ABSTRACT
Fruit crops offer a strong sink for sequestration of atmospheric carbon dioxide, thereby, aid in moderating the impact of climate change-related issues, besides creating a parallel nutrient sink. Mere annual application of organic manures/composts has failed to address different soil fertility functions due to failure to prolong the impact of soil organic pool on long-term basis. The paradigm shifts from purely inorganic to either organic fertilizers or in combination with chemical fertilizers and microbial inoculants (preferably in consortium mode) started gaining wide scale use for enhanced soil health vis-à-vis elevated quality production and reduced rhizosphere CO2 emmission. This change of concept later formed the basis for Integrated Soil Fertility Management (ISFM)-based strategy involving three basic components viz., microbial inoculants (biofertilizers), inorganic fertilizers, and organic fertilizers. Development of microbial consortium exploiting the microbial synergisms with variety of fruit crops as an important component of ISFM is one of the popular methods of managing multiple soil fertility constraints occurring within the rhizosphere. Further advancements in rhizosphere-specific consortia (often by scaling up crop-microbiome) mediated ISFM could further fulfil the nutrient demand and supply by crop. Upscaling such studies through rhizosphere hybridization has provided some initial inroads in harnessing the potential of different rhizosphere microbial communities. ISFM studies carried out in fruit crops in India, Iran, and China showed better agronomic response and soil health-related properties, considered very close to climate-resilient soil fertility management, a gateway to sustainable quality production. The review also highlights the future directions for ISFM in fruit crops catering to their multiple requirements.
Introduction
Fruit crops represent hardly 1% of the global agricultural land area (FAO, Citation2011). Globally, fruit crops occupy an area of 65.29 million ha (citrus>grape>banana) with a production of 883 million tons (citrus>banana>apple) and average productivity of 13.52 tons/ha (FAO, Citation2020). Approximately 1.7 million (2.8%) human deaths worldwide are attributable to micronutrient deficiency induced through suboptimum consumption of fruits and vegetables, and regarded as one of the top 10 selected risks for global mortality (WHO, 2014). In the 21st century, nutrient efficient plants will play a major role in increasing crop yields compared to the 20th century, mainly due to limited land and water resources available for crop production, higher cost of inorganic fertilizer inputs, declining trends in crop yields globally, and increasing environmental concerns (Baligar et al., Citation2001). According to one estimate, at least 60% of the world’s arable lands have mineral deficiencies or elemental toxicity problems, and on such soils, fertilizers, and lime amendments are essential for achieving improved crop yields (Pathak and Nedwell, Citation2011).
Most of the fruit crops by the virtue of their woody framework (Nutrients locked therein), extended physiological stages of growth, differential root distribution pattern (root volume distribution), growth stages from the point of view of nutrient requirement, and preferential requirement of some nutrients by specific fruit crop, collectively make them nutritionally more efficient than the annual crops (Scholberg and Morgan, Citation2012). In the backdrop of demography – driven diminishing per capita availability of land (more so in fruit crops), sustaining soil fertility management has gained a greater significance in meeting the multipronged challenges of sustaining the quality production on one hand and ensuring the carrying capacity of soil health on the other hand (Srivastava et al., Citation2002).
Renewed and intensified efforts are in progress during the past 10–15 years to grow using alternative management practices ever since the depleting soil fertility has attained a serious concern with the practice of high-density orcharding coupled with heavy use of chemical fertilizers that were immediately available to the plants for nutrient uptake (Kohli et al., Citation1998; Srivastava, Citation2010a) bringing substantial reduction in soil organic matter (Intrigliolo and Stagno, Citation2001). However, the fertilizers act in exactly the same way as nutrient from organic resources in the soil, since they are chemically the same (Srivastava et al., Citation2002). The plant itself cannot tell where the nutrient is coming from (Srivastava and Malhotra, Citation2014). In recent years, the nutrient additions have been exclusively in favor of mineral fertilizers due to demographic pressure, of demands related to life styles, and trade involvement. While, the quick and substantial response to fruit yield due to mineral fertilizers eclipsed the use of organic manures, the inadequate supply of the latter sources exacerbated this change (Ghosh, Citation2000).
It is often claimed, traditional way of fruit growing is less energy consuming than conventional method of input intensive production of fruit crops. Considering the economics of fruit production, fertilizers alone on an average, constitutes about 20–30% of total cost of production which is a significant recurring expenditure, a grower needs to invest every year (Srivastava and Singh, Citation2008) an energy intensive exercise too. Many studied have carried out energy analysis to determine the energy efficiency of orchards production, such as citrus (Ozkan et al., Citation2004), cherries (Kizilaslan, Citation2009), and apricot (Esengun et al., Citation2007), sweet cherry (Demircan et al., Citation2006) in Turkey, walnut in Iran (Banaeian et al., Citation2010), apple in Greece (Strapatsa et al., Citation2006), apricot and plum in Italy (Sartori et al., Citation2005). Studies by Namdari et al. (Citation2011) revealed that mandarin production was more energy intensive compared to sweet orange. The major energy inputs in sweet orange and mandarin production were diesel fuel (27 and 24%), chemical fertilizers (22 and 23%), and irrigation water (21 and 23%), respectively. The results showed that 62375.18 MJ/ha energy was consumed by orange orchards and 77501.17 MJ/ha by mandarin orchards. A meta-analysis of agronomic response of major fruit crops (citrus, apple, water melon, banana, grape, kiwi, jujube, mango, papaya, lychee, apple, apricot, pomegranate etc) to fertilization in China by Li et al. (Citation2019a) showed that the yield response to N, P, or K fertilizer were 7.6, 5.2, or 5.9 tons/ha, indicating relative yield of 78.08%, 82.9%, or 82.4%, respectively, with higher relative yield for deciduous fruit trees compared to evergreen fruit trees. A rationale nutrient managements, therefore, crucial for balancing yield and environmental concerns in countries like China, India, and other countries where fertilizers are often over-used.
Soils under fruit crops differ from other annually cultivated soils that after remain fallow for 3–6 months every year forcing unwarranted depletion of soil organic matter (Bhargava, Citation2002). On the contrary, biological oxidation of existing C continues in soil covered under crop. Multiple nutrient deficiencies are considered to have triggering effect on potential source of atmospheric CO2. Soil carbon stock is, hence, considered as an important criterion of determining the impact of ISFM in the longer version of impact assessment (He et al., Citation1997b; Joa et al., Citation2006). The amount of accumulated C within the rhizosphere soil does not continue to increase with time with increasing C outputs. An upper limit of C saturation level occurs, which governs the ultimate limit of soil C sink and rate of C sequestration in mineral soils, independent of C input rate. Therefore, an understanding of mechanisms involved in C stabilization in soils is needed for controlling and enhancing soil C sequestration (Goh, Citation2004) with fruit crop-based land uses.
Over the years, the concepts of integrated nutrient management (INM) and integrated soil fertility management (ISFM) have been gaining acceptance, moving away from a more sectorial and inputs-driven approach (Kirad et al., Citation2010; Srivastava et al., Citation2019, Citation2012). INM advocates the careful management of nutrient stocks and flows in a way that leads to profitable and sustained production. While, ISFM emphasizes the management of nutrient flows, but also highlights other important aspects of soil complex such as maintaining organic matter content, soil structure, moisture, and microbial biodiversity (Srivastava et al., Citation2015b). A dynamic concept of ISFM is looked upon the economic yield in terms of fruit yield coupled with quality on one hand, and soil physico-chemical and microbiological health on other hand as a marker of resistance against the nutrient mining. The current failure to strike a balance between annual nutrient demand and quantum of nutrients applied further complicated the issue of fertility management (Ghosh et al., Citation2012; Srivastava and Singh, Citation2008). Still more attention is needed toward integrated soil biological management as a crucial aspect of ISFM, since providing protection to rhizosphere against the nutrient depletion is of utmost importance for sustained orchard production, in which the objectivity of ISFM could have far reaching consequences (Srivastava, Citation2010a). Exploring microbial diversity perspectives in fruit crops is, therefore, important and equally useful to arrive at measures that can act as indicators of soil quality and sustainable orchard productivity using biological soil management ISFM (Srivastava, Citation2010b). Diagnosis of nutrient constraints and their efficient management has, therefore, now shifted in favor of ISFM through collective use of organic manures, inorganic fertilizers, and beneficial microorganisms.
Soil Fertility and Functions: Important Facts
Soil is a distinct living entity that is one of the core building blocks of land. Land consists of soil, rocks, rivers, and vegetation (Lal, Citation2010). Soil contributes five principal functions within a landscape: i. nutrient cycling; ii. water retention; iii. biodiversity and habitat; iv. storing, filtering, buffering, and transforming compounds; and v. provision of physical stability and support (Blum, Citation1993). A fully functioning soil lies at the heart of solving the big issue of food security, biodiversity, climate change, and fresh-water regulation, but to date there has been no easy way to communicate these linkages. The narrative on soils must be improved and its voice must be raised if the required response is to be achieved. The key aim in securing soil is to maintain and optimize its functionality: its diverse and complex ecosystems of soil biota, its nutrient cycling capacity, its roles as substrate for growing plants, as a regulator, filter and holder of fresh water, and as a potential mediator of climate change through the sequestration of atmospheric carbon dioxide (Koch et al., Citation2013). Soil security is explored as a conceptual framework that could be used as the basis for a soil policy framework with soil carbon as an exemplar indicator
Soil function fertility refers to the ability of soil to support and sustain plant growth, which relates to making all the essential nutrients available for root uptake. This is facilitated by their storage in soil organic matter, nutrient recycling from organic to plant available mineral forms, and physicochemical processes that control their fixation and release (Srivastava, Citation2013). On the other hand, managed soils are highly dynamic system that makes the soil work and supply ecosystem services to humans. Overall, the fertility and functioning of soils strongly depend on interactions between soil mineral matrix, plants, and microbes. These are responsible for both building and decomposing soil organic matter, and therefore for the preservation and availability of nutrients in soils, cycling of nutrients in soils must be preserved (Srivastava and Ngullie, Citation2009).
Soil health considers the physical, chemical, and biological properties of the soil and the disturbance and ameliorative responses of land managers. Soil health also describes the capacity of a soil to meet performance standards relating to nutrient and water storage and supply, biological diversity, and function and resistance to degradation. The most important of these manageable services include BNF (Biological nitrogen fixation), other symbiotic and beneficial organisms, nutrient and moisture supply, carbon storage, and protection from erosion (Srivastava and Singh, Citation2009a). Let us look at soil nutrient imbalances, nutrient mining, and sustainability of nutrient management practices. Managing soil carbon for multiple benefits address to enhance a range of ecological services. Increasing the soil organic matter of degraded soils can boost crop productivity, sequester CO2, enhance soil microbial growth and activities, and improve water capture and retention. Soil carbon stocks, highly vulnerable to human activities, decrease significantly in response to changes in land capability and land use such as deforestation and increased tillage continues (Srivastava and Singh, Citation2009b; Tagaliavini et al., Citation2007). Opportunity to use existing mechanisms to encourage active management of soil carbon – land use planning that excludes vulnerable soils from land uses that lead to soil organic carbon losses. Promotion of proper management practices to protect and enhance soil organic matter as an essential element of good soil and environmental quality. Promotion of sources of plant nutrients (e.g., cover crops, legumes, crop diversification that enhance soil organic carbon stocks. Integration of several crops in a field at the same time to increase soil organic matter, soil biodiversity, and soil health. Decline in soil fertility is the major constraint limiting the productivity of fruit crops. Continuous reduction in nutrient density of different fruit crops is an indication of nutrient mining-induced decline in fruit crop productivity (Srivastava and Ngullie, Citation2009).
Fruit Crops as Carbon Sink and Response at Elevated CO2
Pedospheric, atmospheric, and biotic carbon pools are reported to the tune of 2400, 750, and 550 Gt of carbon, respectively (Brady and Weil, Citation2004). In particular, about 80% of the biotic pool of carbon is fixed in plants and fungi (Kimmins, Citation1997). Modern agricultural practices convert the pedosphere, which is normally a carbon sink, into a significant carbon source, a process which is resulting in significant repercussions on the total amount of CO2 in the atmosphere. This is the case in modern fruit orchards, especially in areas where rainfall is infrequent during the growing season, and the soil is managed with shallow and repeated tillage (Xiloyannis et al., Citation2002).
Perennial fruit trees act as strong carbon sink by sequestering the atmospheric carbon (Sugiura et al., Citation2007). Studies in the past showed carbon sequestration capacity (tons/ha/year) of 7.96 by apple (Qaisar et al., Citation2018), 65.00 by cocoa (Kongsager et al., Citation2013), 49.50 by grape (Nistor et al., Citation2018), 14.72 by oil palm (Pulhin et al., Citation2014), 27.25 by mango (Chavan and Ganesh, Citation2012), 15.73 by coconut (Magat, Citation2009), 6.30 by kiwi (Holmes et al., Citation2015), and 76.00 by sweet orange (Kongsager et al., Citation2013). However, conversion of forest land into fruit orchard cultivation led to 5–23% and 4–21% reduction in soil organic carbon and N-stock, respectively. Compared to other soil uses, fruit crops like guava, mango, and sapota contributed to improvement of soil organic carbon stratification index (Bernardi et al., Citation2007).
There is a little information in the literature on the effects of atmospheric CO2 enrichment on mineral element concentrations in tissues of tropical C3 plants (Hocking and Meyer, Citation1991). Atmospheric CO2 is expected to increase steadily (Ehleringer and Cerling, Citation1995; Houghton and Skole, Citation1990), knowledge of changes in foliar nutrient concentrations in response to CO2 enrichment is important for diagnosing nutrient deficiencies that are based on critical concentrations (Conroy, Citation1992; Hocking and Meyer, Citation1991). Most of the macronutrients (N, P, K, Mg, S, and Na) are highly mobile and leachable except for Ca. Ca being an immobile element, can be used as indicator of carbon content in different tree components (Negi et al., Citation2003).
The total carbon emission and the heterotrophic carbon emission in the grape orchard were estimated to as 422.7 g C/m2/year and 222.5 g C/m2/year, respectively, and both values were one half of those in the peach orchard. The total carbon supply was 401.0 g C/m2/year in the grape orchard (litter from floor vegetation 54.5%; litter from grapevine 34%, and fertilizer 11.5%), and was only one-third of the value in the peach orchard. The study suggested that soil in orchards acts as a carbon sink owing to a large amount of carbon input from the floor vegetation (Sekikawa et al., Citation2003).
Elevated CO2 enhances the photosynthetic rates of fruiting trees; it should also lead to increased biomass production. In the two-year study of Centritto et al. (Citation1999a), cherry seedlings grown at 700 ppm CO2 exhibited photosynthetic rates that were 44% greater than those displayed by seedlings grown in ambient air, independent of a concomitant soil moisture treatment. In the two-year study of Centritto et al. (Citation1999b), for example, well-watered and water-stressed seedlings growing at twice-ambient CO2 concentrations displayed basal trunk areas that were 47 and 51% larger than their respective ambient controls. In a longer three-month study, Keutgen and Chen (Citation2001) noted that cuttings of Citrus madurensis exposed to 600 ppm CO2 displayed rates of photosynthesis that were more than 300% greater than rates observed in control cuttings exposed to 300 ppm CO2. Likewise, Schaffer et al. (Citation1997) reported that atmospheric CO2 enrichment to enhance the rates of net photosynthesis in various tropical and sub-tropical fruit trees, including avocado, banana, citrus, mango and mangosteen. Similarly, in a study spanning more than thirteen years, Idso and Kimball (Citation2001) demonstrated that the aboveground wood biomass of mature sour orange trees growing in air enriched with an additional 300 ppm of CO2 was 80% greater than that attained by control trees growing in ambient air. According to Pan et al. (Citation1998), elevated CO2 enhanced the photosynthesis of apple plants and altered carbohydrate accumulation in mature leaves in favor of starch and sorbitol over sucrose.
In the light of climate change-related issues, perennial fruit trees play an important role in carbon cycle of terrestrial ecosystems and sequestering atmospheric CO2 (Guimarães et al., Citation2014; Lobell et al., Citation2005). According to Wu et al. (Citation2012), net C sink and C storage in biomass of apple orchard ranged from 19 to 32 Tg C, respectively, and from 230 to 475 Tg C in 20 years period, amounting to 4.5% of total net C sink in the terrestrial ecosystems in China. In an estimate, Lakso (Citation2010) observed that an acre of apple orchard fixed about 20 tons of CO2 from the air each season, and provided over 15 tons of O2, equivalent to over 5 billion BTU’s of cooling power. While, Mwamba (Citation2012) showed that citrus trees carbon sequestration in biomass ranged from 23.9 tons CO2/ha for young trees to 109 tons CO2/ha for mature trees. These studies provide strong clues about the utility of perennial framework of fruit trees serving as an effective carbon sink, off-setting CO2 enriched climate.
Nutrient Removal versus Nutrient Requirement
Fruit crops by the virtue of their perenniality longer growth period, and developing fruits acting as major sink, are considered nutrient extracting in nature, and hence so nutrient responsive. Interestingly, nutrient uptake pattern () by major fruit crops display the fact that K–removal is far higher than N or K. However, P-removal is nearly half of N- removal. Crops like banana, citrus, grape, kiwifruit, mango etc are considered highly nutrient exhaustive crops, warranting their replenishment to ensure long-term sustainability in production (Srivastava, Citation2010a; Tandon and Muralidharadu, Citation2010).
Table 1. Nutrient removal pattern by major fruit crops
Nutrient-microbe Synergy in Unlocking Productivity
Recognition of the importance of soil microorganisms has led to an increased interest in measuring the quantum of nutrients held in their biomass (Srivastava et al., Citation2002). An increase in the microbial biomass often goes along with increased nutrient immobilization. Plant growth promoting microorganisms play an important role exerting various mechanisms such as biological nitrogen fixation, growth hormone production, phosphate solubilization siderophore production, hydrolytic enzymes production, antagonistic activity, individually or collectively leading to improved nutrient use efficiency (Singh et al., Citation2018; Wu and Srivastava, Citation2015). These metabolites can be either overproduced or combined with appropriate biocontrol strains to obtain new formulations for their more effective applications. Studies have demonstrated that Azotobacter inoculation alone can substitute upto 50% nitrogen requirement of banana and 25% phosphorus requirement of papaya (Singh and Varu, Citation2013). Arbuscular mycorrhizal fungi has also been reported to substantially improve nutrient acquisition capacity of host plant, and fruit yield in addition to enriching the rhizosphere biologically in a much activated form (Moshiri et al., Citation2019b; Wu et al., Citation2020). Mineral fertilizers on the other hand have limited direct effects, but their application can enhance soil biological activity via increases in system productivity, crop residue return, and soil organic matter (Wu et al., Citation2016). Another important indirect effect especially of nitrogen fertilization is the soil acidification, with considerable negative effects on soil organisms (Wu et al., Citation2019).
There are ample evidences accrued through worldwide research that nutrient-microbe synergy is the launching pad for any fruit crop to mobilize and accumulate the required nutrients as per the metabolic nutrient demand, a pre-requisite to improved NUE (Wu and Srivastava, Citation2015). Many genes play a central role in the acquisition and distribution of nutrients, including many protein-coding genes as well as microRNAs (miR395, miR398, miR397, and miR408) (Chiou, Citation2007; Sunkar et al., Citation2007). Oustric et al. (Citation2019) reported that higher tolerance to nutrient deficiency could be explained by better activation of their antioxidant system. However, for the other genotypes, tetraploidization did not induce greater tolerance to nutrient deficiency. Rengel et al. (Citation1996) observed that the total number of bacterial colony-forming units increased in the rhizosphere of Zn-efficient genotypes of wheat under Zn-deficiency and in Mn-efficient genotypes under conditions of Mn-deficiency. In contrast, a Zn-deficiency treatment acted synergistically with the number of fluorescent Pseudomonas in the rhizospheres. Fruit crops have displayed an excellent synergy with a variety of microbes, which could play an important role in improving the use efficiency of applied nutrients ().
Table 2. Agronomic response of microbial inoculation in different fruit crops
A still bigger question emerges, whether rhizosphere competent microbes could collectively contribute toward improved resilience of plant’s rhizosphere against potential nutrient mining. And if those microbes are so successful in promoting growth response, addition of starter nutrients in such combination may further magnify the magnitude of response called nutrient-microbe synergy. Our earlier studies have shown that rhizosphere effective microbes have the tendency to play multiple roles to overcome various biotic and abiotic stresses while interacting with an environment (Huang et al., Citation2014a, Citation2014b). Rhizosphere modification through roots by soil microorganisms exudation is an important attribute that regulates not only the availability of nutrients in the soil but also their acquisition by plants (Zou et al., Citation2014). A number of studies have suggested that whole range of microorganisms including arbuscular mycorrhizal fungi (AMF) have helped to alleviate different abiotic stresses (Chi et al., Citation2018; Moshiri et al., Citation2019b; Wang et al., Citation2014; Wu et al., Citation2014, Citation2013; Zou et al., Citation2015) in fruit crops and aid in improving the use efficiency of applied nutrients.
Microbial Consortium, a Novel Concept
The most common objective of developing microbial consortium is to capitalize on both the capabilities of individual microbes and their interactions to create useful systems in tune with enhanced productivity, and soil health improvements through efficient metabolic functionality (Brenner et al., Citation2008). Two major underlying principles are applied in the whole process of development of microbial consortium. The first one is resource ratio theory which uses both qualitatively and quantitatively to assess the outcomes between component microorganisms competing for shared limiting resources. This permits coexistence of multiple microbes or the competitive exclusion of all but a single microbe (Brauer et al., Citation2012). And the second principle theory relevant to microbial consortium is maximum power principle initially proposed by Lotka (Citation1992) and later modified at various levels, is value for analyzing consortial interactions. It also dictates that biological systems that maximize fitness by maximizing power, is analogous to metabolic rate or the capacity to capture and utilize energy (Sciubba, Citation2011). The microbial consortium is classified (Handelsman et al., Citation1998; Kim et al., Citation2008; Klitgord and Segré, Citation2011) as artificial (carrying two or more wild type microbes whose interactions do not typically occur naturally), synthetic (carrying microbes which are modified through manipulations of genetic content), and natural (carrying microbes having much wider applications like bioremediation, wastewater treatment, biogas synthesis etc.).
In the past, a number of studies have suggested the co-inoculation of different microbes is more effective than single inoculation summarized as: A. brasilense – P. striata/B. polymyxa, A. lipoferem – Agrobacterium radiobacter/A. lipoferem-Arthrobacter mysorens, A. brasilense – Rhizobium, A. brasilense – A. chroococcum – Klebsiella pneumoniae – R. meliloti, A. brasilense – R. leguminosarum, and A. brasilense/Streptomyces mutabilis – A. chroococcum (Alagawadi and Gaur, Citation1992; Belimov et al., Citation1995; Elshanshoury, Citation1995; Fabbrie and Del Gallo, Citation1995; Hassouma et al., Citation1994; Neyra et al., Citation1995; Yadav et al., Citation1992). Microbes involving AM fungi and bacteria have also been suggested for improvement in both yield and quality. These include: A. brasilense – G. fasciculatum in wheat (Gori and Favilli, Citation1995), strawberry (Bellone and de Bellone, Citation1995), A. brasilense – Pantoea dispersa in sweetpepper, and A. chroococcum – G. mosseae in pomegranate (Aseri et al., Citation2008). Later, Srivastava et al. (Citation2015b) put forward a microbial consortium, Aspergillus flavus MF113270, Bacillus pseudomycoides MF113272, Acinetobacter radioresistens MF113273, Micrococcus yunnanensis MF113274, and Paenibacillus alvei MF113275 developed through isolation, characterization, and evaluation of effective microbes from citrus rhizosphere.
Microbial Response of Rhizosphere Hybridization
Artificially, the rhizosphere can be modified or reconstruct as per the need the of plant to enhance the physiological efficiency by rhizosphere engineering, rhizosphere hybridization, creating an artificial environment suitable for the plant growth-promoting microorganisms (PGPMs) to surplus a protective layer against the pathogenic microbes (Rhizosphere fortification), or by various agronomic practices. Rhizosphere hybridization is new concept to modify the rhizosphere ecology to create an optimum environment for PGPMs to show the positive effect of plant agronomy (Keditsu and Srivastava, Citation2014). The concept of “rhizosphere hybridization” is therefore, advocated to harness the value added benefit of nutrient-microbe synergy, besides providing dynamism to microbial consortium suiting to wide range of perennial fruits (Srivastava et al., Citation2015a, Citation2015b).
Our studies (Cheke et al., Citation2018a, Citation2018b) on response of different treatments involving rhizosphere soil of three perennial trees viz., Ficus racemosa L. (Umber tree), Ficus benghalensis L. (Banyan tree), and Ficus religiosa L. (Pipal tree) along with rhizosphere soil of healthy and highly productive sweet orange trees in sweet orange buddlings showed differential response in terms of agronomic parameters, changes in soil physical properties, and pool of plant available nutrients. However, hybridized rhizosphere of sweet orange and Ficus racemosa L. out-smarted the response over other rhizosphere hybridization treatments (). These studies lend some support to the fact that inoculation of soil or crops with rhizospheric or endophytic microbes, respectively, can enhance the micronutrient contents in various plant tissues including roots, leaves, and fruits (Ku et al., Citation2019).
Table 3. Pre-evaluation response of rhizosphere hybridization in sweet orange (Citrus sinensis Osbeck) buddlings raised on Rangpur lime (Citrus limonia Osbeck)
Integrated Soil Fertility Management (ISFM) Approach
Increasing pressure on soil and water resources and soil nutrients depletion has called into question, the changing strategies and approaches of soil fertility management and plant nutrition. Globally, following the idea of sustainable development, ISFM is a holistic approach which carries a variety of overlapping components (). The conventional method of applying organic manure year after has miserably failed to inflict in a sustainable change in organic carbon of soil compared to green manure (). These observations on the other hand also suggested that this organic manuring have not been able to retain the added organic carbon to passive carbon pool of the soil. This is where, a stronger intervention of both microbial inoculants and limited use of chemical fertilizers come as interplay. Morugán-Coronado et al. (Citation2020) observed the highest response on SOC increased with the growth of permanent crops in the alleys. Soil N showed a similar trend to that of SOC, but the effect of no-tillage was not significant compared to conventional tillage. No effect was observed in tree crop yield due to the growth of alley crops, conservation tillage or organic fertilization.
Table 4. Various components of the integrated soil fertility management in fruit crops
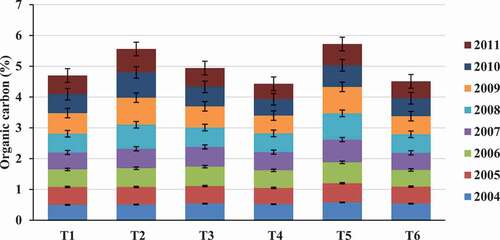
Figure 1. Changes in organic carbon during 8 years of treatment with different organic manures in comparison to inorganic fertilizers in Nagpur mandarin (Citrus reticulata Blanco) (Source: Srivastava et al., Citation2012)
ISFM: Indian Experience
Our long term study on microbial consortium-mediated ISFM (Module I–V) for sustained quality production of Nagpur mandarin (Citrus reticulata Blanco) was carried out on smectile rich black soil (Typic Ustochrept). These five modules consisted of: Module I- 100% recommended dose of fertilizer as RDF (600 g N – 200 g P – 300 g K – 200 g ZnSO4 – 200 g FeSO4 – 200 g MnSO4/tree/year), Module II – 75% RDF + 25% Vermicompost, Module III-75% RDF + 25% Vermicompost + Microbial Consortium, Module IV-50% RDF + 50% Vermicompost, and Module V – 50% RDF + 50% Vermicompost + Microbial Consortium. Pooled results obtained during 2007–16 (10 years) are summarized as follows. Maximum cumulative increase in canopy volume was observed with module-III (18.67 m3) followed by module-IV (17.16 m3), Module-V (16.93 m3), on part with Module-II (16.70 m3), and Module I- T1(11.50 m3). Incorporation of microbial consortium either with module-III or with Module – V invariably induced higher canopy volume suggesting better response on fruit yield as well. The physiological consequences of such increase in canopy volume would be to expand the fruit-bearing area and a nutrient sink for increasing fruit yield with higher number of leaves/fruit. The maximum fruit yield of 88.8 kg/tree was observed with treatment Module-V which was better than 83.2 kg/tree with T4 or 80.5 kg/tree with Module-III than 71.0 kg/tree with treatment Module-II. Different fruit quality parameters, except peel thickness () displayed significant response in relation to five modules, ISFM.
Table 5. Growth attributing parameters in response to different modules of ISFM in Nagpur mandarin (Citrus reticulata Blanco) (Pooled data: 2007–16)
Rational soil use practices must allow economically and environmentally sustainable yields, which will only be reached with the maintenance or recovery of the soil health. Thus, a healthy soil has been defined as “The continued capacity of soil to function as a vital living system, within ecosystem and land-use boundaries, to sustain biological productivity, promote the quality of air and water environments, and maintain plant, animal, and human health” (Doran and Safley, Citation1997). Soil health refers to the ecological equilibrium and the functionality of a soil and its capacity to maintain a well-balanced ecosystem with high biodiversity above and below surface, besides an improvement in productivity (Srivastava and Hota, Citation2020). However, most of soil health-related parameters generally have a slow response, when compared to the biological parameters. Thus, a systemic approach based on different kinds of indicators (physical, chemical, and biological) in assessing soil health would be safer than using only one kind of attribute (Cardoso et al., Citation2013). The soil microbial load in terms total bacterial count and fungal count in response to different ISMF-based treatments showed variable higher soil microbial load with Module III and Module V compared to either Module II or Module IV including Module I. In our study changes in soil fertility indices with regard to plant available macro- as well as micronutrients were observed highly significant (). Sharma et al. (Citation2017) also observed increased in water holding capacity by 40.32% in custard apple cv. Arka Sahan after application of 50% recommended dose of fertilizers + 50% N through vermicompost and biofertilizers (Azotobacter 50 g-PSB 50 g-VAM 20 g).
Table 6. Changes in soil fertility status in response to different modules of ISFM-based treatments in Nagpur mandarin (Citrus reticulata Blanco) (Pooled data: 2007–16)
While, amongst different fractions of soil carbon viz., organic-C (SOC), inorganic-C (SiC), and total-C (TC) only SOC and TC were significantly affected. However, soil C:N ratio in the range of 12.00–12.32, without displaying significant changes in response to different ISFM modules. These soil fertility changes were strongly associated with rhizosphere and endosphere biochemical properties in term of acid phosphates (EC:3.1.3.2), alkaline phosphates (EC:3.1.3.1), peroxides (EC:1.11.1.7), and dehydogenase (EC: 1.3.99.1) (). All the microbial consortium-mediated ISFM modules significantly reduced, the CO2 emission comparison to module involving inorganic fertilizers (). Singha et al. (Citation2014) observed an increase in dehydrogenase activity (2.09 µg TPF/g/hour) in top soil (0–10 cm) with the application of 100 N-50 P2O5-100 K2O g/tree/year of age + Azotobacter + PSM + Trichoderma harzianum + organic mulching in mango cv. Dashehari raised on Typic Ustocrept. In our study from other angle highlighting the CO2 emission rate in response to different IFSM treatments further showed comparatively higher CO2 emission in the morning than showing better carbon accreditation with those ISFM modules having all the three components. We have attempted to review the published literature on various modules of ISFM in different fruit crops (). The results accrued have also confirmed that any mode of ISFM which supplies nutrients at differential rates can sustain the production coupled with quality, besides soil health resilience which are quite synonymous to climate-smart ISFM system.
Different components of IFSM are likely to display a significant difference in magnitude of response under two contrasting agro-pedological conditions requiring intervention of soil and water conservation practices in high altitude orchards compared to plain land orchards. Solanki et al. (Citation2019) studied the ISFM in peach (cv. July Elberta) orchard located at 1275 m altitude in acidic loam soil which revealed maximum biometric response, soil fertility status, leaf nutrient composition, and soil microbial population with 75% RDF (500 N- 250 P2O5- 700 K2O g/tree + 40 kg FYM/tree) + vermicompost (15 kg/tree). In another study, Kumar et al. (Citation2018) observed best combination of integrated fertilization consisting of 75% of RDF+25% N through FYM+ Microphos (a commercial form of phosphate solubilizing bacteria) in apple (cv. Oregon Spur) raised on high altitude (1640 m) sandy loam Inceptisol with regard to increase in yield (14.5 kg/plant), soil organic carbon (10.9 g/kg), and dehydrogenase activity (18.4 µg TPF/hr/g soil) as compared to fruit yield (7.7 kg/plant), soil organic carbon (8.53 g/kg), and dehydrogenase activity (10.3 µg TPF/hr/g soil) with control.
Table 7. Response of different INM-based treatments on profiles of enzymatic activity insoil and index leaves of Nagpur mandarin (Citrus reticulata Blanco) (Pooled data: 2007–16)
Table 8. Different modules of INM/ISFM recommended for important fruit crops
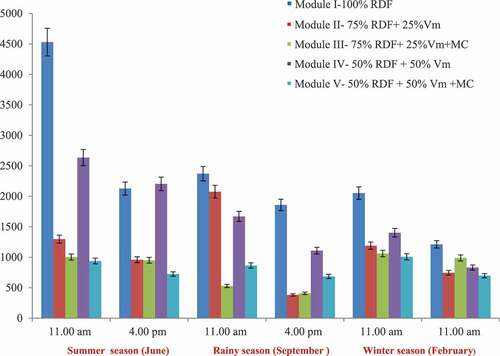
Figure 2. Reduction in CO2 emission rate (mg C/m2/hr) in response to different ISFM modules in citrus
ISFM: Chinese Experience
Plant available nutrients directly affect the growth, fruit set, retention, fruit yield (Huang et al., Citation2014a), leaf nutrient compositions (Qiu et al., Citation2010), and fruit quality (Ling et al., Citation2012). The use of chemical fertilizer, organic fertilizer or biofertilizer has advantages and disadvantages with regard to nutrient supply chain, crop growth, and environmental quality. The advantages need to be integrated to make optimum use of each type of fertilizer and achieve balanced nutrient management for crop growth (Chen, Citation2006). It is widely recognized that it is not cost-effective to apply large amounts of fertilizers, since crop yield responses to the last part of a high nutrient application become almost nil, when the yield has almost attained the theoretically highest level (Wang et al., Citation2016a). Organic fertilizer alone significantly promotes the absorption of calcium, and promote the transformation of nitrate nitrogen to adjust the soil nitrate content changes (Hu et al., Citation2011). It was also suggested that organic manure increased soil organic matter, total N, available P, available K, exchangeable Ca, exchangeable Mg, pH value, and soil fertility (Zang et al., Citation2016). The orchards with increased organic fertilizer significantly improved the leaf nutrient composition vis-à-vis quality production (Chu et al., Citation2012; Wang et al., Citation2018).
Farmers in China often apply large amounts of inorganic fertilizers to the soil to ensure high banana yields under intensive plantation. For instance, the application rates of N, P, and K are 585, 511, and 912 kg/ha, respectively, in Puer city (Yunnan province), southwest China (Wen, Citation2013). Proper application of N, P, and K fertilizers significantly increased the yield and soluble solids content of fruit by 10–20%, sugar by 15–30%, and Vitamin C content by 13–57% (Han et al., Citation2008). Bag-controlled release fertilizer (BCRF) having micro hole density of 0.2 mm x 0.5 cm, are made with environmentally friendly paper-plastic composites (50 g paper and 15 g polyethylene) instead of using common granular coated fertilizers, which maintained a stable supply of nutrients to plant, decreased nitrogen leaching, ammonia volatilization, and greenhouse gas emissions, while volatilization loss of N was significantly reduced from peach orchard soil. A 5-year study revealed that application of BCRF promoted the formation of a dense root system in peach trees by the development of fine roots and a more concentrated root distribution, thereby, aided in improving the fruit quality. 15N tracer experiments further showed that BCRF significantly increased the absorption and utilization rate of nitrogen by peach trees and reduced the amount of nitrogen fertilizers by 65–82% compared to conventional fertilizer application (Xiao et al., Citation2019)
Increased in the dose of nitrogen fertilization from 0 to 2.72 kg/year showed a differential growth pattern in gene expression in citrus cv Huangguogan (Citrus reticulata Blanco × Citrus sinensis Osbeck). Genes encoding nitrate reductase (NR), nitrite reductase (NiR), glutamine synthetase (GS), glutamate dehydrogenase (GDH), and asparagine synthetase (AS) were considerably lower at low than at high-N supply. However, they were all inhibited by excess nitrogen (2.72 kg/year). Therefore, 1.81 kg N/year as the optimal N application rate for young ‘Huangguogan’ trees was recommended (Liao et al., Citation2019). Field experiments were conducted in citrus orchards in Danjiangkou, Hubei Province to assess the effects of N (0, 0.3, 0.6, and 0.9 kg/plant), P (0, 0.1, 0.2, and 0.3 kg/plant), K (0, 0.15, 0.30, and 0.45 kg/plant) and their interactions on residual nutrient content in soils, and the yield and quality of satsumas. The results showed that application rates of N (0.6 kg/plant), P (0.3 kg/plant), and K (0.15 kg/plant) significantly improved the fruit yield and quality. But the response was on par with 0.3 kg N- 0.2 kg P- 0.3 kg K/plant, lower than the current recommended application rates (0.5–0.8 kg N, 0.2–0.5 kg P, 0.25–0.64 kg K/plant) according to Li et al. (Citation2019b).
The Nanfeng tangerine (Citrus reticulata Blanco cv. Kinokuni) fruit pulp’s shear force and insoluble dietary fiber (IDF) were significantly lower with RF + CaMg (500 g lime and 100 g magnesium oxide/plant) than RF (0.68 kg N, 0.31 kg P2O5, 0.5 kg K2O/tree) alone treatment. The fruit pulp’s shear force (higher shear forces meant inferior mastication characteristics) was significantly positively related with IDF but negatively related with the soluble dietary fiber (SDF). It was further concluded that the fruit pulp mastication characteristic was significantly related to both IDF and SDF of the fruit pulp, predominantly influenced by the fruit pulp B, P, Ca, and K contents, further suggesting an improvement in mastication characteristic of fruit pulp improved by combining N, P, and K fertilization rates with 500 g lime and 100 g magnesium oxide/tree (Zheng et al., Citation2015). The details ISFM followed in different fruit crops grown in China have been further summarized ().
Table 9. Response of different components of ISFM in fruit crops grown in China
ISFM: Iranian Experience
Soil fertility and plant nutrition management in Iran began in 1960 with a focus on the development of strategies of chemical fertilizers applications. In recent decades with effective steps in the development of soil testing, has followed the application of organic and biofertilizers. In Iran, integrated management of soil fertility and plant nutrition is defined as the intelligent use of the optimal combination of organic, chemical, and biological nutrients along with adaptation to environmental and local conditions with the aim of optimizing the use of natural soil resources in fruit-based production system to achieve sustainable soil fertility, improve nutrient efficiency and optimal production without harming the soil ecosystem (Asadi Kangarshahi et al., Citation2017).
Most of the researches done in Iran are in the field of optimal management of chemical fertilizers. However, due to the positive effect of the use of fertilizers and organic matter in improving the physical, chemical, and biological properties of soil. It is appropriate for the first period of moving toward integration, i.e., the simultaneous application of chemical and organic fertilizers. Number of studies (Ebrahimiyan and Baybordi, Citation2012; Golchin et al., Citation1999; Kazemini et al., Citation2008; Majidi et al., Citation1999; Mirzashahi, Citation2007; Mousavi et al., Citation2013, Citation2017a, Citation2010; Rezaeyan Bajgiran, Citation2009; Zolfi Bavaryani, Citation2006) showed the positive effect of applying organic fertilizers with chemical fertilizers alone compared to the application of chemical fertilizers in supply of nutrients and crop production. The conducted researches in Iran have shown the positive effects of the simultaneous application of biological and chemical fertilizers on increasing the yield and decreasing the use of chemical fertilizers application (Amirabadi et al., Citation2010; Hassanabadi et al., Citation2012). Also, integrated application of biological and chemical fertilizers has reduced the environmental stress such as drought (Naseri et al., Citation2017), soil salinity (Daei et al., Citation2009), and soil compaction (Miransari et al., Citation2008) and soil contamination with toxic metals (Mousavi et al., Citation2018a, Citation2017b, Citation2018b). Mycorrhizal inoculants are the most widely used and known microbial inoculum used in different fruit crops of Iran (). The best time for its application is when establishing new orchards and planting seedlings (Asadi Kangarshahi et al., Citation2017; Hosseini et al., Citation2017; Mostashari et al., Citation2016).
Table 10. Some published works on integrated management of soil fertility and plant nutrition (IMSFPN) on Iranian fruit crops
Shirinzadeh et al. (Citation2020) studied the arbuscular mycorrhizal fungi performance on some growth indices improvement of micro-propagated pear rootstock (Pyrodwarf) under drought stress. The pear seedlings treated by arbuscular mycorrhizal fungi had better acclimatization, growth, and more tolerance at normal and drought stress condition, evident from 3.6, 1.3, 3.1, and 1.9 times increase in total leaf area, stem height, total leaf fresh weight, and root dry weight, respectively, besides higher concentration of nutrients like P, Mn, Cu, Zn, and K in shoot and root tissues.
Effects of organic, biological, and chemical N-fertilizers on some quantity and quality characteristics of Thompson seedless grape (Vitis vinifera L.) studied by Majidi and Doulati Baneh (Citation2020) indicated that maximum fruit yield resulted with liquid inoculum of N-fixing bacteria (200 ml/vine) + animal manure (1 kg/vine) and solid inoculum of N-fixing bacteria (200 g/vine) with 11.73 and 11.83 kg/vine, respectively. The application of N-bio-inoculation coupled with animal manure was observed more effective than urea as N-fertilizer alone in meeting the nutrient requirement of grape and producing the highest yield. However, there was no significance difference in response on any of the fruit quality parameters.
Rezvani Nasab et al. (Citation2019) studied the influence of different soil conditioners (organic materials at 400 g/tree in the form of municipal solid waste compost (MSWC), cow manure, and chemical materials (270 g/vine) sulfur and gypsum) on the nutritional status of pistachio. The results showed that MSWC and sulfur together caused the increase of available K in 40–60 cm depth, due to higher mobility of K in comparison to other ions. MSWC and gypsum enhanced Mg of the soil solution in 20–40 cm depth. While, integrated application of cow manure and sulfur imparted highest concentration of N in index leaves.
In a study on evaluation of storage life and postharvest quality of pomegranate cv. ‘Rabbab-e-Shiraz’ fruits produced in conventional, integrated, and organic systems was studied by Meighani et al. (Citation2017). The results showed that the weight loss in organic fruits was significantly lower than conventional and integrated management system during storage. The highest total soluble solid/titratable acidity ratio of pomegranate fruits were obtained from integrated management system. While, total phenolics and flavonoids content of organic fruits were higher at harvest and during storage, which decreased in all fruits during the storage.
Jamshidi et al. (Citation2016) studied the effect of integrated plant nutrition management system on some morphological characteristics and nutrient uptake in citrus seedlings. The results showed that wet and dry weight of root and shoot and the P, Fe, Zn concentrations and the lateral branches number increased with substrate enriched (combined use of organic matter treated with Pseudomonas putida (6 x 108 cfu/g)) compared to the control treatment. Some instructions have been published on the integrated management of soil fertility and plant nutrition in fruit crops in Iran (). Despite the researches done on integrated application of chemical fertilizers with organic and biofertilizers in providing nutrients for different crop/plants, these studies have shortcomings in terms of multiplicity as well as climatic and soil diversity of the studied regions.
Suggested Future Viewpoints
A cultivar displaying sustainable quality production under both intensive and organic farming system may not perform with similar magnitude of success when compared with inorganic fertilization. The major difference lies between the nutrient availability pattern and form in through various modes of nutrient delivery. The plants suitable for intensive (conventional) farming get high amount of nutrients at its peak stage whereas in organic farming, the manure applied need to be decomposed first by microorganisms and follow mineralization process on which conversion to available forms like NO3− and NH4+, hence its availability was low when it was highly required. It is, hence, highly desirable to breed the fruit trees for organic cultivation such that it can change the trees’ nutrients absorption pattern, increase the nutrient absorption capacity, reduced root losses due to pathogens, ability to maintain a high mineralization activity in rhizosphere via root exudates, increased rooting depth, and associated ability to recover N leached from the topsoil. A considerable approach is urgently required to sustain the rising organic food requirement (Sharma and Bardhan, Citation2017).
Despite many cutting edge technologies addressing a variety of core issues of nutrient management, many more issues are yet to be attempted with respect to ISFM-based production vis-à-vis rhizosphere dynamics fitting 4 R- Nutrient Stewardship. Simultaneously, concerted efforts would be required to develop ISFM-based yield monitors and soil quality indicators in order to develop a comprehensive system, whereby the concept of soil security could be effectively brought into a reality with an emphasis on development of minimum data set to define crop-based Soil Health Card, the efforts on these lines are still in infancy. The efforts such as these are likely to transform climate smart ISFM as like a common conventional management system using fruit crops-based land uses.
The role of Zn in flowering, fruit set, fruit quality (external and internal) and fruit shelf life; models defining the critical periods of Zn-supply to assure sustained response and its uptake for helping the management decision under different fruit crop-based cropping systems; and devising means for improved Zn-uptake efficiency need to be attempted to unravel many of the complexities involved with Zn-nutrition under ISFM-based production management (Srivastava and Singh, Citation2004, Citation2009b).
Out of different soil properties, the microbial biomass is the one biological property of soil that undergoes immediate change in response to fertilizer like input (Srivastava and Singh, Citation2009a). Studies, therefore, need to be undertaken with a view to explore the possibility whether microbial properties could be used as a potential tool for finding out soil fertility constraint instead of available supply of nutrients in soil. Simultaneously, an eye should be kept on long-term changes in total carbon pool of soil to arrive at the logistic conclusion that sequestration of carbon through improved production level could rejuvenate the lost productivity potential of nutritionally depleted soil (Srivastava and Hota, Citation2020). While, the genetic, functional and metabolic diversity of soil microorganisms within the rhizosphere of wide range of fruit crops is important, the capacity of soil microbial communities to maintain functional diversity of those critical soil processes could ultimately be more important to ecosystem productivity and stability than mere taxonomic diversity. In this context, it remains to be assessed how secondary metabolites-mediated nutrient-microbe synergism is associated with productivity of fruit crops.
With the availability of more technical know-how on combined use of organic manures compost, prolonged shelf life of microbial bio-fertilizers, and inorganic chemical fertilizers, an understanding on nutrient acquisition and regulating the water relations would help switch orchards to large CO2 sink (expanding carbon capturing capacity of rhizosphere) with emphasis on nutrient-use-efficiency, so that a more sustainable fruit-based integrated crop production system under biotic and abiotic stress could be evolved. The molecular approach to breeding of mineral deficiency resistance and mineral efficiency would facilitate produce nutritionally efficient biotypes in order to maximize the quality production of fruit crops on sustained basis. However, it remains to be established whether or not polyploidy could render a given fruit crop better tolerance against mineral deficiencies. Role of AMF in providing an additional resilience to rhizosphere’s ability of carbon accredition within rhizosphere and associated development of plant’s antioxidant profile as a defense mechanism should divert the research studying strong mycorrhizal dependency of fruit crops (Bora and Bora, Citation2020; Srivastava et al., Citation2002). Crop rhizosphere-specific AMF-based microbial consortium would add a new dimension in providing newer options for raising the productivity potential of fruit crops. The framework on soil biodiversity effects from field to fork comprises: i. recognizing both direct and indirect mechanisms of soil biodiversity effects on crops properties, ii. identifying postharvest processes that affect biodiversity legacy effects on crop properties; and iii. pinpointing biodiversity-related crop properties that influence the efficacy and success of operations occurring in the agrifood chain (Rillig et al., Citation2018)
Impacts due to environmental changes and anthropogenic activity are the potential threats to the conservation of soil quality, while expanding fruit culture to marginal soils having a wide range of limitations. With the availability of more technical know-how on efficient use of bulky organic manures, prolonged shelf life of microbial bio-fertilizers, and better understanding on citrus – mycorrhiza symbiosis with regard to nutrient acquisition and regulating the water relations, a more effective integrated citrus production system could be evolved in future. Fertilizer applications are currently managed to protect environmentally sensitive areas by using controlled release fertilizers (use of organic manures, a befitting option), frequent low concentration fertigation, multiple applications, and variable rate application technology in order to improve fertilizer use efficiency using another very prudent concept, called biofertigation. These futuristic directions are anticipated to perch fruit industry on sound scientific footing.
References
- Alagawadi, A.R., and A.C. Gaur. 1992. Inoculation of Azospirillium brasilense and phosphate-solubilizing bacteria on yield of sorghum (Sorghum bicolor (L.) Moench) in dry land. Trop. Agri 69:347–350.
- Amirabadi, M., F. Rejali, M. Ardakani, and S. Borji. 2010. Investigation the effects of Azotobacter chroococcum and mycorrhizal fungus along with different levels of phosphorus on qualitative and morphological characteristics of forage maize (KSC 704). Soil and Water Sci 41:49–56.
- Arikan, S., M. Ipek, and L. Pirlak. 2013. Effect of plant growth promoting rhizobacteria (PGPR) on yield and fruit quality of Quince. Int. Conf. Agri. Biotech. 60:97–106.
- Asadi Kangarshahi, A., M. Basirat, N. Akhlaghi Amiri, H. Haghighatnia, A. Sheikh Ashkvari, S. Salimpour, A. Sabah, M. Shahabian, J. Saleh, O. Ghasemi, et al. 2017. Integrated management of soil fertility and plant nutrition in northern and southern citrus of Iran. Soil and Water Research Institute, Karaj, Iran 1–93. (ISBN: 978-600-98070-0-0)
- Aseri, G.K., N. Jain, J. Panwar, A.V. Rao, and P.R. Meghwal. 2008. Biofertilizers improve plant growth, fruit yield, nutrition, metabolism and rhizosphere enzyme activities of pomegranate (Punica granatum L.) in Indian Thar desert. Sci. Hort 117:130–135.
- Aslantas, R., R. Cakmakci, and F. Sahin. 2007. Effect of plant growth promoting rhizobacteria on young apple tree growth and fruit yield under orchard conditions. Sci. Hort. 111:371–377.
- Baligar, V.C., N.K. Fageria, and Z.L. He. 2001. Nutrient use efficiency in plants. Commun. Soil Sci. Plant Anal. 32:921–950.
- Banaeian, N., M. Zangeneh, and M. Omid. 2010. Energy use efficiency for walnut producers using data envelopment analysis (DEA). Aust. J. Crop Sci. 4(5):359–362.
- Belimov, A.A., A.P. Kojemiakov, and C.V. Chuvarliyeva. 1995. Interaction between barley and mixed cultures of nitrogen fixing and phosphate solubilizing bacteria. Plant Soil 173:29–37.
- Bellone, C.H., and S.C. de Bellone. 1995. Morphogenesis of strawberry roots infected by Azospirilium brasilense and VA mycorrhiza. I. Fendrik, M. de Gallo, J. Vanderleyden, and M. de Zamaroczy, eds.. 1994 Azospirillum VI and related microorganism, genetics-physiology-ecology. North Atlantic Treaty Organisation, Advance Study Institute, Series G (Ecological Science): (ISBN-13: 978-3-642-79908-2)Vol. 37; 251–255. Springer, Berlin, Heidelberg, Germany.
- Bernardi, A.C.C., P.L.O. Almeida Machdo, B.E. Madari, S.R. Lucena Tavares, D.V.B. Campos, and L. Araƀújo Crisóstomo. 2007. Carbon and nitrogen stocks of an arenosol under irrigated fruit orchards in semiarid Brazil. Sci. Agric. (Piracicaba, Braz) 64(2):169–175.
- Bhargava, B.S. 2002. Leaf analysis for nutrient diagnosis recommendation and management in fruit crops. J. Indian Soc. Soil Sci. 50(4):352–373.
- Blum, W.E.H.1993. Soil protection concept of the council of Europe and integrated soil research. p. 37–47. In: eds. H.J.P. Eijsackers and T. Hamer. Integrated soil and sediment research: A basis for proper protection, soil and environment. Vol. 1, Springer:Dordrecht, Netherland.
- Bora, P., and L.C. Bora. 2020. Disease management in horticultural crops through microbial interventions: An overview. Indian J. Agri. Sci. 90:1389–1396.
- Brady, N.C., and R.R. Weil. 2004. Elements of the nature and properties of soils. second ed. Pearson Prentice Hall, New Jersey, USA.
- Brauer, V.S., M. Stomp, and J. Huisman. 2012. The nutrient-load hypothesis: Patterns of resource limitation and community structure driven by competition for nutrients in light. Am. Nat. 179:721–740.
- Brenner, K., L. You, and F.H. Arnold. 2008. Engineering microbial consortia: A new frontier in synthetic biology. Trends Biotechnol. 26:483–489.
- Cardoso, E.J.B.N., R.L.F. Vasconcellos, D. Bini, M.Y.H. Miyauchi, C.A.D. Santos, P.R.L. Alves, A.M. Paula, A.S. Nakatani, J.M. Pereira, and M.A. Nogueira. 2013. Soil health: Looking for suitable indicators. What should be considered to assess the effect of use and management on soil health? Sci. Agric 70(4):274–289.
- Centritto, M., H.S.J. Lee, and P.G. Jarvis. 1999a. Interactive effects of elevated [CO2] and drought on cherry (Prunus avium) seedlings. I. Growth, whole-plant water use efficiency and water loss. New Phytol. 14(1):129–140.
- Centritto, M., F. Magnani, H.S.J. Lee, and P.G. Jarvis. 1999b. Interactive effects of elevated [CO2] and drought on cherry (Prunus avium) seedlings. II. Photosynthetic capacity and water relations. New Phytol. 14(1):141–153.
- Chadha, K.L. 2007. Handbook of Horticulture (Ed). Indian Council of Agricultural Research (ICAR): New Delhi, India, 1–1031.
- Chadha, K.L., and B.S. Bhargava. 1997. Plant nutrient supply, needs, efficiency and policy issues for fruit crops in Indian agriculture from 2000 to 2025 AD, p. 114–132. In: J.S. Kanwar and J.C. Katyal, (eds.). Plant nutrient needs, supply, efficiency and policy issues:2000-2025 National academy of agricultural sciences. New Delhi, India.
- Chavan, B., and R. Ganesh. 2012. Total sequestered carbon stock of Mangifera indica. J. Environ. Earth Sci 2(1):37–48.
- Cheke, A.S., V.D. Patil, H.K. Kausadikar, and A.K. Srivastava. 2018a. Rhizosphere hybridization: Soil nutrient availability. J. Pharmacogn. Phytochem SP1:3113–3117.
- Cheke, A.S., V.D. Patil, and A.K. Srivastava. 2018b. Studies on rhizosphere hybridization and nutrient dynamics in sweet orange seedling from pot culture experiment. J. Pharmacogn. Phytochem. SP1:3077–3082.
- Chen, J.H. 2006. The combined use of chemical and organic fertilizers and/or biofertilizers for crop growth and soil fertility. International workshop on sustained management of the soil rhizosphere system for efficient crop production and fertilizer use land development, Bangkok, Thailand. pp. 1–11.
- Chi, G.G., A.K. Srivastava, and Q.S. Wu. 2018. Exogenous easily extractable glomalin-related soil protein improves drought tolerance of trifoliate orange. Arch. Agron. Soil Sci. 64(10):1341–1350.
- Chiou, T.J. 2007. The role of microRNAs in sensing nutrient stress. Plant Cell Environ 30:323–332.
- Chu, C.B., S.H. Wu, X.Y. Zhang, D.P. Zhou, J.Q. Fan, and Z.F. Jiang. 2012. Effects of organic fertilizer application on soil fertility and leaf nutrients and fruit quality of citrus. Acta Agriculturae Shanghai 28:65–68.
- Conroy, J. 1992. Influence of elevated atmospheric CO2 on concentrations of plant nutrients. Aust. J. Bot. 40:445–456.
- Daei, H., M.R. Ardekani, F. Rejali, S. Teimuri, and M. Miransari. 2009. Alleviation of salinity stress on wheat yield, yield component, and nutrient uptake using arbuscular mycorrhizal fungi under field conditions. J. Plant Physiol. 166:617–625.
- Demircan, V., K. Ekinci, H.M. Keener, D. Akbolat, and C. Ekinci. 2006. Energy and economic analysis of sweet cherry production in Turkey: A case study from Isparta province. Energ. Convers. Manage 47:1761–1769.
- Doran, J.W., and M. Safley. 1997. Defining and assessing soil health and sustainable productivity, p. 1–28. In: C.E. Pankhurst, B.M. Doube, and V.V.S.R. Gupta (eds.). Biological indicators of soil health. CAB International, Wallingford, U.K.
- Dutta, P., S. Kundu, and S. Biswas. 2010. Integrated nutrient management in litchi cv Bombai in new alluvial zone of West Bengal. Ind. J. Hort. 67:181–184.
- Ebrahimiyan, E., and A. Baybordi. 2012. Effect of organic and chemical fertilizer combinations on yield, yield components, oil and protein percentage of two canola cultivars. Agron. J. 8(26):11–22.
- Ehleringer, J., and T.E. Cerling. 1995. Atmospheric CO2 and the ratio of intercellular to ambient CO2 concentrations in plants. Tree Physiol. 15:105–111.
- Elshanshoury, A.R. 1995. Interactions of Azotobacter chroococcum, Azospirillum brasilense and Streptomyces mutabilis in relation to their effect on wheat development. J. Agron. Crop Sci. 175:119–127.
- Esengun, K., G. Erdal, O. Gunduz, and H. Erdal. 2007. An economic analysis and energy use in stake-tomato production in Tokat province of Turkey. Renew. Energy. 32:1873–1881.
- Esitken, A., H. Karlidag, S. Ercisli, M. Turan, and F. Sahin. 2003. The effect of spray a growth promoting bacterium on the yield, growth, and nutrient element composition of leaves of apricot (Prunus armeniaca L. cv. Hacihaliloglu). Aust. J. Agric. Res. 54:377–380.
- Esitken, A., L. Pirlak, M. Turan, and F. Sahin. 2006. Effect of floral and foliar application of plant growth promoting rhizobacteria (PGPR) on yield, growth and nutrition of sweet cherry. Sci. Hort. 110:324–327.
- Fabbrie, P., and M. Del Gallo. 1995. Specific interaction between chickpea (Cicer arietinum) and three chickpea-Rhizobium strains inoculated singularly and in combination with Azospirillum brasilense. I. Fendrik, M. de Gallo, J. Vanderleyden, and M. de Zamaroczy, eds.. 1994 Azospirillum VI and related microorganism, genetics-physiology-ecology. North Atlantic Treaty Organisation, Advance Study Institute, Series G (Ecological Science): (ISBN-13: 978-3-642-79908-2) Vol. 37; 267–277. Springer, Berlin, Heidelberg, Germany.
- FAO. 2011. Statistical year book of food and agricultural organization, FAO state div. 2011. p.36. Metalink: P3.Reu.FAO.ESS.FRU.AH.Sc., p.108.
- FAO. 2020. Food and agriculture organisation of United Nation, Rome, Italy. http://www.fao.org/faostat/en/#data/QC
- Ghosh, S.N., S.N. Roy, and B. Bora. 2012. Nitrogen and potassium nutrition in sapota grown in laterite soil. J. Crop Weed. 8:152–154.
- Ghosh, S.P. 2000. Nutrient management in fruit crops. Fertilizer News 45:71–76.
- Godara, R.K., R.P. Awasthi, and N.S. Kaith. 1996. Interaction effect of VA-mycorrhizae and Azotobacter inoculation on micronutrient content of peach seedlings. J. Hill Res. 9:5–10.
- Goh, K.M. 2004. Carbon sequestration and stabilization in soils: Implications for soil productivity and climate change. J. Soil Sci. Plant Nutr. 50:467–476.
- Golchin, A., M. Esmaeeli, and M.J. Malakouti. 1999. Influence of organic materials, Mn, Cu on yield and quality of Wheat in cold provinces. final report of research project. Soil and Water Research Institute, Karaj, Iran 1074:1–82. Issue
- Gori, A., and F. Favilli. 1995. First results on individual and dual inoculation with Azospirillum – Glomus on wheat. I. Fendrik, M. de Gallo, J. Vanderleyden, and M. de Zamaroczy, eds.. 1994 Azospirillum VI and related microorganism, genetics-physiology-ecology. North Atlantic treaty organisation, advance study institute, series G (ecological science). (ISBN-13: 978-3-642-79908-2)Vol. 37; 245–249. Springer, Berlin, Heidelberg, Germany.
- Goswami, A.K., S. Lal, and K.K. Misra. 2012. Integrated nutrient management improves growth and leaf nutrient status of guava cv. Pant Prabhat. Indian J. Hort 69(2):168–172.
- Guimarães, V., M. Danielle, I.S. Gonzaga, and J.O. Melo Neto. 2014. Management of soil organic matter and carbon storage in tropical fruit crops. Rev. Bras. Eng. Agríc. Ambient 18:301–306.
- Han, S.G., L. Hancheol, J. Jaeho, M. Kyunghwan, T.W. Kang, and S.J. Song. 2008. Effects of long-term application of N, P, K fertilizers on fruit quality and yield of citrus tree (Citrus unshiu Marc.). Korean J. Hort. Sci. Technol 26:203–208.
- Handelsman, J., M.R. Rondon, S.F. Brady, J. Clardy, and R.M. Goodman. 1998. Molecular biolgoical access to the chemistry of unknown soil microbes: A new frontier for natural products. Chem. Biol. 5:R245–R49.
- Hasan, M.A., M. Manna, P. Dutta, K. Bhattacharya, S. Mandal, H. Banerjee, S.K. Ray, and S. Jha. 2012. Integrated nutrient management in improving fruit quality of Mango ‘Himsagar’. IX International Mango Symposium, ISHS Acta Hort. 992: 167–172. Sanya, Hainan Island, China.
- Hassanabadi, T., M.R. Ardakani, F. Rejali, and F. Paknejad. 2012. Effect of Nitrogen fixation and solubilizing phosphate inoculation on yield and Nitrogen uptake indices of barley (Hordeum ulgare L.) under different levels of Nitrogen. Iranian J. Agron. Plant Breeding 8(3):161–174.
- Hassouma, M.G., M.T. Hassan, and M.A. Madkour. 1994. Increased yield of alfalfa (Medicago sativa) inoculated with N2-fixing bacteria and cultivated in a calcareous soil of North-western Egypt. Arid Soil Res. Rehab. 8:389–393.
- Hazarika, B.N., and S. Ansari. 2010. Effect of integrated nutrient management on growth and yield of banana cv. Jahaji. Indian J. Hort. 67:270–273.
- He, Z.L., H. Yao, G. Chen, J. Zhu, and C.Y. Huang. 1997. Relationship of crop yield to microbial biomass in highly weathered soils of China, p. 745–746. In: T. Ando, K. Fujita, T. Mae, H. Matsumoto, S. Mori, and J. Sekiya (eds.). Plànt nutrition for sustainable food production and environment. Kluwer Academic Publishers, Tokyo, Japan.
- Hocking, P.J., and C.P. Meyer. 1991. Carbon dioxide enrichment decreases critical nitrate and nitrogen concentration in wheat. J. Plant Nutr. 14:571–584.
- Holmes, A., M. Karin, C. Brent, and D. Markus. 2015. Carbon sequestration in kiwi fruit orchard soils at depth to mitigate carbon emissions. Commun. Soil Sci. Plant Anal. 46:122–136.
- Hosseini Fard, S.J., M. Basirat, N. Sedaghati, and A. Akhyani. 2017. Integrated management of soil fertility and plant nutrition in pistachio trees. Soil and Water Research Institute, Agricultural Research Education and Extension Organization, Karaj, Iran, (ISBN: 9786009516865) pp. 1–101.
- Hosseini, Y., A.H. Mohebi, M. Puzesh Nejad Shirazi, M. Basirat, and F. Rejali. 2017. Integrated management of soil fertility and plant nutrition of date palm. Soil and Water Research Institute, Karaj, Iran, (ISBN: 9786009516872) pp. 1–68.
- Houghton, R.A., and D.L. Skole. 1990. The earth as transformed by human action, global and regional changes in the biosphere over the past 300 years, p. 393–408. In: B.L. Turner, W.C. Clark, R.W. Kates, J.F. Richards, J.T. Mathews, and W.B. Meyer (eds.). Carbon. In. Cambridge Univ. Press, Cambridge, U.K.
- Hu, L.P., T.Z. Xie, Y.L. Zou, T.P. Wang, Q. Chen, and X.Y. Ye. 2011. Effects of different fertilizations on the calcium content in the apple fruits and soil nutrient. Northern Hort 14:16–19.
- Huang, C.H., X.Y. Qu, K.P. Liu, J.H. Leng, G.Q. Tu, B.M. Li, and X.B. Xu. 2014a. Analysis of soil physicochemical properties, leaf nutrients and fruit qualities in the orchards of “Jinkui” kiwifruit (Actinidia deliciosa). J. Fruit Sci 31:1091–1099.
- Huang, Y.M., A.K. Srivastava, Y.N. Zou, Q.D. Ni, Y. Han, and Q.S. Wu. 2014b. Mycorrhizal-induced calmodulin mediated changes in antioxidant enzymes and growth response of drought-stressed trifoliate orange. Front. Microb 5:682–688.
- Idso, S.B., and B.A. Kimball. 2001. CO2 enrichment of sour orange trees: 13 years and counting. Environ. And Exp. Bot. 46:147–153.
- Intrigliolo, F., and F. Stagno. 2001. Organic fertilizers in citrus crops. Rivista di Frutticolturae di Ortofloricoltura 63(11):75–79.
- Jamshidi, B., M. Ramezanpour, and A. Ladan Moghadam. 2016. The impact of integrated plant nutrition management system on some morphological characteristics and nutrient uptake in citrus seedlings. Int. J. New Tech. Res. 2(6):40–44.
- Jeeva, S., M. Kulasekaran, K.G. Shanmugavelu, and G. Oblisami. 1988. Effect of Azospirillum on growth and development of banana cv. Poovan (AAB). South Indian Hort 36:1–4.
- Joa, J.H., L. Han-Choel, H. Seung-Gap, K.H. Moon, and J. Seung-Jong. 2006. Microbial activities in dark brown volcanic ash soils was affected by different fertilizer application methods in citrus orchard. Abstract 138-84, 18th World Congress on Soil Science, Pennsylvania, USA
- Karlidag, H., A. Esitken, M. Turan, and F. Sahin. 2007. Effects of root inoculation of plant growth promoting rhizobacteria (PGPR) on yield, growth, and nutrient element contents of leaves of apple. Sci. Hort. 114:16–20.
- Kazemini, S.A., H. Ghadiri, N.A. Karimiyan, A.A. Kamgar Haghighi, and M. Khordnam. 2008. Interaction effect of nitrogen and organic matter on growth and yield of wheat. J. Water and Soil Sci. 12(45):461–472.
- Keditsu, R., and A.K. Srivastava. 2014. Substrate dynamics: developments and issues. Ann. Plant Soil Res. 16(1):1–8.
- Kemmler, G., and H. Hobt. 1985. Potassium a product of nature. Kali and Salz, Kassel, Federal Republic of Germany. pp. 1–108.
- Kerni, P.N., and A. Gupta. 1986. Growth parameters affected by azotobacterization of mango seedlings in comparison to different nitrogen doses. Res. Dev. Rep. 3:77–79.
- Keutgen, N., and K. Chen. 2001. Responses of citrus leaf photosynthesis, chlorophyll fluorescence, macronutrient and carbohydrate contents to elevated CO2. J. Plant Physio 158:1307–1316.
- Khehra, S., and J.S. Bal. 2014. Influence of organic and inorganic nutrient sources on growth of lemon (Citrus limon (L.) Burm.) cv. Baramasi. J. Exp. Biol. Agric. Sci 2:126–129.
- Kim, H.J., J.Q. Boedicker, J.W. Choi, and R.F. Ismagilov. 2008. Defined spatial structure stabilizes a synthetic multispecies bacterial community. Proc. Natl. Academy Sci. 105:188–193.
- Kimmins, J.P. 1997. Forest Ecology- A foundation for sustainable management. Pearson Prentice Hall, New Jersey, USA (ISBN: 9780023640711) pp. 1–596.
- Kirad, K.S., S. Barche, and D.B. Singh. 2010. Integrated nutrient management in papaya cv. Surya. Acta Hort. 1:377–380.
- Kizilaslan, H. 2009. Input–output energy analysis of cherries production in Tokat Province of Turkey. Appl. Energy 86:1354–1358.
- Klitgord, N., and D. Segré. 2011. Ecosystems biology of microbial metabolism. Curr. Opin. Biotechnol. 22:541–546.
- Koch, A., M.C. Bratney, M. Adams, D. Field, R. Hill, J. Crawford, B. Minasny, R. Lal, L. Abbott, A.G. O’Donnell, et al. 2013. Soil security: solving the global soil crisis. Glob. Policy. 4(4):434–441.
- Kohli, R.R., A.K. Srivastava, and V.J. Shivankar. 1998. Organic culture in citrus cultivation. Ind. Hort. 43:12–15.
- Kongsager, R., N. Jonas, and M. Ole. 2013. The carbon sequestration potential of tree crop plantations. Mitig. Adapt. Strateg. Glob. Chang 18(8):1197–1213.
- Ku, Y.S., H.M. Rehman, and H.M. Lam. 2019. Possible roles of rhizospheric and endophytic microbes to provide a safe and affordable means of crop biofortification. Agron 9:764.
- Kumar, S., A. Sharma, V.K. Sharma, N. Ahmed, K.G. Rosin, and O.C. Sharma. 2018. Integrated fertilization: An approach for higher apple (Malus domestica) productivity and ecological health of soil. Indian J. Agri. Res 88(2):222–227.
- Lakso, A.L. 2010. Estimating the environmental footprint of New York apple orchards. New York Fruit Quarterly 18(1):26–28.
- Lal, R. 2010. Managing soils and ecosystems for mitigating anthrogenic carbon emissions and advancing global food security. Biosciences 60:708–712.
- Li, R.F., Y.D. Chang, T. Hu, X.Y. Jiang, G.L. Liang, Z.M. Lu, Y.W. Yi, and Q.G. Guo. 2017. Effects of different fertilization treatments on soil, leaf nutrient and fruit quality of Citrus grandis var. Longanyou. World J. Eng. Tech 5:1–14.
- Li, W., M. Yang, J. Wang, Z. Wang, Z. Fan, F. Kang, Y. Wang, Y. Luo, D. Kuang, Z. Chen, et al. 2019a. Agronomic responses of major fruit crops to fertilization in China: A meta-Analysis. Agron 10:15.
- Li, Z., R. Zhang, S. Xia, L. Wang, C. Liu, R. Zhang, Z. Fan, F. Chen, and Y. Liu. 2019b. Interactions between N, P and K fertilizers affect the environment and the yield and quality of satsumas. Glob. Ecol. Conserv 19:e00663.
- Liao, L., T. Dong, X. Liu, Z. Dong, X. Qiu, Y. Rong, G. Sun, and Z. Wang. 2019. Effect of nitrogen supply on nitrogen metabolism in the citrus cultivar ‘Huangguogan’. Plos One 14(5):e0216639.
- Ling, L.L., L.Z. Peng, C.P. Chun, C.L. Jiang, and L. Cao. 2012. The relationship between leaf nutrients and fruit quality of navel orange in southern Jiangxi province of China. Plant Nut. Fert. Sci. 18:947–954.
- Lobell, D.B., C.B. Field, K.N. Chahill, and C. Bonfils. 2005. Impacts of future climate changes on California perennial crop yields: model projections with climate and crop uncertainties. Agric. For. Meteorol. 141:208–218.
- Lotka, A.J. 1992. Contribution to the energetic of evolution. Proc. Natl. Acad. Sci. 8:147–151.
- Magat, S.S. 2009. Productive and sustainable coconut farming ecosystems as potential carbon “Sinks” in climate change minimization: A review and advisory notes. 4th Scientific symposium. 01 Dec. 2009, Richmonde Hotel, 21 San Miguel Ave., Ortigas Center. Pasig City, Metro Manila. pp.1–12.
- Mahmoud, H.M., and F.A.F. Mahmoud. 1999. Studies on effect of some biofertilizers on growth of peach seedlings and root rot disease incidence. Egyptian J. Hort. 26:7–18.
- Majidi, A., and H. Doulati Baneh. 2020. Effects of organic, biological and chemical N-fertilizers on some quantity and quality characteristics of Thompson seedless grape. Iranian J. Hort. Sci 50(4):947–957.
- Majidi, A., A. Sarayi, and M.J. Malakouti. 1999. Effect of different amounts and resources of zinc and compost on yield and uptake of zinc in wheat. final report of research project. Soil and Water Research Institute, Karaj, Iran 1073:1–180. Issue
- Meighani, H., M. Ghsemnezhad, and M. Ahmadi. 2017. Evaluation of storage life and postharvest quality of pomegranate cv, ‘Rabbab–e-Shiraz’ fruits produced in conventional, integrated and organic agricultural systems. Iranian J. Hort. Sci 48(3):635–644.
- Mir, M., and S.D. Sharma. 2012. Influence of biofertilizers on plant growth, fruit yield, nutrition and rhizosphere microbial activity of pomegranate (Puica granatum L.) cv. Kandhari Kasali. J. App. Hort 14:129–136.
- Miransari, M., H.A. Bahrami, F. Rejali, and M.J. Malakouti. 2008. Using arbuscular mycorrhiza to alleviate the stress of soil compaction on wheat (Triticum aestivum L.) growth. Soil Biol. Biochem 40:1197–1206.
- Mirzashahi, K. 2007. Influence of simultaneous application of organic materials (farmyard manure) and chemical fertilizers on corn yield (Single Cross 704 cultivar) and soil organic matter. final report of research project. Soil and Water Research Institute, Karaj, Iran, Issue 1540, pp. 1–210.
- Morugán-Coronado, A., C. Linares, M.D. Gómez-López, Á. Faz, and R. Zornoza. 2020. The impact of intercropping, tillage and fertilizer type on soil and crop yield in fruit orchards under mediterranean conditions: A meta-analysis of field studies. Agri. Sys. 178:102736.
- Moshiri, F., M.R. Balali, and F. Rejali. 2019a. A framework for integrated soil fertility and plant nutrition management in Iran. 16th Iranian Soil Science Congress, University of Zanjan, Zanjan, Iran. pp. 42–50.
- Moshiri, F., H. Ebrahimi, M.R. Ardakani, F. Rejali, and S.M. Mousavi. 2019b. Biogeochemical distribution of Pb and Zn forms in two calcareous soils affected by mycorrhizal symbiosis and alfalfa rhizosphere. Ecotoxicol. Environ. Saf. 179:241–248.
- Mostashari, M., A. Khosravinejad, A. Baybordi, M. Basirat, A. Akhyani, M.H. Sedri, and A. Majidi. 2016. Integrated management of soil fertility and plant nutrition of grape trees. Final Report, Publications of Soil and Water Research Institute, Agricultural Research Education and Extension Organization, Karaj, Iran, pp. 1–33.
- Mousavi, S.M., M.A. Bahmanyar, and H. Pirdashti. 2013. Phytoavailability of some micronutrients (Zn and Cu), heavy metals (Pb, Cd), and yield of rice affected by sewage sludge perennial application. Commun Soil Sci. Plant Anal 44(22):3246–3258.
- Mousavi, S.M., M.A. Bahmanyar, H. Pirdashti, and S. Moradi. 2017a. Nutritional (Fe, Mn, Ni, and Cr) and growth responses of rice plant affected by perennial application of two bio-solids. Environ. Monit. Assess 189(7):340.
- Mousavi, S.M., M.A. Bahmanyar, H. Pirdashti, and S. Salek-Gilani. 2010. Trace metals distribution and uptake in soil and rice grown on a 3-year vermicompost amended soil. Afr. J. Biotechnol 9(25):3780–3785.
- Mousavi, S.M., F. Moshiri, and S. Moradi. 2018a. Mobility of heavy metals in sandy soil after application of composts produced from maize straw, sewage sludge and biochar: Discussion of Gondek et al. (2018). J. Environ. Manage 222:132–134.
- Mousavi, S.M., B. Motesharezadeh, H. Mirseyed Hosseini, H. Alikhani, and A.A. Zolfaghari. 2017b. Geochemical fractions and phytoavailability of zinc in a contaminated calcareous soil affected by biotic and abiotic amendments. Environ. Geochem. Health 40(4):1221–1235.
- Mousavi, S.M., B. Motesharezadeh, H. Mirseyed Hosseini, H. Alikhani, and A.A. Zolfaghari. 2018b. Root-induced changes of Zn and Pb dynamics in the rhizosphere of sunflower with different plant growth promoting treatments in a heavily contaminated soil. Ecotoxicol. Environ. Saf. 147:206–216.
- Mwamba, B.J. 2012. Estimation of net carbon sequestration potential of citrus under different management systems using the life cycle approach. Master Research Thesis, Department of Agronomy. University of Zambia, Lusaka, Zambia, pp.1–92.
- Namdari, M., A.A. Kangarshahi, and N.A. Amiei. 2011. Input-output energy analysis of citrus production in Mazandaran province of Iran. African J. Agri. Res. 6(11):2558–2564.
- Naseri, R., M. Barari, M. Zare, K. Khavazi, and Z. Tahmasebi. 2017. Effect of plant growth promoting bacteria and mycorrhizal fungi on growth and yield of wheat under dry-land conditions. J. Soil Biol. 1:49–67.
- Negi, J.D.S., R.K. Manhas, and P.S. Chauhan. 2003. Carbon allocation in different components of some tree species of India: A new approach of carbon estimation. Curr. Sci. 85:1528–1531.
- Neyra, C.A., A. Atkinson, and O. Olubayi. 1995. Co-aggregation of Azospirillum with other bacteria: Basis for functional diversity. In: I. Fendrik, M. Del Gallo, J. Vanderleyden, and M. de Zamaroczy, eds.. Azospirillum VI and related microorganisms. North Atlantic treaty organisation, advance study institute, series G (ecological science). ISBN-13: 978-3-642-79908-2). Vol. 37; 429–439. Springer, Berlin, Heidelberg, Germany.
- Nistor, E., A. Dobrei, A. Dobrei, D. Camen, F. Sala, and H. Prundeanu. 2018. Nitrous oxide (N2O), CO2 production, and C sequestration in vineyards: A review. Water Air Soil Pollut 229(9):1–9.
- Oustric, J., R. Morillon, F. Luro, S. Herbette, P. Martin, J. Giannettini, L. Berti, and G. Santini. 2019. Nutrient deficiency tolerance in citrus is dependent on genotype or ploidy level. Front. Plant Sci 10:1–13.
- Ozkan, B., H. Akcaoz, F., and Karadeniz. 2004. Energy requirement and economic analysis of citrus production in Turkey. Energ. Convers. Manage 45:1821–1830.
- Padma, T.M.R., and D. Kandasamy. 1990. Effect of interaction between VA-mycorrhizae and graded levels of phosphorous on the growth of papaya (Carica papaya). In: Proc. Natl. Conf. Trends in Mycorrhizal Res, 14-16 February, Haryana Agricultural University, Hisar, Haryana, India, pp. 133–134.
- Pan, Q., Z. Wang, and B. Quebedeaux. 1998. Responses of the apple plant to CO2 enrichment: Changes in photosynthesis, sorbitol, other soluble sugars, and starch. Aust. J. Plant Physio 25:293–297.
- Patel, V.B., S.K. Singh, A. Ram, N. Lata, A.K. Singh, and L. Singh. 2009. Microbial and inorganic fertilizers application influenced vegetative growth, yield, leaf nutrient status and soil microbial biomass in sweet orange cv Mosambi. Indian J. Hort. 66:163–168.
- Pathak, H., and D.B. Nedwell. 2011. Nitrous oxide emission from soil with different fertilizer, water levels and nitrification inhibitors. Water, Air Soil Pollut 129:217–228.
- Pirlak, L., M. Turan, F. Sahin, and A. Esitken. 2007. Floral and foliar application of plant growth promoting rhizobacteria (PGPR) to apples increases yield, growth and nutrient element contents of leaves. J. Sust. Agri. 30:145–155.
- Pulhin, F., R.D. Lasco, and J. Ureta. 2014. Carbon sequestration potential of oil palm in Bohol, Philippines. Ecosys. Develop. J 4(2):14–19.
- Qaisar, K.N., P.A. Khan, S. Ahmad, J.A. Mugloo, and T.A. Rather. 2018. Biomass production and carbon sequestration potential of apple based agri-horti-pastoral systems under temperate conditions of Kashmir valley. Int. J. Chem. Studies 6(1):1934–1938.
- Qiu, X.K., Y.J. Dong, Y.S. Wan, G.Q. Hu, and Y.H. Wang. 2010. Effects of different fertilizing treatments on contents of soil nutrients and soil enzyme activity. Soils 42:249–255.
- Quiroga-Rojas, L.I., N. Ruiz-Quinones, G. Munoz-Motta, and M.D. Lozano-Tovar. 2012. Rhizosphere microorganism, potential antagonists of Fusarium sp. causing agent of root rot in passion fruit (Passiflora edulis Sims). Acta Agron. 61:244–250.
- Raman, J. 2012. Response of Azotobacter, Pseudomonas, and Trichoderma on growth of apple seedling. Inter. Proc. Chem. Biol. Environ. Eng. 40:3–90.
- Rengel, Z., R. Gutteridge, P. Hirsch, and D. Hornby. 1996. Plant genotype, micronutrient fertilization and take-all infection influence bacterial populations in the rhizosphere of wheat. Plant Soil 183:269–277.
- Rezaeyan Bajgiran, S. 2009. Investigation the influence of chemical fertilizers of Fe and Zn and farmyard manure and their interaction effect on quantitative and qualitative yield of saffron. final report of research project. Soil and Water Research Institute, Karaj, Iran 1425:1–63. Issue
- Rezvani Nasab, A., A. Fotovat, A. Astarayi, and A. Tajabadi Pour. 2019. Influence of chemical and organic amendments on concentration of some nutrients in soil and leaf of pistachio. J. Agric. Eng. 42(1):47–59.
- Rillig, M.C., A. Lehmann, J. Lehmann, T. Camenzind, and C. Rauh. 2018. Soil biodiversity effects from field to fork. Trends Plant Sci. 23(1):17–24.
- Sartori, L., B. Basso, M. Bertocco, and G. Oliviero. 2005. Energy use and economic evaluation of a three-year crop rotation for conservation and organic farming in NE Italy. Biosyst. Eng. 91(2):245–256.
- Schaffer, B., A.W. Whiley, C. Searle, and R.J. Nissen. 1997. Leaf gas exchange, dry matter partitioning, and mineral element concentrations in mango as influenced by elevated atmospheric carbon dioxide and root restriction. J. Amer. Soc. Hort. Sci. 122:849–855.
- Scholberg, J., and K.T. Morgan. 2012. Nutrient use efficiency in citrus, p. 205–229. In: A.K. Srivastava (ed.). Advances in Citrus Nutrition. Springer Verlag: The Netherlands. (ISBN: 978-94-007-4170-6)
- Sciubba, E. 2011. What did lotka really say? A critical reassessment of the maximum power principle. Ecol. Modell. 222(8):1347–1353.
- Sekikawa, S., T. Kibe, H. Koizumi, and S. Mariko. 2003. Soil carbon sequestration in a grape orchard ecosystem in Japan. J. Japan. Agric. Syst. Soc. 19:141–150.
- Shah, M.S., M. Wisal, S. Azam, and N. Haq. 2006. Integrated nitrogen management of young deciduous apricot orchard. Soil Environ 25:59–63.
- Shamseldin, A., H. El-Sheikh Mohamad, H.S.A. Hassan, and S.S. Kabeil. 2010. Microbial biofertilization approaches to improve yield and quality of Washington navel orange and reducing the survival of nematode in the soil. J. Amer. Sci. 6:264–271.
- Sharma, A., P. Bhatnagar, and S. Kumar. 2017. Study the correlation effect of integrated nutrient sources and their interaction on soil properties of custard apple (Annona squamosa) field. Int. J. Pure App. Biosci 5(3):1–4.
- Sharma, K.M., and K. Bardhan. 2017. Can high yielding verities perform similarly in organic farms? Plant Arch 17(1):675–680.
- Shen, Z., S. Zhong, Y. Wang, B. Wang, X. Mei, R. Li, Y. Ruan, and Q. Shen. 2013. Induced soil microbial suppression of banana fusarium wilt disease using compost and biofertilizers to improve yield and quality. Eur. J. Soil Biol. 57:1–8.
- Shirinzadeh, K., E. Sedaghati, A.A. Mohammadi Mirik, and H.R. Karimi. 2020. Study of arbuscular mycorrhizal fungi performance on some growth indices improvement of micro-propagated pear rootstock (Pyrodwarf) under drought stress. Iranian J. Horti. Sci. 51(1):255–271.
- Singh, C., and B.B. Sharma. 1993. Leaf nutrient composition of sweet orange as affected by combined use of bio-and chemical fertilizers. South Indian Hort 41:131–134.
- Singh, J.K., and D.K. Varu. 2013. Effect of integrated nutrient management in papaya (Carica papaya L.) cv. Madhubindu. Asian J. Hort 8:667–670.
- Singh, N., D.P. Sharma, V. Kumar, and D. Hota. 2018. Impact of different rootstocks and soil agro-techniques on rhizospheric biological activities and growth traits under apple replant sick soil. Multilogic in Science 7:88–92.
- Singha, A., T. Adak, K. Kumar, S.K. Shukla, and V.K. Singh. 2014. Effect of integrated nutrient management on dehydrogenase activity, soil organic carbon and soil moisture variability in a mango orchard ecosystem. J. Anim. Plant Sci 24(3):843–849.
- Solanki, S.P.S., N.C. Sharma, J.S. Chandel, and P. Kumar. 2019. Effect of conjoint bio-organic and inorganic nutrient sources on plant growth, leaf nutrient content and soil properties of peach (Prunus peersica). Indian J. Agri. Res 89(1):16–21.
- Sparks, D. 1989. Pecan nutrition - a review. Proc. South-eastern Pecan Growers Association 82:101–122.
- Srivastava, A.K. 2010a. Development of INM to harmonize with improved citrus production: Changing scenario. J. Adv. Plant Physiol. 12:294–368.
- Srivastava, A.K. 2010b. Integrated nutrient management in citrus: Frontier developments. Indian J. Fert. 6(11):34–44.
- Srivastava, A.K. 2013. Recent developments in diagnosis and management of nutrient constraints in acid lime. J. Agric. Sci. 2(3):86–96.
- Srivastava, A.K., and D. Hota. 2020. Fruit crops under nutrient-capped scenario: A timeless journey. Curr. Hort. 8(2):14–17.
- Srivastava, A.K., and S.K. Malhotra. 2014. Nutrient management in fruit crops: Issues and strategies. Indian J. Fert. 10(12):72–88.
- Srivastava, A.K., S.K. Malhotra, and N.K. Krishna Kumar. 2015a. Exploiting nutrient-microbe synergy in unlocking productivity potential of perennial fruits: A review. Indian J. Agri. Sci. 85(4):459–481.
- Srivastava, A.K., and E. Ngullie. 2009. Integrated nutrient management: Theory and practice. Dyn. Soil, Dyn. Plant 3(1):1–30.
- Srivastava, A.K., D.H. Paithankar, K.T. Venkataramana, B. Hazarika, and P. Patil. 2019. INM in fruit crops sustainable quality production and soil health. Indian J. Agric. Sci. 89(3):379–395.
- Srivastava, A.K., and S. Singh. 2004. Zinc nutrition, a global concern for sustainable citrus production. J. Sust. Agri 25(3):5–42.
- Srivastava, A.K., and S. Singh. 2008. Citrus nutrition research in India: problems and prospects. Indian J. Agric. Sci. 78:3–16.
- Srivastava, A.K., and S. Singh. 2009a. Citrus decline: Soil fertility and plant nutrition. J. Plant Nutr 32(2):197–245.
- Srivastava, A.K., and S. Singh. 2009b. Zinc nutrition in Nagpur mandarin on haplustert. J. Plant Nutr 32(7):1065–1081.
- Srivastava, A.K., S. Singh, and A.D. Huchche. 2012. Evaluation of INM in citrus (Citrus reticulata Blanco): Biometric response, soil carbon and nutrient dynamics. Inter. J. Innov. Hort 1(2):126–134.
- Srivastava, A.K., S. Singh, and A.D. Huchche. 2015b. Evaluation of INM in citrus on Vertic Ustochrept: biometric response and soil health. J. Plant Nutr. 38(5):1–15.
- Srivastava, A.K., S. Singh, and R.A. Marathe. 2002. Organic citrus: Soil fertility and plant nutrition. J. Sust. Agri. 19(3):5–29.
- Strapatsa, A.V., G.D. Nanos, and C.A. Tsatsarelis. 2006. Energy flow for integrated apple production in Greece. Agric. Ecosyst. Environ. 116:176–180.
- Sugiura, T., H. Kuroda, and H. Sugiura. 2007. Influence of the current state of global warming on fruit tree growth in Japan. Horticultural Research (Japan) 6:257–263.
- Sunkar, R., V. Chinnusamy, J. Zhu, and J.K. Zhu. 2007. Small RNAs as big players in plant abiotic stress responses and nutrient deprivation. Trends Plant Sci. 12:301–309.
- Suresh, C.P., and M.A. Hasan. 2001. Studies on the response of Dwarf Cavendish banana (Musa AAA) to biofertilizer inoculation. Hort. Journal. 14:35–41.
- Tagaliavini, M., G. Tonon, F. Scandellari, A. Quinones, S. Palmieri, G. Menarbin, P. Gioacchini, and A. Masia. 2007. Nutrient recycling during the decomposition of apple leaves (Malus domestica) and mowed grasses in an orchard. Agr, Ecosys. Environ 118:191–200.
- Taheri, M., M. Basirat, T. Khoshzaman, M. Mostashari, and M.A. Shakeri. 2017. Instruction of integrated management of soil fertility and plant nutrition in olive orchards. Final Report, Soil and Water Research Institute, Agricultural Research Education and Extension Organization, Karaj, Iran (ISBN: 978-600-95168-8-9), pp. 1–105.
- Tandon, H.L.S., and Y. Muralidharadu. 2010. Nutrient uptake, removal and recycling by crops. Fertilizer Development and Consultation Organization. New Delhi, India. p.1–140.
- Tao, C., R. Li, W. Xiong, Z. Shen, S. Liu, B. Wang, Y. Ruan, S. Geisen, Q. Shen, and G.A. Kowalchuk. 2020. Bio-organic fertilizers stimulate indigenous soil Pseudomonas populations to enhance plant disease suppression. Microbiome 8:137.
- Tiwari, D.K., M.A. Hasan, and P.K. Chattopadhyay. 1999. Leaf nutrient and chlorophyll content in banana (Musa AAA) under influence of Azotobacter and Azospirillum inoculation. Environ. Ecol. 17:346–350.
- Wang, L., F.E.Y. Yang, J. Yuan, W. Raza, Q. Huang, and Q. Shen. 2016b. Long-term application of bioorganic fertilizers improved soil biochemical properties and microbial communities of an apple orchard soil. Front. Microbiol 7:1893.
- Wang, N., W. Joost, and F.S. Zhang. 2016a. Towards sustainable intensification of apple production in China-yield gaps and nutrient use efficiency in apple farming systems. J. Integrative Agri. 15(4):716–725.
- Wang, S., A.K. Srivastava, Q.S. Wu, and R. Fokom. 2014. The effect of mycorrhizal inoculation on the rhizosphere properties of trifoliate orange (Poncirus trifoliata L. Raf.). Sci. Hort. 170:137–142.
- Wang, Z.Y., G.N. Zhang, J.X. Yu, Y.Q. Zhen, and X.H. Xu. 2018. Effects of EM bacteria combined with organic materials on soil physical and chemical properties and photosynthetic characteristics of peach leaves. J. Ecol. 37(9):2657–2662.
- Wange, S.S., and D.B. Ranawade. 1998. Effect of microbial inoculants on fresh root development on grape var. Kishmis Chorni. Recent Hort 4:27–31.
- Wen, L. 2013. Present situation of fertilizer application to banana plants in Puer city. Chinese J. Trop. Agric. 33:5–8.
- World Health Organisation. 2014. Promoting fruit and vegetable consumption around the world. In: Global strategy on diet, physical activity and health, Report of a Joint FAO/WHO Workshop, 1–3 September 2014, Kobe, Japan. pp. 14–18.
- Wu, Q.S., W.Q. Gao, A.K. Srivastava, F. Zhang, and Y.N. Zou. 2020. Nutrient acquisition and fruit quality of Ponkan mandarin in response to AMF inoculation. Indian. J. Agric. Sci. 90(8):1563–1567.
- Wu, Q.S., J.D. He, A.K. Srivastava, Y.N. Zou, and K. Kuca. 2019. Mycorrhizas enhance drought tolerance of citrus by altering root fatty acid composition and their saturation levels. Tree Physiol. 39:1149–1158.
- Wu, Q.S., and A.K. Srivastava. 2015. Effect of mycorrhizal symbiosis on growth behavior and carbohydrate metabolism of trifoliate orange under different substrate P levels. J. Plant Growth Regul. 34:499–508.
- Wu, Q.S., A.K. Srivastava, M.Q. Cao, and J. Wang. 2014. Mycorrhizal function on soil aggregate stability in root zone and root-free hyphae zone of trifoliate orange. Arch. Agron. Soil Sci. 61(6):813–825.
- Wu, Q.S., A.K. Srivastava, and Y.N. Zou. 2013. AMF-induced tolerance to drought stress in citrus. A Review. Sci. Hort. 164:77–87.
- Wu, Q.S., S. Wang, and A.K. Srivastava. 2016. Mycorrhizal hyphal disruption induces changes in plant growth, glomalin-related soil protein and soil aggregation of trifoliate orange in a core system. Soil & Tillage Research 160:82–91.
- Wu, T., W. Yi, Y. Changjilang, R. Chiarawipa, Z. Xinzhoing, H. Zhenhai, and L. Wu. 2012. Carbon sequestration by fruit trees – Chinese apple orchards as an example. Plos One 7:e38883.
- Xiao, Y., F. Peng, Y. Zhang, J. Wang, Y. Zhuge, S. Zhang, and H. Gao. 2019. Effect of bag-controlled release fertilizer on nitrogen loss, greenhouse gas emissions, and nitrogen applied amount in peach production. J. Clean. Prod 234(10):258–274.
- Xiloyannis, C., G. Montanaro, and A. Sofo. 2002. Proposte per contenere i danni da siccita` alle piante da frutto. Frutticoltura 7/8:19–27.
- Xuan, Y., X. Liu, T.H. Zhu, G.H. Liu, and C. Mao. 2011. Isolation and characterization of phosphate solubilising bacteria from walnut and their effect on growth and phosphorous mobilization. Biol. Fert. Soils. 47:437–446.
- Yadav, K., V. Prasad, K. Mandal, and N. Ahmed. 1992. Effect of co-inoculation (Azospirillum and Rhizobium strains) on nodulation, yield, nutrient uptake and quality of lentil (Lens culinaris). Lens Newsletter 19:29–31.
- Yadav, R., B.H. Singh, H.K. Singh, and A.L. Yadav. 2007. Effect of integrated nutrient management on productivity and quality of aonla (Emblica officinalis Gaetm.) fruits. Plant Arch 1&2:881–883.
- Yu, X., X. Liu, and T.H. Zhu. 2014. Walnut growth and soil quality after inoculating soil containing rock phosphate with phosphate-solubilizing bacteria. Science Asia 40:21–27.
- Zang, X.P., Z.X. Zhou, X.E. Lin, M.J. Dai, Y. Ge, Y.X. Liu, and W.H. Ma. 2016. Effects of different organic manure application rate on mango fruit quality and soil fertility. Chinese Soil and Fert 1:98–101.
- Zhao, X., Q. Dong, S.B. Ni, X.Y. He, H. Yue, L. Tao, Y. Nie, C.S. Tang, F.Q. Zhang, and J. Shen. 2019. Rhizosphere processes and nutrient management for improving nutrient-use efficiency in macadamia production. Hort. Sci. 54:603–608.
- Zhao, Z.P., S. Yan, F. Liu, P.H. Ji, X.Y. Wang, and Y.A. Tong. 2014. Effects of chemical fertilizer combined with organic manure on Fuji apple quality, yield and soil fertility in apple orchard on the Loess Plateau of China. Int. J. Agri. & Biol. Eng 7(2):45–55.
- Zheng, C., X. Lan, Q. Tan, Y. Zhang, H. Gui, and C. Hu. 2015. Soil application of calcium and magnesium fertilizer influences the fruit pulp mastication characteristics of Nanfeng tangerine (Citrus reticulata, Blanco cv. Kinokuni). Sci. Hort. 191:121–126.
- Zolfi Bavaryani, M. 2006. Investigation the effects of farmyard manure on availability and recovery of residual phosphorus. Final report of research project. Soil and Water Research Institute, Karaj, Iran, Issue 1291:1–85.
- Zou, Y.N., A.K. Srivastava, Q.D. Ni, and Q.S. Wu. 2015. Disruption of mycorrhizal extraradical mycelium and changes in leaf water status and soil aggregate stability in rootbox-grown trifoliate orange. Front. Microb 6:20–31.
- Zou, Y.N., A.K. Srivastava, Q.S. Wu, and Y.M. Huang. 2014. Glomalin-related soil protein and water relations in mycorrhizal citrus (Citrus tangerina) during soil water deficit. Arch. Agron. Soil Sci 60(8):1103–1104.
