ABSTRACT
The perishable nature and seasonality of fruits make them harder to use in the food industry. Hence, the aim of this study is to preserve antioxidant-rich Syzygium caryophyllatum (L.) fruit using different drying techniques and evaluate the efficiency of drying methods in terms of antioxidant capacity. Fruits were dried using five different drying methods, namely, sun drying, dehumidified air drying, oven drying, vacuum drying, and freeze-drying. Highest 2,2-diphenyl-2-picryl-hydrazyl free radical scavenging activity (DPPH%) and Ferric reducing antioxidant power (FRAP) value retention were observed in vacuum drying and lowest in sun drying. Furthermore, during fruit drying, it is important to maintain the drying time as minimum as possible to minimize the loss of antioxidant activity. Therefore, vacuum drying was selected as the best drying method in preserving antioxidant-rich Syzygium caryophyllatum (L.) fruit pulp.
Introduction
Fruits are a rich source of essential nutrients and a wide range of phytochemicals and economical sources of nutrients for developing countries. Phytochemicals are bioactive, non-nutrient plant compounds in fruits and other plant foods with health benefits (Liu, Citation2004). Phytochemicals including phenolics, flavonoids, and anthocyanins are significantly contributed to the antioxidant activity of fruits (Nayak et al., Citation2015). Anthocyanins are a major part of natural antioxidants, but they are quite unstable during processing and storage. Temperature, pH, oxygen, and water activity are crucial factors that influencing the stability of these anthocyanins (Tsai, et al. Citation2004).
Because of the seasonal nature of fruits, which mainly produce from March to May, the availability of fruits in the year around is a problem for the food industry in Sri Lanka. Hence, the preservation of fruits is an important task. During the past few decades, the attention of researchers has focused more on to monitor the nutritional value during processing, preservation, and storage of fruits. One of the popular methods in preserving fruits is applying different modes of drying methods that are most common, economical, and easy to apply (Kwok et al., Citation2004; López et al., Citation2010; Mejia-Meza et al., Citation2010; Prasad et al., Citation2013). Further, drying increases the shelf life and decreases the cost of packaging, storage, and transporting of the foods (Gümü Şay et al., Citation2014). Several researchers have reported that during drying, it causes the reduction of nutraceutical properties (Katsube et al., Citation2009; Kuljarachanan et al., Citation2009; Miranda et al., Citation2009) and oxidative decomposition and thermal degradation of polyphenols (Kamiloglu and Capanoglu, Citation2015; López-Vidaña, et al., Citation2017). Degradation and polymerization of phenolics during heating also lead to discoloration of fruits (Markakis, Citation1982; Tsai et al., Citation2004). Several studies have been conducted to identify the effect of different drying methods, such as freeze-drying (Wang et al., Citation2009; Zubia et al., Citation2009), hot air drying (Katsube et al., Citation2009; Lewicki, Citation2006), microwave drying (Chauhan et al., Citation2015), vacuum drying (Wijewardana et al., Citation2015), and sun-drying (Chauhan et al., Citation2015; Wijewardana et al., Citation2015).
Syzygium caryophyllatum (L.) is an endemic tropical tree in Sri Lanka that belongs to the family Myrtaceae, bearing fruits during April – June, and they are blackish-purple color, which has a delicate but characteristic flavor combination of mildly sweet, sour, and astringent taste (Shilpa et al., Citation2015). These fruits can impart unique sensory characteristics into processed foods along with functional properties which can be used to upgrade human health as well (Shilpa et al., Citation2015). However, this fruit has been barely analyzed for its functional properties. Shilpa et al. (Citation2015) have reported that S. caryophyllatum fruits contain higher antioxidant activity compared to other commonly consumed fruits such as mango, papaya, apple, orange, and pomegranate. Although S. caryophyllatum fruit pulp possesses several beneficial characteristics relevant to sensorial and functional properties, this fruit is not utilized in the food industry up to now. This is mainly due to the seasonality and perishable nature of the fruit. Thus, it is important to identify a suitable preservation method with minimal damage to the nutritional and functional properties of fruits because it can help to integrate S. caryophyllatum fruits as a functional ingredient to the food industry in the future.
Therefore, in view of upholding the functional properties of S. caryophyllatum fruit while preserving them to secure continuous supply as raw material for the food industry, this study aims at preserving Syzygium caryophyllatum (L.) fruit by using different types of drying methods and also to evaluate the efficacy of the drying methods in terms of antioxidant capacity of dried fruits.
Material and Methods
Chemicals and Equipment
Methanol, sodium hydroxide, sodium acetate tetrahydrate, glacial acetic acid, ferric chloride, 6-hydroxy-2-5-7-8-tetramethylchroman-2-carboxylic acid (Trolox), 2,2-diphenyl-2-picryl-hydrazyl (DPPH), 2,4,6-tripyridyl-s-triazine (TPTZ) and dimethyl sulfoxide (DMSO) were purchased from Sigma–Aldrich (USA). All chemicals and reagents used in the experiment were of analytical grade. All the bio-assays were carried out using high throughput 96-well microplate readers (Spectra Max Plus 384, Molecular Devices, USA and Spectra Max-Gemini EM, Molecular Devices Inc, USA) hot air oven (UN 30, Universal oven, Mammert GmbH + Co. KG, Schwabach, Germany), dehumidified air dryer (010MT, Joysun Pharma Equipment, Wenzhou, China), freeze dryer (Telstar– LYOBRTA 4PS, Parc Scientific Technologic, Terrassa, Spain), vacuum dryer (Memmert VO200, Memmert GmbH + Co. KG, Schwabach, Germany)
Plant Material
Fruits of Syzygium caryophyllatum (L.) were collected from Galle district in Sri Lanka (80°13ʹ15ʹ’ E 6°3ʹ12ʹ’ N), where the plant is being grown in the natural habitat (from about 50 trees; Average temperature: 27°C, Humidity: 73%, Soil type: clay loam soil, and Rainfall: 1500–2500 mm). The voucher specimen of the plant was deposited at Herbal Technology Section, Industrial Technology Institute, Sri Lanka (BP002). All fruits were handpicked using protective gloves in the fresh form (during June 2019) and transferred to the laboratory (under cold condition using Rigifoam containers with ice cubes) during the same day of plucking for the subsequent use of the study. Fruits were stored at −20°C until they were subjected to different modes of drying.
Preparation of Plant Material for Drying
After receiving the fruits, they were sorted out to get fully ripen, uniform size, and fruits in dark purple color. Thereafter, they were cleaned, washed and water droplets on the surface were dried by blowing ambient air at 25°C. The edible portion of fruits was collected (seeds were removed) and fruit pulp (approximately 5 kg) was blended with distilled water using a laboratory-scale blender for 1 minute. Prepared fruit pulp was divided into five portions and each portion was subjected to five different drying methods.
Drying Methods
Sun–drying (SD)– Fruit pulp was spread as a thin layer (2–5 mm thickness) on the stainless-steel drying trays and kept under direct sunlight (temperature between 27°C and 30°C) for 3 days (total exposed time to the sun was approximately 15 hours) between 12–15 June 2019.
Dehumidified air drying (DD) – Fruit pulp was spread as a thin layer (2–5 mm thickness) on the stainless-steel drying trays and dried in a dehumidified air dryer at 4°C for 3 days.
Oven drying (OD) – Fruit pulp was spread as a thin layer (2–5 mm thickness) on the stainless-steel drying trays and dried in a hot air oven at 40°C for 10 hours and 60°C for 4 hours.
Vacuum drying (VD) – Fruit pulp was spread as a thin layer (2–5 mm thickness) on a Pyrex petri dish and dried in a vacuum oven at 40°C and 6 mbar for 4 hours.
Freeze drying (FD) – Fruit pulp was spread as a thin layer (2–5 mm thickness) on the stainless-steel drying trays and dried in a freeze dryer at −50°C for 24 hours.
All dried samples were ground using a laboratory-scale grinder (mesh size 150 micron) and packed in airtight containers separately and stored at −20°C for the subsequent use of the study. Antioxidant analysis of dried fruit samples was performed within a week of drying.
Antioxidant Analysis
Sample Preparations for TPC and Antioxidant Analysis
The stock solution of dried fruit pulp was prepared by accurately weighing 1.5 mg of dried fruit powder and it was dissolved in 1000 µL of corresponding solvent (stock concentration 1500 ppm). Samples were vortexed (ZX3, VELP Scientifica, Italy) for 5 min and sonicated (750D, VWR Intl. Ltd., Montreal, QC, Canada) for 15 min. Thereafter, the prepared solution was centrifuged (Durafuge 300, Precision Scientific, Richmond, VA, USA) at 5000 rpm for 15 min to get a clear supernatant solution (Zuhair et al., Citation2013).
Total Polyphenolic Content (TPC)
Total polyphenolic content of dried fruit powder was measured according to the modified Folin–Ciocalteu method of Singleton et al. (Citation1999). Therein, the stock solution (110 µL, powdered dried fruit sample dissolved in distilled water) was added to ten times dilute folin-ciocalteu reagent and incubated with sodium carbonate solution (10% w/v, 70 µL) for 30 min at room temperature (25 ± 2°C) and the absorbance was measured at 765 nm wavelength with the microplate reader. Gallic acid was used as the reference standard. Results were expressed as mg Gallic acid equivalents (GAE)/g of dried noodles using the calibration curve of Gallic acid standard (y = 0.000432x + 0.02436, R2 = 99.8%).
2,2-diphenyl-2-picryl-hydrazyl (DPPH) Free Radical Scavenging Activity
The DPPH free radical scavenging activity of fruits was conducted according to the method of Blois (Citation1958) with some modifications. The stock solution was made by dissolving the dried fruit powder in methanol (stock concentration 150 µg/mL). The 150 µL DPPH solution (40 µg/mL, absorbance of 0.70 ± 0.01 at 517 nm) was incubated with 20 µL of stock solution and 30 µL of methanol at room temperature (25 ± 2°C) in dark for 10 min. The absorbance of the mixture was recorded at 517 nm wavelength using a microplate reader. The percentage of DPPH scavenging activity was calculated as follows.
Scavenging activity (%) = [(A control–A sample)/A control] ×100, where A is the absorbance.
Ferric Reducing Antioxidant Power (FRAP)
The FRAP assay was determined according to the method described by Benzie and Szeto (Citation1991). The freshly prepared FRAP reagent was made by mixing 300 mM acetate buffer (3.1 g sodium acetate trihydrate and 16 mL glacial acetic acid, pH 3.6), 10 mM TPTZ solution (2,4,6-tripyridyl-s-triazine) in 40 mM HCl, and 20 mM ferric chloride hexahydrate solution (10:1:1, v/v/v) and incubating it at 37°C for 10 min. The stock solution was made by dissolving the dried fruits in acetate buffer (stock concentration of 150 µg/mL) and 20 µL of the stock solution were incubated with acetate buffer (30 µL) and FRAP solution (150 µL) at room temperature (25 ± 2°C) for 8 min. The absorbance of the ferrous–TPTZ complex was recorded at 593 nm wavelength with a microplate reader. Trolox was used as the reference standard. Results were expressed as mg Trolox equivalents (TE)/g of dried fruit sample using a calibration curve of Trolox (y = 0.0773x + 0.1595, r2 = 0.9976).
Statistical Analysis
The analysis was performed in triplicate for each drying method of fruit pulps and the obtained results were expressed as mean ± standard error (SE). The results were statistically analyzed according to the one-way ANOVA by using IBM SPSS v. 22.0 Statistical software. Turkey’s multiple-range tests were used for mean separation when the F value was significant (p < .05) for each drying method.
Results and Discussion
Syzygium caryophyllatum (L.) is an evergreen tropical tree that bears dark purple colored fruit, cherished with a juicy, mildly sweet, and sour taste when it ripens. The S. caryophyllatum trees are naturally grown in low-altitude and fruiting occurs in late March to early August. Even though S. caryophyllatum fruit is not used in the food industry yet, S. caryophyllatum plants are being cultivated on a commercial scale recently as a potential exporting fruit. demonstrates the appearance of S. caryophyllatum fruit and physiochemical characteristics of fresh ripened fruit are tabulated in .
Table 1. Physiochemical properties of S. caryophyllatum fruit pulp
Due to the perishable and seasonal natures of S. caryophyllatum fruit, industrial application of it in the sphere of fruit processing is less common. Hence, five drying methods were adopted in order to preserve S. caryophyllatum fruit pulp as a raw material. Since the method of drying may affect the major bioactive compounds in fruit pulp particularly on polyphenolic compounds, the effectiveness of it in the drying of S. caryophyllatum fruit pulp was evaluated using Folin–Ciocalteu method, and results are illustrated in .
Figure 2. Total polyphenolic content (TPC) of Syzygium caryophyllatum fruit pulps dried with different drying methods. Data represented as mean ± SE (n = 4). Drying methods: 40°C OD – Oven drying at 40°C, 60°C OD – Oven drying at 60°C, FD – Freeze drying, VD – Vacuum oven drying, SD – Sun drying, DD – Dehumidified air drying
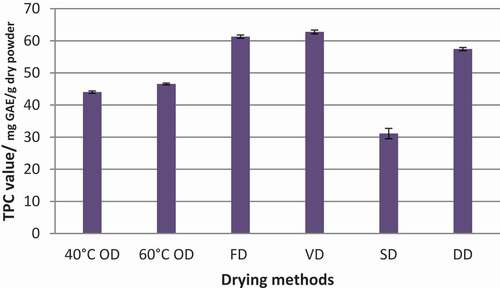
According to the results given in , drying methods were significantly affected (p < .05) to the TPC of dried S. caryophyllatum fruit pulp. While the highest TPC was observed in the VD method (62.75 mg GAE g−1DW), the second-highest was exhibited by the FD method (61.30 mg GAE g−1DW). However, these two values were not significantly different from each other (p > .05). Cooling with dehumidified drying method also exhibited a considerable amount of TPC (57.41 mg GAE g−1DW) against VD and FD methods, but this value was significantly different (p < .05) to the values of FD and VD methods. OD showed a significant reduction in TPC and when comparing the two temperatures used for drying, drying at 60°C contributed to retention a significant amount of TPC against 40°C (TPC; 46.55 and 44.02 mg GAE g−1DW at 60°C and 40°C, respectively, forOD). This may be due to the time–temperature relationship as high drying temperature takes low drying time compared to the drying at low temperature. Finally, in comparing the five drying methods, the lowest TPC was observed in the SD method (31.11 mg GAE g−1DW).
Nevertheless, the different drying methods may affect the antioxidant activity of S. caryophyllatum fruit pulp as well. Antioxidant activity of S. caryophyllatum fruit pulp dried with different drying methods was evaluated with respect to 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity and ferric reducing antioxidant power (FRAP), and results obtained are illustrated in and .
Figure 3. DPPH radical scavenging activity percentage of Syzygium caryophyllatum (150 ppm) fruit pulps dried with different drying methods. Data represented as mean ± SE (n = 4). Drying methods: 40°C OD – Oven drying at 40°C, 60°C OD – Oven drying at 60°C, FD – Freeze drying, VD – Vacuum oven drying, SD – Sun drying, DD – Dehumidified air drying
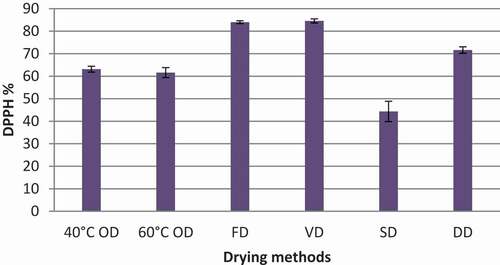
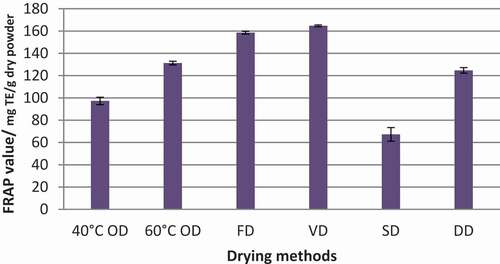
Figure 4. FRAP value of Syzygium caryophyllatum fruit pulps dried with different drying methods. Data represented as mean ± SE (n = 4). Drying methods: 40°C OD – Oven drying at 40°C, 60°C OD – Oven drying at 60°C, FD – Freeze drying, VD – Vacuum oven drying, SD – Sun drying, DD – Dehumidified air drying
According to the results given in , DPPH radical scavenging activity percentage was significantly changed (p < .05) during different types of drying methods for S. caryophyllatum fruit pulp. The highest DPPH radical scavenging% was recorded in the VD method (84.54%). So also FD pulps exhibit the second-highest DPPH radical scavenging% (83.99%). However, there was no significant difference in DPPH radical scavenging% between VD and FD fruit samples (p > .05). Cooling with dehumidified drying method was also recorded a considerable amount of DPPH radical scavenging% retention (71.61%) in comparison with OD and SD. The lowest DPPH radical scavenging% was exhibited in the SD method (44.34%). Hot air oven drying with two temperatures (40°C and 60°C) depicts that drying at 40°C having a little higher scavenging% than that of at 60°C OD.
According to the results, the temperature is a deciding factor in maintaining the antioxidant capacity of fruit pulp because high temperatures can contribute to irreversible oxidative processes for natural antioxidants. According to Hung and Duy (Citation2012), there is a strong correlation between free phenolics and DPPH scavenging in vegetables. Researchers suggested that most of the phenolic acids are bonded to carbohydrate and proteinaceous moieties and can be liberated from the bonds of cell walls at increased temperatures (Vashisth et al., Citation2011; Sun et al., Citation2015; Mejia-Meza et al., Citation2010; López-Vidaña, et al., Citation2017). Therefore, drying at a low temperature, such as FD, VD, and DD shows a higher antioxidant capacity than high-temperature drying, such as OD. Further, considering the results of dehydrating the fruit pulps, drying time was also a critical factor because low-temperature preservation minimizes the degradation of heat-sensitive molecules but required a long drying period to complete it. However, a long period of drying would result in oxidation or degradation of polyphenols and anthocyanins, thereby significant loss in the antioxidant activity of phenols (Esteban et al., Citation2009; Georgetti et al., Citation2008; Mueller-Harvey, Citation2001). The fruit pulp is directly exposed to oxygen for a long period during drying, which may lead to oxidation of phytochemicals within the fruits and also destroy the color, vitamins, and flavor (López-Vidaña, et al. Citation2017). Therefore, it is crucial to keep the drying time minimum to avoid the loss of the antioxidant capacity of the fruits (Cruces et al., Citation2015). This may be the reason for VD showing the highest antioxidant capacity than FD because FD took place at −50°C and VD took place at 40°C for 24 hours and 4 hours respectively. SD was the easy and economical method compared to the other four types of drying but it exhibits the lowest antioxidant capacity that may be due to the prolonged drying process. Moreover, under sun-drying, fruit pulp is directly exposed to oxygen for a long period and also other external factors, such as light conditions, UV rays, and so forth, can contribute to the degradation of antioxidant vigor.
FRAP values of S. caryophyllatum fruits showed a significant difference against different methods of drying (p < .05) and results pertaining to the FRAP values are illustrated in . In the case of FRAP value, this also showed the same pattern as DPPH radical scavenging% for VD, FD, and SD. The highest FRAP value was given by the VD method (164.70 mg TE g−1DW) and the second-highest was recorded by FD (158.56 mg TE g−1DW). FRAP value of fruit powder obtained from FD showed a significant reduction over VD (p < .05). DD also exhibited a sharp decrement of FRAP value against FD. However, the FRAP value of DD was in line with a similar value range of the FRAP value of the OD method. In the case of oven drying at 40°C, it shows a sharp decrement in FRAP value against 60°C and it was also not in the line with DPPH radical scavenging%. However, the lowest FRAP value (67.31 mg TE g−1DW) of fruit pulp was given by SD.
In general, during the drying process, a lot of reactions take place, which can lead to changes in the composition of polyphenolic compounds. Some researchers have suggested that during high-temperature heating increase the phenolic content due to faster releasing of compounds through the cell walls, degradation of biologically active compounds, and formation of new compounds which contains higher antioxidant capacity than native compounds, such as hydroxyphenols (Gümü Şay et al., Citation2014; Larrauri et al., Citation1996; Lavelli et al., Citation2007; Moure et al., Citation2001). Moreover, at high temperatures oxidative and hydrolytic enzymes may deactivate and reduce the loss of phenolic acids as well (Gümü Şay et al., Citation2014).
Hossain et al. (Citation2010) reported that the total antioxidant capacity of vacuum-dried rosemary and thyme herbs showed higher TP and FRAP values than freeze-dried herbs and also air-dried samples showed the highest TP and antioxidant capacity. Sun et al. (Citation2015) reported that freeze-drying was an effective method to retain phenolic compounds, synephrine, and antioxidants, whereas hot air-drying was effective for retaining flavonoids and sun-drying, hot air-drying and freeze-drying methods can be used for retaining limonoids in physiologically dropped un-matured citrus fruits. Gümü Şay et al. (Citation2014) also reported that freeze-dried tomato and ginger samples exhibit better antioxidant properties than thermally dried (SD, OD, VD) tomato and ginger samples. Chan et al. (Citation2009) also reported that all methods of thermal drying (microwave, OD, SD) resulted in drastic declines in antioxidant activity, whereas non-thermal drying methods, such as freeze-drying, resulted in increase in antioxidant activity; however, air-dried leaves lost the antioxidant activity significantly. Joshi et al. (Citation2011) reported that oven drying of red-fleshed apple slices showed the lowest antioxidant capacity than freeze-dried and air-dried samples and freeze-dried slices showed the best antioxidant capacity. Sogi et al. (Citation2013) reported that freeze-dried mango peel and kernel exhibit the highest antioxidant capacity than hot air, vacuum, and infrared dried mango peel and kernel.
Not only was the antioxidant activity of S. caryophyllatum fruit pulp powder produced from different drying methods, the color of the fruit pulps also changed with different drying techniques as illustrated in .
Figure 5. Appearance of dried S. caryophyllatum fruit pulp (a) 40°C oven drying (b) 60°C oven drying (c) freeze drying (d) vacuum drying (e) sun drying (f) dehumidified air drying

The appearance of dried fruit pulp also showed that the colors of VD, FD, and DD fruit pulps exhibit a similar range of color values, and SD fruit pulp showed a significantly lighter color (p < .05) that indicates that color fadeness occurs during SD. OD fruit pulps do not show a significant color change (p > .05) but fruit pulp powder produced from OD at 40°C showed a significantly darker color (p < .05) than the pulp powder produced at 60°C. Therefore, color retention of fruit pulp powder depends on the drying method, and VD, FD, and DD methods showed more attractive color retention than that of other methods.
In this study, five different drying methods were used to preserve the S. caryophyllatum fruit pulp with a view to secure a continuous supply of raw material for the food industry. However, in selecting the best drying method for supplying raw materials at a commercial scale, the quality of dried raw material alone is not sufficient; the other factors particularly cost and time must also be taken into account. The data given in illustrate a comparison of some detective factors involved in different drying methods used in the food industry.
Table 2. Comparison of some determining factors of drying methods
According to , while the lowest drying time was observed in both OD at 60°C and VD methods, the highest one was recorded by DD. When comparing the cost of drying of each method, SD was the least as it utilizes the sunrays as a renewable energy source. However, this method has several drawbacks because sun-drying required hot, dry, and breezy weather conditions, therefore drying cannot be performed in the rainy season (Ahmed et al., Citation2013). Moreover, the weather is uncontrollable; thus, the drying condition cannot be controlled because drying temperature, humidity, air pollution, and UV radiation are varied with the weather conditions. Ahmed et al. (Citation2013) also reported that for sun drying, a minimum temperature of 30°C and humidity level preferably below 60% were required. However, this humidity level cannot be found in most instances in the wet zone where this plant (S. caryophyllatum) grows very well. In the case of all other drying methods, which utilized electrical power as their energy source; however, the FD method imparted the highest drying cost. According to the results of this study, the VD method exhibited the highest TPC and antioxidant activity along with relatively low drying cost and low drying time against FD. According to , the lowest drying cost except SD was exhibited in the OD method, and the drying cost at 40°C was higher than that of at 60°C because drying at 40°C required relatively a high drying time. However, the nutritional quality of fruit pulp in the OD method was comparatively low than VD, FD, and DD methods. Further, S. caryophyllatum fruit pulp dried out of DD method exhibited rather a high TPC and antioxidant activity, but it required a considerably high drying cost as well as capital cost. According to , the lowest moisture content of dried fruit pulp was observed in the FD method and the highest value was given by the SD. VD also showed a considerably low moisture content compared to the FD method.
Finally, in comparing the nutritional quality, particularly bioactive compounds, and factors related to different drying methods, vacuum drying was selected as the best drying method to preserve S. caryophyllatum fruit pulp as a potential antioxidant source for the future food industry.
Conclusion
The effects of five different types of drying methods (vacuum drying, freeze-drying, dehumidified drying, hot air oven drying at 40°C, 60°C, and sun-drying) on TPC and antioxidant capacity of ripened fruits of Syzygium caryophyllatum (L.) were studied to identify potential use as a preservation method. The results of the study suggest that the effect of the five drying methods on TPC and antioxidant activity was significantly affecting (P < .05), whereas the vacuum drying showed the highest antioxidant retention capacity than that of sun-drying which shows the least retention of antioxidants. Furthermore, vacuum drying exhibited the lowest drying time, and comparatively, low cost compared to other drying methods. Long-time drying imparts to declines the TPC and antioxidant activity rather than high-temperature drying methods. Therefore, during fruit drying, it is important to keep the drying time as minimum as possible to minimize the loss of antioxidant activity and preserve them with minimum adverse effects.
Declaration of Interest
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ahmed, N., J. Singh, H. Chauhan, P.G.A. Anjum, and H. Kour. 2013. Different drying methods: Their applications and recent advances. Int. J. Food Nutr. Saf. 4(1):34–42.
- Benzie, I.F.F., and Y.T. Szeto. 1991. Total antioxidant capacity of teas by the ferric reducing antioxidant power assay. J. Agric. Food Chem. 47:633–636. doi: https://doi.org/10.1021/jf9807768.
- Blois, M.S. 1958. Antioxidant determination by use of stable free radical. Nature 181:1199–1200. doi: https://doi.org/10.1038/1811199a0.
- Chan, E.W.C., Y.Y. Lim, S.K. Wong, K.K. Lim, S.P. Tan, F.S. Lianto, and M.Y. Yong. 2009. Effects of different drying methods on the antioxidant properties of leaves and tea of ginger species. Food Chem. 113:166–172. doi: https://doi.org/10.1016/j.foodchem.2008.07.090.
- Chauhan, A., B. Tanwar, and I. Arneja. 2015. Influence of processing on physiochemical, nutritional and PHYTOCHEMICAL COMPOSITIOn of Carissa spinarum (Karonda) fruit. Asian J. Pharm. Clin. Res. 8(6):254–259.
- Cruces, E., Y. Rojas-Lillo, E. Ramirez-Kushel, E. Atala, C. López-Alarcón, E. Lissi, and I. Gómez. 2015. Comparison of different techniques for the preservation and extraction of phlorotannins in the kelpLessonia spicata (Phaeophyceae): Assays of DPPH, ORAC-PGR, and ORAC-FL as testing methods. J. Appl. Phyco. 28(1):573-580. doi: https://doi.org/10.1007/s10811-015-0602-9
- Esteban, R., L. Balaguer, E. Manrique, R. Rubio De Casas, R. Ochoa, I. Fleck, M. Pintó-Marijuan, I. Casals, D. Morales, M.S. Jiménez, et al. 2009. Alternative methods for sampling and preservation of photosynthetic pigments and tocopherols in plant material from remote locations. Photosyn. Res. 101::77–88. doi: https://doi.org/10.1007/s11120-009-9468-5.
- Georgetti, S.R., R. Casagrande, C.R.F. Souza, W.P. Oliveira, and M.J.V. Fonseca. 2008. Spray drying of the soybean extract: Effects on chemical properties and antioxidant activity. LWT–Food Sci. Technol. 41:1521–1527. doi: https://doi.org/10.1016/j.lwt.2007.09.001.
- Gümü Şay, O.A., A.A. Borazan, N. Ercal, and O. Demirkol.2014. Drying effects on the antioxidant properties of tomatoes and ginger. Food Chem. 173: 156-162. doi:https://doi.org/10.1016/j.foodchem.2014.09.162
- Hossain, M., C. Barry-Ryan, A. Martin-Diana, and N. Brunton. 2010. Effect of drying method on the antioxidant capacity of six Lamiaceae herbs. Food Chem. 123(1):85–91. doi: https://doi.org/10.1016/j.foodchem.2010.04.003.
- Hung, P.V., and T.L. Duy. 2012. Effects of drying methods on bioactive compounds of vegetables and correlation between bioactive compounds and their antioxidants. Int. Food Res. J. 19(1):327–332.
- Joshi, A.P.K., H.P.V. Rupasinghe, and S. Khanizadeh. 2011. Impact of drying processes on bioactive phenolics, vitamin c and antioxidant capacity of red-fleshed apple slices. J. Food Process. Preserv. 35:453–457. doi: https://doi.org/10.1111/j.1745-4549.2010.00487.x.
- Kamiloglu, S., and E. Capanoglu. 2015. Polyphenol content in figs (Ficus carica L.): Effect of sun-drying. Int. J. Food Prop. 18:521–535. doi: https://doi.org/10.1080/10942912.2013.833522.
- Katsube, T., Y. Tsurunaga, M. Sugiyama, T. Furuno, and Y. Yamasaki. 2009. Effect of air-drying temperature on antioxidant capacity and stability of polyphenolic compounds in mulberry (Morus Alba L.) leaves. Food Chem. 113:964–969. doi: https://doi.org/10.1016/j.foodchem.2008.08.041.
- Kuljarachanan, T., S. Devahastin, and N. Chiewchan. 2009. Evolution of antioxidant compounds in lime residues during drying. Food Chem. 113:944–949. doi: https://doi.org/10.1016/j.foodchem.2008.08.026.
- Kwok, B.H.I., T. Durance, and D.D. Kitts. 2004. Dehydration techniques affect phytochemical contents and free radical scavenging activities of Saskatoon berries (Amerlachier alnifolia Nutt). J. Food Sci. 69(3): SNQ122-SNQ126. doi:https://doi.org/10.1111/j.1365-2621.2004.tb13381.x.
- Larrauri, J.A., P. Ruperez, B. Borroto, and F. Saura-Calixto. 1996. Mango peels as a new tropical fibre: Preparation and characterisation. LWT–Food Sci. Technol. 29:729–733. doi: https://doi.org/10.1006/fstl.1996.0113.
- Lavelli, V., B. Zanoni, and A. Zaniboni. 2007. Effect of water activity on carotenoid degradation in dehydrated carrots. Food Chem. 104:1705–1711. doi: https://doi.org/10.1016/j.foodchem.2007.03.033.
- Lewicki, P.P. 2006. Design of hot air drying for better foods. Trends Food Sci. Technol. 17(4):153–163. doi: https://doi.org/10.1016/j.tifs.2005.10.012.
- Liu, R.H. 2004. Potential synergy of phytochemicals in cancer prevention: Mechanism of action. J. Nutr. 134:3479–3485. doi: https://doi.org/10.1093/jn/134.12.3479S.
- López, J., E. Uribe, A. Vega-Gálvez, M. Miranda, J. Vergara, E. González, and K. Di Scala. 2010. Effect of air temperature on drying kinetics, vitamin c, antioxidant activity, total phenolic content, non-enzymatic browning and firmness of blueberries variety O´Neil. Food Bioprocess. Tech. 3:772–777. doi: https://doi.org/10.1007/s11947-009-0306-8.
- López-Vidaña, E.C, I.P. Figueroa, F.B. Cortés, B.A. Rojano, and A.N. Ocaña. 2017. Effect of temperature on antioxidant capacity during drying process of mortiño (Vacciniummeridionale Swartz). Int. J. Food Prop. 20(2):294–305. doi: https://doi.org/10.1080/10942912.2016.1155601.
- Markakis, P. 1982. Stability of anthocyanins in Foods, p. 163–180. In: P. Markakis (ed.). Anthocyanins as food colors. Academic Press, New-York.
- Mejia-Meza, E.I., J.A. Yañez, C.M. Remsberg, J.K. Takemoto, N.M. Davies, B. Rasco, and C. Clary. 2010. Effect of dehydration on raspberries: Polyphenol and anthocyanin retention, antioxidant capacity, and antiadipogenic activity. J. Food Sci. 75:5–12. doi: https://doi.org/10.1111/j.1750-3841.2009.01383.x.
- Miranda, M., H. Maureira, K. Rodríguez, and A. Vega-Gálvez. 2009. Influence of temperature on the drying kinetics, physicochemical properties, and antioxidant capacity of Aloe Vera (Aloe Barbadensis Miller) gel. J Food Eng 91:297–304. doi: https://doi.org/10.1016/j.jfoodeng.2008.09.007.
- Moure, A., J.M. Cruz, D. Franco, J.M. Domı́nguez, J. Sineiro, H. Domı́ Nguez, M.J. Núñez, and J.C. Parajó. 2001. Natural antioxidants from residual sources. Food Chem. 72:145–171.
- Mueller-Harvey, I. 2001. Analysis of hydrolysable tannins. Anim. Feed Sci. Technol. 91:3–20. doi: https://doi.org/10.1016/S0377-8401(01)00227-9.
- Nayak, B., R.H. Liu, and J. Tang. 2015. Effect of processing on phenolic antioxidants of fruits, vegetables, and grains—a review. Crit. Rev. Food Sci. Nutr. 55(7):887–919. doi: https://doi.org/10.1080/10408398.2011.654142.
- Prasad, K.N., A. Azlan, and N.M.Y. Barakatun. 2013. Neutraceutical properties of dried tropical fruits: Guavas and papayas, p. 444–456. In: C. Alasalvar and F. Shahidi (eds.). Dried fruits: Phytochemicals and health effects. Hoboken, NJ, USA: Wiley-Blackwell.
- Shilpa, K.J., G. Krishnakumar, and F. Yildiz. 2015. Nutritional, fermentation and pharmacological studies of Syzygium caryophllatum (L.) alston and Syzygium zeylanicum (L.) DC fruits. Cogent Food Agri. 1:1018694. doi: https://doi.org/10.1080/23311932.2015.1018694.
- Singleton, V. L., R. Orthofer, and R. M. Lamuela-Raventos. 1999. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocaltue reagent. Methods Enzymol. 299: 152–178.
- Sogi, D.S., M. Siddiq, I. Greiby, and K.D. Dolan. 2013. Total phenolics, antioxidant activity, and functional properties of ‘Tommy Atkins’ mango peel and kernel as affected by drying methods. Food Chem. 141:2649–2655. doi: https://doi.org/10.1016/j.foodchem.2013.05.053.
- Sun, Y., Y. Shen, D. Liu, and X. Ye. 2015. Effects of drying methods on phytochemical compounds and antioxidant activity of physiologically dropped un-matured citrus fruits. LWT - Food Sci. Technol. 60:1269–1275. doi: https://doi.org/10.1016/j.lwt.2014.09.001.
- Tsai, P.J., Y.Y. Hsieh, and T.C. Huang. 2004. Effect of sugar on the anthocyanin degradation and water mobility in roselle anthocyanin model system using 17O NMR. J. Agric. Food Chem. 52:3097–3099. doi: https://doi.org/10.1021/jf0306587.
- Vashisth, T., K. Singh Rakesh, and B. Pegg Ronald. 2011. Effects of drying on the phenolic content and antioxidant activity of muscadine pomace. LWT–Food Sci. Technol. 44:1649–1657. doi: https://doi.org/10.1016/j.lwt.2011.02.011.
- Wang, T., R. Jónsdóttir, and G. Ólafsdóttir. 2009. Total phenolic compounds, radical scavenging and metal chelation of extracts from Icelandic seaweeds. Food Chem. 116:240–248. doi: https://doi.org/10.1016/j.foodchem.2009.02.041.
- Wijewardana, R.M.N.A., S.B. Navarathne, and I. Wickramasinghe. 2015. Comparison of antioxidant properties of dehydrated fruits and vegetables with different drying techniques. Int. J. Res. Biol. Sci. 5(4):40–44.
- Zubia, M., M.S. Fabre, V. Kerjean, K. Le Lann, V. Stiger-Pouvreau, M. Fauchon, and E. Deslandes. 2009. Antioxidant and antitumoural activities of some phaeophyta from Brittany coasts. Food Chem. 116:693–701. doi: https://doi.org/10.1016/j.foodchem.2009.03.025.
- Zuhair, R.A., A. Aminah, A.M. Sahilah, and D. Eqbal. 2013. Antioxidant activity and physicochemical properties changes of papaya (Carica papaya L. cv. Hongkong) during different ripening stage. Int. Food Res. J. 20(4):1653–1659.

