogens causing the deterioration of apple, namely: Alternaria sp., Penicillium expansum and Rhizopus stolonifera by implementing the poisoned food (PF) and the volatile activity (VA) methods.
Materials and Methods
Plant Material and EO Isolation
The aerial parts of T. luteum subsp. flavovirens were collected wildly, at the full flowering stage, from Tizi n’talghoumt (32°37ʹ48” N and 4°31ʹ45” O) located in mountain zones with altitudes up to 1907 m from the south-east of Errachidia (Morocco). A voucher specimen (CIM HERB # 41) was deposited in the Herbarium of Faculty of Sciences and Techniques, Errachidia, Morocco. The EO was prepared from 100 g of dried vegetal material by hydrodistillation for 3 h using a Clevenger type apparatus, according to the method recommended in the European Pharmacopoeia (European Pharmacopeia, Citation1997).
Gas Chromatography (GC-FID) Analysis
The GC-FID analysis of EO components was carried out using a Perkin-Elmer Autosystem XL GC equipped with fused-silica capillary columns Rtx-1 (polydimethylsiloxane) and Rtx-wax (polyethyleneglycol) (60 m × 0.22 mm i.d., film thickness 0.25 μm). Temperature program: from 60°C to 230°C at 2°C/min and then held isothermally at 230°C for 35 min. Injector and detector temperatures were held at 280°C. Samples were injected in the split mode (1/50), the carrier gas was H2 (1 ml/min); the volume injected was 0.2 μl of pure oil. The retention index (RI) of the compounds was determined relative to the retention time of a series of n-alkanes (C5-C30) using the Van den Dool and Kratz equations (Dool and Kratz, Citation1963).
Gas Chromatography-Mass Spectroscopy (GC-MS) Analysis
Samples were analyzed using a PerkinElmer Turbo mass detector (quadrupole) coupled to a PerkinElmer Autosystem XL. chromatograph equipped with Rtx-1 and Rtx-wax fused-silica capillary columns. The carrier gas was helium (1 mL/min), the ion source temperature was 150 C, the oven temperature was programmed from 60 to 230 C at 2 C/min and then held at 230 C for 35 min. The injector was operated in the split (1/80) mode at a temperature of 280 C, the injection volume was 0.2 mL of pure oil, the ionization energy was 70 eV, and the EI/MS data were acquired over the mass range 356350 Da (Znini et al., Citation2013a).
Components Identification
The identification of the components was based: (i) on the comparison of their GC retention indices (RI) on nonpolar and polar columns with those of authentic compounds or literature data (Joulain and König, Citation1998) and (ii) on computer matching with commercial mass spectral libraries (Adams, Citation2004) and comparison of spectra with those of our library.
Antifungal Activity Assay (Mycelial Growth Inhibition)
Three fungal isolates causing apples rot: Alternaria sp., Penicillium expansum and Rhizopus stolonifer were isolated directly from rooted apples collected from different rooms in Midelt station (Morocco), according to the methodology described in our previous works (Laghchimi et al., Citation2014a, Citation2014b, Citation2013b; Znini et al., Citation2011; Znini et al. Citation2013a). The antifungal activities of the studied EO against mycelial growth of fungi isolated were undertaken using PF technique and VA assays according to the methodology described in our previous works (Manssouri et al., Citation2016) with some modifications in VA assay. Indeed, the Petri dishes (90 × 20 mm) were filled with 20 mL (PDA) medium and then seeded with a mycelial disc (6 mm diameter), cut from the periphery of 7-days–old mycelium culture of the tested fungi. The Petri dishes were inverted and sterile filter paper discs (9 mm in diameter) impregnated with different above concentrations of essential oil (0.125, 0.25, 0.5, 1, and 2 μL/mL distilled water with sterile agar suspension (0.2%)) of EO were attached to the inverted lid (1 disc per lid). For corresponding control, an equal amount of distilled water with sterile agar suspension (0.2%) was poured on the sterilized paper filter. The plates were then sealed immediately by parafilm to prevent loss of essential oil vapors and incubated for 11 day for Alternaria sp., 7 days for P. expansum and 30 hours for R. stolonifer at 25 ± 2°C.
The fungitoxicity of EO was expressed in terms of percentage of mycelial growth inhibition (I %) and calculated following the formula (Pandey et al., Citation1982):
where Dt and Di represent mycelial growth diameter in control and treated Petri plates, respectively. The measurements were used to determine the Minimum Inhibitory Concentration (MIC) (lowest concentration of the EO that will inhibit the visible growth of a microorganism after overnight incubation). The EC50 values (concentration causing 50% inhibition of mycelial growth on control media) were calculated using probit analysis (SPSS program version 15.0 for Windows A).
In both types of experiments, the fungistatic–fungicidal nature of essential oil was determined after transferring the discs from the Petri dish which had 100% inhibition to a new plate with fresh PDA medium not supplemented by essential oil. After given incubation time, the resumption of mycelial growth means that the essential oil exerts a fungistatic action, while the absence of this growth indicates the death of the fungal strain, hence the essential oil has a fungicidal effect. The minimum fungicidal concentration (MFC) was also determined by parallel experiments.
Spore Production Assay
Fungal spore production was tested using the modified method of Tzortzakis and Economakis (Citation2007). The spores of the previously exposed colonies by EO in both techniques were collected by adding 5 mL sterile water containing 0.1% Tween-20 to each Petri dishes and rubbing the surface three times with the sterile L-shaped spreader to free spores. The spore suspensions obtained were filtered through sterilize cheesecloth into a sterile 50 mL glass beaker and homogenized by manual shaking. Spore concentration was estimated using a hemocytometer slide (depth 0.1 mm, 0.0025 mm2). The percentage reduction of spore production was computed by the following equation:
where Nt and Ni represent the number of spores in control and treated Petri plates, respectively.
Data Analysis
Data were performed by an analysis of variance (ANOVA). The mean and standard error of data were calculated using SPSS program version 15.0 for Windows. The separation of means was done by using the Least Significant Difference (LSD) test at p< .05.
Results and Discussion
Essential Oil Composition
The hydrodistillation of aerial parts of T. luteum subsp. flavovirens gave yellow oils with pleasant odor and yield was found as 0.94% (v/w). This result fits into the overall context of the yield of T. polium, which ranged from 0.05% to 1.5% (Djabou et al., Citation2012). The analysis of EO composition was carried out using GC and GC-MS and the components were identified by comparison of their EI-mass spectra and their retention indices (RI) with those of our authentic compound library with those reported in the literature. The composition and the chromatogram of the oil are given in and .
Table 1. Composition of the EO of T. luteum subsp. flavovirens from Southeast of Morocco
Figure 2. Chromatographic profile on apolar column (a) and polar column (b) of T. luteum subsp. flavovirens EO
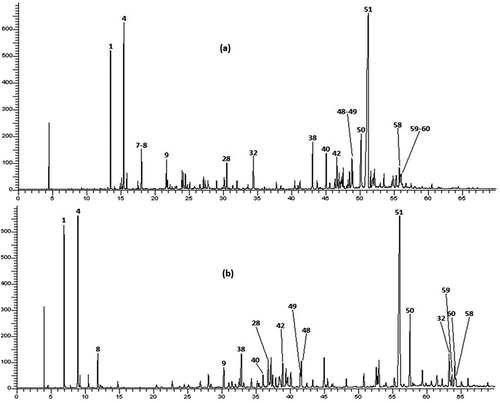
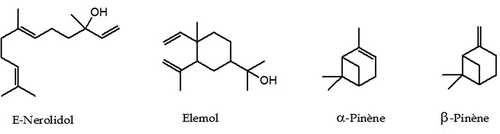
As shown in , 63 components were identified, representing 89.9% of the total oil content which among them, 6 monoterpene hydrocarbons, 27 oxygenated monoterpenes, 12 sesquiterpene hydrocarbons, and 17 oxygenated sesquiterpenes. This EO was characterized by a large amount of sesquiterpenic fraction with 65.3% of the total oil, which the oxygenated sesquiterpenes account 41.7% and hydrocarbon sesquiterpenes represent 23.6%. However, the content of monoterpenic fraction was 27.4% of the total oil, mostly attributed to hydrocarbon monoterpenes with a percentage of 20%. E-Nerolidol was the main constituent accounting for 31.0% of the total oil content, followed by β-pinene (6.9%), elemol (5.0%), and α-pinene (4.5%). The chemical structures of the most abundant compounds identified in. T. luteum subsp. flavovirens EO are presented in .
In the literature, there is only one report on the composition of the EO of T. luteum subsp. flavovirens from Morocco harvested on 10 stations from Southern Morocco (Ouknin et al., Citation2019). Fifty-five compounds were identified in using GC and GC-MS, which the major components were elemol (16.4%), α-pinene (12.0%), trans-caryophyllene (7.0%), α-humulene (6.4%), β-pinene (5.7%), and γ-eudesmol (5.3%). For comparison purposes, the chromatographic profile indicates that quantitative and semi qualitative differences between the chemical compositions of both samples were observed. Indeed, the highest percentage of E-nerolidol (31.0%) was detected in our Sample, whereas it was detected with the lowest percentage in Sample B (1.7%). Elemol found in sample B as a major constituent (16.4%) was about three times that produced by our sample (5.0%). In the case of α-Pinene, it was detected with a large amount (12.0%) in Sample B than (4.5%) in our Sample. For β-Pinene, the percentages were detected in quasi-similar amounts in both Samples (6.9% and 5.7%, respectively). The differences observed in EO composition of two samples could be attributed to several factors such as ecological conditions, geographical locations, plant organ type and stage of vegetative cycle and edaphic conditions. These factors can act on the terpenes biosynthetic pathways, thus contributing to the emergence of different chemical profiles (Croteau and Gershenzon, Citation1994). In addition, the differences in two chemoprofiles obtained from two samples may be attributed to the effect of the preparation procedure. Indeed, Ouknin et al. (Citation2019) reported that their sample is a collective EO represents the average of a mixture of equal quantities of oils collected from 10 different stations. Contrariwise, our sample is obtained by hydrodistillation of the aerial parts of T. luteum subsp. flavovirens harvested at a single station.
Mycelial Growth Inhibition
The results obtained in assays of antifungal activities at different concentrations of T. luteum subsp. flavovirens EO by PF and VA techniques against mycelial growth of three studied isolates are summarized in . The data obtained, in both techniques, demonstrated that this EO exhibited antifungal activity against tested phytopathogens, with varying effects at different concentrations. The mycelial radial growth of fungal isolates tested was inhibited significatively (p < .05) and in a dose-dependent manner.
Table 2. Mycelial radial growth inhibition of Alternaria sp., P. expansum and R. stolonifer at various concentrations of T. luteum subsp. flavovirens EO in liquid and vapor-phase
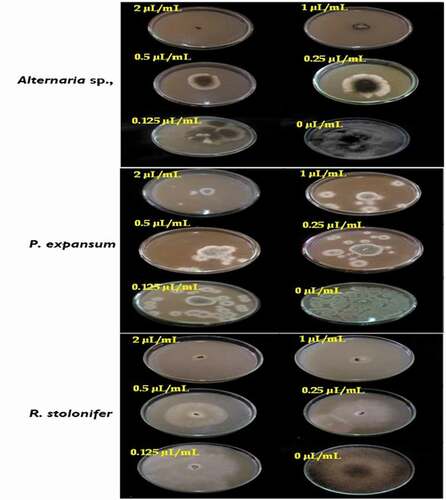
In PF technique, the results showed that Alternaria sp. and P. expansum are the fungal pathogens susceptible to the T. luteum subsp. flavovirens EO in liquid phase with the EC50 values are 0.32 and 0.34 µL/mL, respectively. Additionally, the mycelial growth inhibition of both pathogens increases gradually with the concentration reaching the maximum value of 86.24 ± 0.17 and 83.80 ± 1.27% at 2 µL/mL, respectively. However, R. stolonifer had a high resistance to the EO with the EC50 value is 1.25 µL/mL. The mycelial growth of this strain was suppressed percentage of inhibition at 0.125 µL/mL (0.00%), whereas, from 1 µL/mL the action of the oil was moderate (42.44 ± 1.21%) and had a significant effect at high concentrations (71.22 ± 2.39% at 2 µL/mL). These obtained results indicated that the MICs values of studied oil were >2 µL/mL against all tested strains ().
Figure 4. Effect of the concentrations of T. luteum subsp. flavovirens EO in liquid phase on the mycelial radial growth of fungal isolates tested
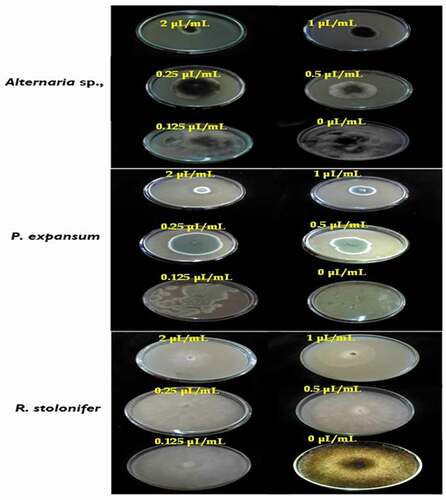
Using VA assay, the results showed that all the strains tested are susceptible to the EO in the volatile phase. Indeed, the vapor activity was more pronounced for Altenaria sp. (EC50 = 0.22 µL/mL) where the percentage of inhibition reaches a maximum of 100% at 2 µL/mL, indicating that this dose corresponds to the MIC of oil against Altenaria sp. Moreover, the results obtained after the transfer of mycelial discs where growth inhibition was complete by EO vapor into fresh PDA medium without this oil showed that mycelial growth of Altenaria sp., grew after incubation for the second day, indicating a fungistatic effect of this oil against this isolate (MFC >2 µL/mL).
However, the T. luteum subsp. flavovirens EO in vapor phase exhibited a moderate to high antifungal activity against the other 2 fungi with the EC50 values were 0.25 and 0.37 µL/mL, respectively. The EO vapor had a significant effect at 2 µL/mL (87.11 ± 1.77 and 81.79 ± 1.29% for P. expansum and R. stolonifer, respectively), suggesting that the MICs values against these fungal strains were >2 µL/mL (). Furthermore, it is important to note that the inhibitory effect of the VA assay was more effective than PF technique. In this latter assay, relatively higher concentrations were required to inhibit mycelial growth.
Spore Production Assay
The effects of the T. luteum subsp. flavovirens EO on spore production of each fungi tested are shown in . As observed in mycelial growth inhibition experiments, the VA assay of EO vapor was found to be more effective on spore production inhibition than the PF technique with complete inhibition was observed at 2 µL/mL. The results showed that spore production was significantly (p< .05) inhibited by different concentrations of EO vapor.
Figure 6. Effects of different concentrations of S T. luteum subsp. flavovirens EO in vapor phase on the spore production of the three fungal strains
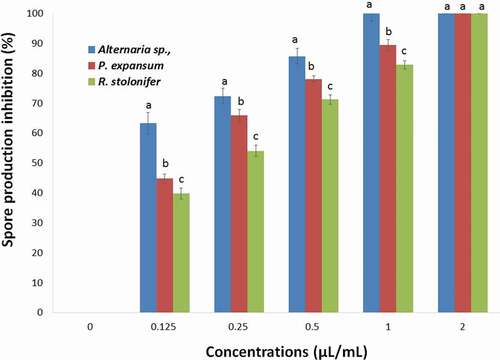
Figure 7. Effects of different concentrations of T. luteum subsp. flavovirens EO in liquid phase on the spore production of the three fungal strains
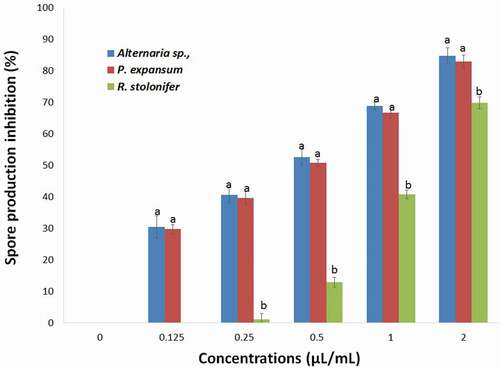
However, no significant difference was observed for the effect of EO in liquid phase on the spore production of Alternaria sp., and P. expansum. This phase exhibited a moderate to potent inhibitory effect and R. stolonifer in the range of 29.77–84.74% at a concentration ranging from 0.125 to 2 µL/mL.
To the best of our knowledge, the antifungal activity against phytopathogenic fungi of this oil has not yet been reported. In this context, this study showed that T. luteum subsp. flavovirens EO exhibited antifungal activity against the radial growth of all the phytopathogenic fungi studied. This activity is related to its chemical composition rich in oxygenated terpenes (49.1%) and hydrocarbons terpenes (43.6%), especially the major components E-nerolidol (31.0%), β-pinene (6.9%), elemol (5.0%), and α-pinene (4.5%). For instance, it was reported that the W. saharae EO with E-Nerolidol as among major compounds (23%) showed antifungal activities in liquid and vapor phase against various pathogens tested (Znini et al., Citation2013b). Also, the antifungal activity of nerolidol against hyphal growth of Trichophyton mentagrophytes was confirmed by the work of Park et al. (Citation2009). Moreover, the inhibitory activity of an EO results from a complex interaction between its different constituents. For this, synergistic effects between the major and minor constituents present in this natural mixture should be taken into consideration to account for its overall biological activity. It has been reported in the literature that the oxygenated terpenes and their respective hydrocarbons have enormous potential for strongly inhibiting the growth of fungal pathogens inducing lysis and cytoplasmic evacuation in fungi (Fiori et al., Citation2000). Additionally, the antifungal activity of these compounds may be attributed to their interference with certain enzymatic reactions during cell-wall synthesis, causing changes in cell permeability by disrupting lipid packing and changes to membrane properties and functions (Zambonelli et al., Citation1996).
According to the results obtained in this study, EO vapor phase assay shows better antifungal activity against the pathogens tested than that observed in the liquid phase. These results are in agreement with those reported in the literature. Indeed, the EO from Salvia aucheri var. mesatlantica inhibited significatively the mycelial growth of all fungal strains tested with the MICs were 2 µL/mL in VA assay, whereas >2 µL/mL in PF technique (Laghchimi et al., Citation2014a). Also, the percentage of inhibition of Warionia saharae EO against mycelia growth of Alternaria sp, was 84.44% at 2 µL/mL using PF technique, indicating that the MIC was >2 µL/mL. Whereas, the percentage of inhibition reaches a maximum of 100% at 2 µL/mL, indicating that this latter value was the MIC of Altenaria sp., using VA assay (Znini et al., Citation2013b). This behavior can be attributed to the high absorption of volatile hydrophobic molecules of EO by the mycelial tissue thanks to its lipophilic nature, against their low absorption in the agar-based medium thanks to its high water content (Inouye et al., Citation2001). Also, the same authors reported, in another study, that in the liquid phase, the lipophilic molecules associate to form micelles and suppress thus the attachment of the EOs to the organism, whereas in vapor phase, these molecules allow free attachment (Inouye et al., Citation2003). Consequently, these observations suggest that the physical and chemical properties (solubility and volatility) of EOs can have a considerable effect on the in vitro antimicrobial activity (Tullio et al., Citation2007). Indeed, in liquid phase, the antimicrobial activity of EO compounds depends on their diffusibility and their solubility in agar while in the vapor phase, their activity depends on the volatility of each compound (Goni et al., Citation2009).
In the same way, the inhibitory effect of the EO against the sporulation of tested fungi in vapor phase was found to be more effective in liquid phase. This reflects the impacts of volatile components produced by oil to limit the spread of pathogens by suppressing spore production or lowering spore load on surfaces or in storage (Tataoui-Elaraki et al., Citation1993; Tzortzakis and Economakis, Citation2007).
Conclusion
In conclusion, 63 compounds, accounting for 89.9% of the total of T. luteum subsp. flavovirens EO, were identified. The oxygenated sesquiterpene content possessed the highest value 41.7% of the oil, followed by the hydrocarbon sesquiterpene (23.6%), the hydrocarbon monoterpene (20.0%), and the oxygenated monoterpene (7.4). E-nerolidol (31.0%), β-pinene (6.9%), elemol (5.0%), and α-pinene (4.5%) were the major components of this EO. Furthermore, this EO was found to be an effective antifungal against mycelial growth of three apple phytopathogenic fungi in postharvest (Alternaria sp., Penicillium expansum, and Rhizopus stolonifer). The results indicated that this EO inhibited the mycelial growth of three fungal isolates tested significatively (p < .05) and in a dose-dependent manner. In vapor phase, the MIC value was 2 µL/mL against Alternaria sp., whereas >2 µL/mL in liquid phase for all studied strains. Fungal sporulation was also completely inhibited at 2 µL/mL in vapor phase for three pathogens. Therefore, this oil can be exploited as an ideal alternative to synthetic fungicides for using in the treatment of many fungal phytopathogens causing severe destruction to apples. But a further study especially under in vivo conditions is warranted to confirm the preservative capacity of this essential oil.
Acknowledgments
I wish to express my thanks to Mr Jalal El oualidi from Laboratoire of Botanique, Scientific Institute of Rabat (Morocco) for his help in plant identification.
References
- Adams, R.P. 2004. Identification of essential oil components by gas chromatography/quadrupole mass spectroscopy. Allured Publ. Crop, Carol Stream, IL, USA.
- Aminifard, M.H., and H. Bayat. 2018. Antifungal activity of black caraway and Anise essential oils against penicillium digitatum on blood orange fruits. Int. J. Fruit Sci. 18:307–319. doi: https://doi.org/10.1080/15538362.2017.1409682.
- Castro, A., S. Moco, J. Coll, and J. Vervoort. 2010. LC-MS-SPE-NMR for the isolation and characterization of neo-clerodane diterpenoids from Teucrium luteum subsp. flavovirens. J. Nat. Prod. 73:962–965. doi: https://doi.org/10.1021/np9005025.
- Croteau, R., and J. Gershenzon. 1994. Genetic control of monoterpene biosynthesis in mints (Mentha: Lamiaceae), p. 193–228. In: B.E. Ellis, G. Kuroki, and H.A. Stafford (eds.). Genetic engineering of plant secondary metabolism. Plenum Press, New York, NY.
- Djabou, N., A. Muselli, H. Allali, M. Dib, T. Boufeldja, V. Laurent, and J. Costa. 2012. Chemical and genetic diversity of two Mediterranean subspecies of Teucrium polium L. Phytochemistry. 83:51–62. doi: https://doi.org/10.1016/j.phytochem.2012.05.019.
- Dool, H., and P. Kratz. 1963. A generalization of the retention index including linear temperature programmed gas liquid partition chromatography. J. Chromatog. 11:463–471. doi: https://doi.org/10.1016/S0021-9673(01)80947-X.
- El Oualidi, J., S. Puech, and T. Navarro. 2002. Geographical variation and successive adaptive of yellow-flowered Teucrium (Labiateae) in the Mediterranean region. Bot. Rev. 68:209–234. doi: https://doi.org/10.1663/0006-8101(2002)068[0209:GVASAR]2.0.CO;2.
- European Pharmacopeia. 1997, 2004. 5th ed., Council of Europe. 3th ed. Strasbourg, Cedex. p. 217–218.
- Fallahi, E., D. Bakhshi, and B. Fallahi. 2013. Postharvest fruit quality and growth of ‘Pacific Gala’ apple trees at different ages as influenced by irrigation and rootstock. Int. J. Fruit Sci. 13:478–491. doi: https://doi.org/10.1080/15538362.2013.789279.
- Fiori, A.C., K.R. Schwan-Estrada, J.R. Stangarlin, J.B. Vida, C.A. Scapim, M.E. Cruz, and S.F. Pascholti. 2000. Antifungall activity of leaf extracts and essential oils of some medicinal plants against Didymella bryoniae. J. Phytopathol. 148:483–487. doi: https://doi.org/10.1046/j.1439-0434.2000.00524.x.
- Goni, P., P. Lopez, C. Sanchez, R. Gomez-Lus, R. Becerril, and C. Nerin. 2009. Antimicrobial activity in the vapour phase of a combination of cinnamon and clove essential oils. Food Chem. 116:982–989. doi: https://doi.org/10.1016/j.foodchem.2009.03.058.
- Inouye, S., S. Abe, H. Yamaguchi, and M. Asakura. 2003. Comparative study of antimicrobial and cytotoxic effects of selected essential oils by gaseous and solution contacts. Int. J. Aromather. 13:33–41. doi: https://doi.org/10.1016/S0962-4562(03)00057-2.
- Inouye, S., K. Uchida, and H. Yamaguchi. 2001. In-vitro and in-vivo anti-trichophyton activity of essential oils by vapour contact. Mycoses. 44:99–107. doi: https://doi.org/10.1046/j.1439-0507.2001.00618.x.
- Joulain, D., and W.A. König. 1998. The atlas of spectral data of sesquiterpene hydrocarbons. EbVerlag Hamburg.
- Laghchimi, A., M. Znini, L. Majidi, J. Paolini, J.M. Desjobert, and J. Costa. 2014a. Liquid and vapour-phase antifungal activities of essential oil of Salvia aucheri Boiss. var. mesatlantica Maire. (endemic from Morocco) against fungi commonly causing deterioration of apple. Der. Pharma. Chem. 6:370–378.
- Laghchimi, A., M. Znini, L. Majidi, F. Renucci, A. El Harrak, and J. Costa. 2014b. Chemical composition and effect of liquid and vapor phase of Lavandula multifida essential oil on mycelial growth of fungi responsible for the rot of apple. J. Mater. Environ. Sci. 5:1770–1780.
- Manssouri, M., M. Znini, A. El Harrak, and L. Majidi. 2016. Antifungal activity of essential oil from the fruits of Ammodaucus leucotrichus Coss. & Dur., in liquid and vapour phase against postharvest phytopathogenic fungi in apples. J. Appl. Pharmac. Sci. 6:131–136. doi: https://doi.org/10.7324/JAPS.2016.60520.
- Marzouk, R.I., and G.E. El-Badan. 2018. Molecular characterization of Teucrium L. (Lamiaceae) as a prerequisite for its conservation. Asian J. Biol. Sci. 11:16–23. doi: https://doi.org/10.3923/ajbs.2018.16.23.
- Nartvaranant, P. 2019. Fruit growth, fruit carbohydrate concentration and fruit nutrient concentration in dropped pummelo (Citrus grandis (L.) Osbeck) Fruit cv. Thong Dee. Int. J. Fruit Sci. 19:91–103. doi: https://doi.org/10.1080/15538362.2018.1528924.
- Navarro, T., and J. El Oualidi. 1999. Synopsis of the genus Teucrium L. (Lamiaceae) in Morocco. Acta Botan. Malac. 24:63–75. doi: https://doi.org/10.24310/abm.v24i0.8518.
- Ouknin, M., M. Chibane, J.M. Desjobert, J. Costa, and L. Majidi. 2019. Chemical profiling study and antioxidant activity of wild Teucrium luteum subsp. flavovirens essential oil from Morocco. J. Appl. Pharmac. Sci 9:98–102.
- Pandey, D.K., N.N. Tripathi, R.D. Tripathi, and S.N. Dixit. 1982. Fungitoxic and phytotoxic properties of the essential oil of H. suaveolens. Zeitschrift für Pflanzenkrankheiten und Pflanzenschutz. 89:344–349.
- Park, M.J., K.S. Gwak, I. Yang, K.W. Kim, E.B. Jeung, J.W. Chang, and I.G. Choi. 2009. Effect of citral, eugenol, nerolidol, and α-terpineol on the ultrastructural changes of Trichophyton mentagrophytes. Fitoterapia. 80:290–296. doi: https://doi.org/10.1016/j.fitote.2009.03.007.
- Saravanan, S., and T. Parimelazhaga. 2014. In vitro antioxidant, antimicrobial and anti-diabetic properties of polyphenols of Passiflora ligularis Juss. fruit pulp. Food Sci. Hum. Well. 3:56–64. doi: https://doi.org/10.1016/j.fshw.2014.05.001.
- Suhr, K.I., and P.V. Nielsen. 2003. Antifungal activity of essential oils evaluated by two different application techniques against rye bread spoilage fungi. J. Appl. Microbiol. 94:665–667. doi: https://doi.org/10.1046/j.1365-2672.2003.01896.x.
- Tataoui-Elaraki, A., H. Ferhout, and A. Errifi. 1993. Inhibition of the fungal asexual reproduction stages by three Moroccan essential oils. J. Ess. Oil Res. 5:535–545. doi: https://doi.org/10.1080/10412905.1993.9698274.
- Tripathi, P., N.K. Dubey, and A.K. Shukla. 2008. Use of some essential oils as post-harvest botanical fungicides in the management of grey mould of grapes caused by Botrytis cinerea. World J. Microbiol. Biotechnol. 24:39–46. doi: https://doi.org/10.1007/s11274-007-9435-2.
- Tullio, V., A. Nostro, N. Mandras, P. Dugo, G. Banche, M.A. Cannatelli, A.M. Cuffini, V. Alonzo, and N.A. Carlone. 2007. Antifungal activity of essential oils against filamentous fungi determined by broth microdilution and vapour contact methods. J. Appl. Microbiol. 102:1544–1550. doi: https://doi.org/10.1111/j.1365-2672.2006.03191.x.
- Tzortzakis, N.G., and C.D. Economakis. 2007. Antifungal activity of lemongrass (Cympopogon citratus L.) essential oil against key postharvest pathogens. Innovative Food Sci. Emerging Technol. 8:253–258. doi: https://doi.org/10.1016/j.ifset.2007.01.002.
- Zain, M.E. 2011. Impact of mycotoxins on humans and animals. J. Saudi Chem. Soc. 15:129–144. doi: https://doi.org/10.1016/j.jscs.2010.06.006.
- Zambonelli, A., A. Zechini d’Aulerio, A. Bianchi, and A. Albasini. 1996. Effects of essential oil on phytopathogenic fungi. Phytopathology. 144:491–494. doi: https://doi.org/10.1111/j.1439-0434.1996.tb00330.x.
- Znini, M., G. Cristofari, L. Majidi, A. El Harrak, J. Paolini, and J. Costa. 2013b. In vitro antifungal activity and chemical composition of Warionia saharae essential oil against 3 apple phytopathogenic fungi. Food Sci. Biotechnol. 22:113–119. doi: https://doi.org/10.1007/s10068-013-0056-2.
- Znini, M., G. Cristofari, L. Majidi, H. Mazouz, P. Tomi, J. Paolini, and J. Costa. 2011. Antifungal activity of essential oil from Asteriscus graveolens against postharvest phytopathogenic fungi in apples. Nat. Prod. Commun. 6:1763–1768.
- Znini, M., G. Cristofari, L. Majidi, J. Paolini, J.M. Desjobert, and J. Costa. 2013a. Essential oil composition and antifungal activity of Pulicaria mauritanica Coss., against postharvest phytopathogenic fungi in apples. Food Sci. Technol. 54:564–569.