ABSTRACT
The aim of this study was to adjust the basic culture medium for germination of pollen grains for the different species from the genus Hylocereus. Pollen grains from the species Hylocereus polyrhizus Weber and Hylocereus undatus (Haw.) Britton & Rose were withdrawn of anthers from flowers bagged the previous day, before its opening. Then, with a brush, the pollen grains were spread on the surface of Petri dishes containing 20 mL of culture medium. Four experiments were sequentially installed, considering and selecting the best results from the previous experiment to install the next one. Measurements were made on the length of pollen tube under different concentrations of boric acid. Moreover, germination rate readings were performed after 24 h. The pollen viability of species from the genus Hylocereus varies according to species and the pollen germination was influenced by composition and concentration of medium. The culture medium for the germination of pollen grains from H. polyrhizus species must be increased with 100 g L−1 of sucrose, 518 mg L−1 of calcium nitrate and 636 mg L−1 of boric acid, the pH being measured for 5 and the medium solidified with 6 g L−1 of agar. For H. undatus, the medium should be composed of 100 g L−1 of sucrose, 616 mg L−1 of calcium, 619 mg L−1 of boric acid, 6 g L−1 of agar and pH 6. Both species increased the length of pollen tube with the addition of boric acid.
Introduction
The awareness of the population in the search for a healthier diet considerably increased fruit consumption, especially exotic fruits (Chang et al., Citation2019). Taking into consideration nutritional characteristics, especially its high contents of vitamin C (20.69 mg 100 g−1) and phenolic compounds (124.55 mg 100 g−1) (Abreu et al., Citation2012), pitahaya emerges as an interesting alternative for producers and consumers. It is a cactaceous fruit originating in the tropical forests of Mexico and Central and South America (Hernandez, Citation2000). Pitahayas can be divided into four great groups: Stenocereus Britton & Rose, Cereus Mill., Selenicereus (A. Beger) Riccob, and Hylocereus Britton & Rose, being Hylocereus the most cultivated genus, with emphasis on two species, white-fleshed pitahaya [Hylocereus undatus (Haw.) Britton & Rose] and Costa Rican pitahaya (Hylocereus polyrhyzus Weber).
One of the basic premises for success in genetic improvement programs of any kind is the prior knowledge on the pollen viability, whose objectives in the case of pitahaya is the search for more attractive esthetically and nutrient rich cultivars (Fragallah et al., Citation2019). There are methods cited in the literature which can be used to obtain information on pollen viability, such as staining techniques (De Jesus et al., Citation2018; Nunes et al., Citation2012), in vivo germination (Abdelgadir et al., Citation2012; Soares et al., Citation2014) and in vitro germination (Sharafi, Citation2010, Citation2011). Among these methods, evaluation by in vitro germination is considered a practical and accurate form (Einhardt et al., Citation2006). In this sense, it is necessary to prepare a culture medium containing organic and inorganic elements that reproduce similarly the conditions offered by the feminine flower structure when receiving the pollen grain, which is different for each species (Silva et al., Citation2017).
Pollen germination is influenced by several factors, such as species, season, composition and concentration of medium, temperature and incubation time, flower development stage when collected and collection method, and storage conditions (Sharafi, Citation2019, Citation2010; Sharafi et al., Citation2017). The search for fruits of the genus Hylocereus has increased and in the literature there is little information about the establishment of a culture medium for in vitro germination of pollen grains to provide information about the species. Considering the foregoing, the aim of this study was to adjust the basic culture medium for the verification of germination capacity of pollen grains for species from Hylocereus genus.
Materials and Methods
The experiments were conducted at the Laboratory of Tissue Culture of the Federal University of Lavras (UFLA), located in Lavras, MG, 21º14ʹ06” S and 45º00ʹ00” W, Brazil, with an average altitude of 919 m. The municipality has rainy temperate (mesothermal) climate, characterized as Cwb according to Köppen-Geiger classification. The annual average precipitation are 1,493.2 mm and temperature are 19.3°C, with 66% of the precipitation occurring from November to February (Vilela and Ramalho, Citation1979). Pollen grains from Costa Rican pitahaya (Hylocereus polyrhzius Weber) and white-fleshed pitahaya [Hylocereus undatus (Haw.) Britton & Rose] were collected from healthy plants with four implantation years in the Fruit-growing sector/UFLA.
Considering that the opening of flowers only occurs at dawn, flower buds that showed at the ends white staining between the sepals and the petals were selected during the afternoon, indicating that they would open at dawn of the next day. Thereafter, they were protected with paper bags in order to prevent future losses and contaminations from other species. The flower buds were collected in the following morning and taken to the laboratory for in vitro germination tests immediately.
Determination of the Culture Medium
The basic culture medium for in vitro pollen germination of both species was determined based on the testing steps described below (). The concentration of components which provided the best index of pollen grains germination in the previous step was used in the following steps. The pH of the culture medium was adjusted by using solutions of sodium hydroxide (1 M) and hydrochloric acid (1 M).
Table 1. Tested concentrations of the components at each step of the experiment to obtain the optimum basic culture medium for the in vitro pollen germination of pitahaya species
Evaluations
Number of Pollen Grains per Anther and per Flower
The number of pollen grains per anther and per flower was obtained by simple counting in order to characterize the studied species. The number of anthers per flower was evaluated in 10 flowers from different species. From these flowers, six anthers were randomly collected and each set of anthers was stored in Eppendorf tubes uncovered at controlled temperature at 27°C for 24 hours in the dark to promote dehiscence and thus the release of pollen grains, according to methodology established by Ramos et al. (Citation2008).
After 24 hours, 1,000 μL lactic acid solution was added inside the tubes. After 48 h, a 10 μL sample from each Eppendorf was placed on a Neubauer reading slide for quantification of pollen grains with the aid of an optical microscope in the 100x objective lens. This experiment was conducted with five replicates, each replication consisting of four readings on the slide.
The amount of pollen grains per anther was obtained by multiplying the average number of pollen grains from each sample by the volume of lactic acid in the solution (1,000 μL), then dividing this value by the product between the volume of lactic acid of the sample (10 μL) and the number of anthers from each tube. The number of pollen grains per flower was calculated by multiplying the average estimate of pollen grains per anther by the average number of anthers per flower.
Germination Rate
According to methodology described by Chagas et al. (Citation2010) and Figueiredo et al. (Citation2013), pollen grains were uniformly distributed with brush on Petri dishes containing 20 mL of medium for each step, and were incubated at 27°C. After 24 hours of incubation, the germination rate of the pollen grains was evaluated by using an optical microscope with 10x objective lens. The pollen grains which length of the pollen tube exceeded twice its diameter were considered as germinated (Figueiredo et al., Citation2013).
During the definition of the suitable basic medium in the step of determining the concentration of boric acid for the pollen grains germination, the length of the pollen tube was also evaluated through measurements made by Motic Image Plus 3.0 software.
All steps were performed as a factorial experiment in a completely randomized design with four replicates, and each replicate was accomplished to four fields of view observed by using an optical microscope.
The obtained data were submitted to analysis of variance and the averages were compared by the Tukey test at 5% probability and the quantitative data were submitted to regression. The analyses were conducted by the SISVAR statistical software (Ferreira, Citation2011).
Results and Discussion
Number of Pollen Grains per Anther and per Flower
The following values were observed in the Costa Rican pitahaya (Hylocereus polyrhzius): average of 993 anthers per flower, 180.67 pollen grains per anther and 180,847.3 pollen grains per flower, in contrast, in white-fleshed pitahaya (Hylocereus undatus) the found average values were 873.3 anthers per flower, 322.2 pollen grains per anther and 281,380.6 pollen grains per flower (). It is verified that the species H. undatus despite having a smaller number of anthers, has a greater number of pollen grains per flower. Similar data were presented by Albuquerque Junior et al. (Citation2010) in a study that evaluated the number of anthers per flower, the number of pollen grains per anther and the pollen germination capacity of different cultivars of apple trees. In this work, it is highlighted that despite the high average value of anthers, it cannot be stated that it indicates a greater amount of pollen grains per flower, since the number of anthers per flower of a cultivar can vary annually due to the environmental conditions. The large amount of pollen grains per flower can be considered an advantage in pitaya orchards, since the supply of pollen grains facilitates pollination to obtain in fruiting.
Table 2. Number of anthers per flower, number of pollen grains per anther and per flower of different species from genus Hylocereus. Means from ten flowers followed by standard error
Germination Rate
For H. polyrhzius there was no significant interaction between pH and agar, however, pH values differed significantly at 5% level by the Tukey test. It was observed that when the pH was adjusted to 5, a higher pollen germination rate (32.24%) occurred, and with pH elevation to 6 and 7, the recorded values were 23.92% and 16.05% (). However, in the present study there was no significant difference among the agar concentrations, so the concentration of 6 g L−1 was used for the continuity of study, since this showed a higher germination rate (25.42%). For H. undatus, there was a significant interaction between the pH and agar factors, where the maximum germination was obtained in a medium containing 6 g L−1 of agar and with adjustment of pH 6, reaching 48.45% of germination. In the other pH levels (5 and 7), the germination rate was lower, with values of 37.55% and 25.97%, respectively (). This relation was also investigated by Ramos et al. (Citation2008), which studied the germination rate of pollen grains of Citrus sinensis L. Osbeck and Nogueira et al. (Citation2016) with in vitro pollen germination of different pear cultivars. Both researches stated that there is an increase in the germination rate of pollen grains with increasing pH in the culture medium. However, studies with in vitro pollen germination of cultivars of Ricinus communis L. performed by Cuchiara et al. (Citation2015) showed a pollen germination inhibition at higher pH values.
Figure 1. In vitro germination rate of pollen grains of Hylocereus polyrhzius Weber. subjected to different pH in the culture medium
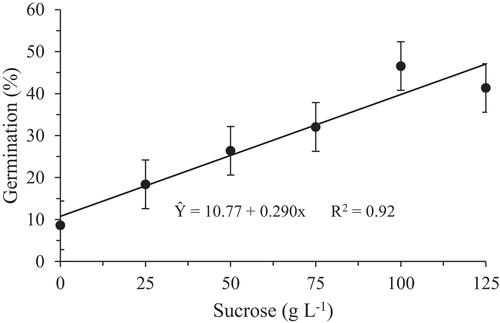
Figure 2. In vitro germination rate of pollen grains of Hylocereus undatus (Haw.) Britton & Rose subjected to different pH and agar concentrations in the culture medium
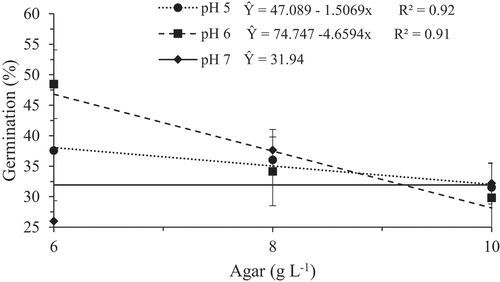
The pH is an important factor in determining the basic medium for the in vitro germination of pollen grains, since it may influence the availability of nutrients and plant growth regulators, besides interfering in the degree of agar solidification (Pasqual et al., Citation2002). According to Pio et al. (Citation2006), which aimed established the culture medium for pollen germination of C. sinensis, the pH has utmost importance in the physiological processes involved in the pollen grains germination, also influencing the chances of fertilization and hence fruiting and production. The agar, together with pH, acts as a solidifying agent of the medium, besides influencing the osmotic equilibrium and the absorption of nutrients. Each fruit shows different results in its need for agar concentration in the medium for the maximization of germination.
There was a trend of linear increase in the germination rate of pollen grains inasmuch as the sucrose concentration for both species was increased, registering the highest value when using 100 g L−1, and the lowest in the absence of sucrose for both species, being (46.6%) and (60.83%) the highest averages and (8.63%) and (6.92%) the lowest for H. polyrhzius and H. undatus, respectively ().
Figure 3. In vitro germination rate of pollen grains of Hylocereus polyrhzius Weber. (a) and Hylocereus undatus (Haw.) Britton & Rose (b) submitted to different sucrose concentrations in the culture medium
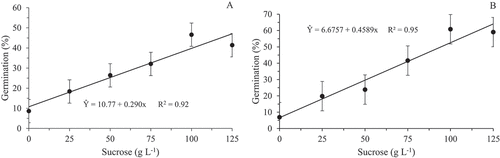
Similar results were observed by Chagas et al. (Citation2010) with pear tree rootstocks, Figueiredo et al. (Citation2013) with blackberry (Rubus spp.) and Nogueira et al. (Citation2016) with pear cultivars, which higher germination rates of pollen grain were achieved with higher sucrose concentrations added to the medium. However, some species may require even larger sucrose amounts to achieve maximum in vitro germination. This is the case of Ateyyeh (Citation2005), who obtained better results in pollen germination of Citrus maximus and Citrus paradisi using 200 g L−1 sucrose. On the other hand, in other species, such as the apple tree, the required sucrose concentration may be lower (Dantas et al., Citation2005). This fact can be justified because the sucrose aimed to supply energy in the biosynthetic processes involved in cell growth, differentiation and morphogenesis. Thus, high germination rates achieved with the addition of sugar concentration may be a consequence of the higher energy supply in the form of carbohydrate, favoring the growth of the pollen tube (Chagas et al., Citation2010; Figueiredo et al., Citation2013).
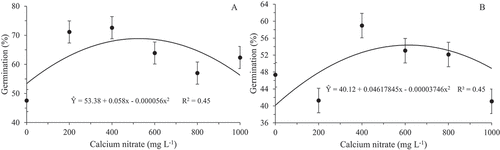
With the adjustment of the quadratic equation, the maximum germination was obtained in the concentration of 518 mg L−1 of calcium nitrate (68.87%), an increase of 29% in relation to the absence of this component for the H. polyrhzius species () whereas the increment for H. undatus was 36% with the concentration of 616 mg L−1, obtaining an average of 54.36% (). At higher concentrations, the rates gradually decreased regardless of the species. These results corroborate those by Silva et al. (Citation2017), Santos et al. (Citation2011) and Gonçalves et al. (Citation2008), which studied the pollen grains germination from different species of physalis, ornamental banana tree (Musa Valentina H. Wendl. & Drude) and pear (Pyrus communis L.) cv. Packham’s 01, respectively, and differ from those found by Pio et al. (Citation2004) for the citrus pollen germination, which obtained a higher rate with 800 mg L−1 of calcium nitrate. According to Silva et al. (Citation2017), the response to calcium will depend on the ability of the species or taxon to support this element.
Figure 4. In vitro germination rate of pollen grains of Hylocereus polyrhzius Weber. (a) and Hylocereus undatus (Haw.) Britton & Rose (b) subjected to different concentrations of calcium nitrate in the culture medium
The maximum germination with the increase of different boric acid doses was obtained with 636 mg L−1, reaching 86.97%, with a gradual decrease in the upper doses (), showing an increase of approximately 87% in the germination rate of the H. polyrhizus. For H. undatus, the highest average obtained was (99.87%), adjusted for 619 mg L−1 boric acid, which represents an increase of more than 100% in relation to the absence of this substance (). Metz et al. (Citation2000) worked with these two pitahaya species and found similar results for H. polyrhizus with an average germination of 82.8%, however, their results were divergent to the present study for the H. undatus species, which reached an average of 79.2%. Boric acid had the same effect for pollen grains germination of pear (Nogueira et al., Citation2016). In addition, increased in vitro pollen germination was observed in other fruit species, as blackberry (Figueiredo et al., Citation2013) and rootstock of the pear (Chagas et al., Citation2010).
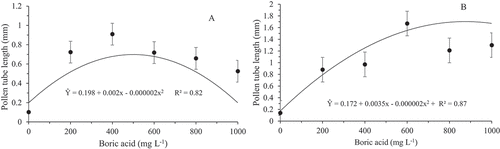
Figure 5. In vitro germination rate of pollen grains of Hylocereus polyrhzius Weber. (a) and Hylocereus undatus (Haw.) Britton & Rose (b) submitted to different boric acid concentrations in the culture medium

A possible explanation for such an accentuated increase in germination may be the fact that boric acid forms an ionizable complex with sugar (sugar borate), which reacts with the plasma membrane, promoting greater growth of the pollen tube and thus possibly increasing the germination index (Dantas et al., Citation2005). Besides, boron has an effect in the reproductive phases of plants (Alva et al., Citation2015), participates in the germination process and formation of the pollen tube wall (Tanaka and Fujiwara, Citation2008) and reduces the possibility of the pollen splitting (Franzon and Raseira, Citation2006).
Regarding the size of the pollen tube, the greatest pollen tube length (0.85 mm) was reached using 500 mg L−1 boric acid for the species H. polyrhizus () whereas a length of 1.70 mm with a concentration of 875 mg L−1 was observed for H. undatus (). Both species obtained an increase in the length of the pollinic tube, reaching an increase of four times and 13 times greater than in the absence of boric acid, for the Costa Rican pitahaya and white-fleshed pitahaya, respectively. Such a discrepant variation among species can be justified because each species has different needs in relation to boric acid. However, since both species have increased may be attributed to the fact that boron stimulates the growth of pollen tube and decreases the likelihood of its disruption (Franzon and Raseira, Citation2006). An investigation about the effect of boron in pollen grain germination and tube growth in Malus domestica L., revealed that this compound has an essential performance in those process (Sharafi and Raina, Citation2020).
Conclusions
This study was able to established the basic medium for germination of pollen grains of Costa Rican pitahaya (Hylocereus polyrhzius Weber.) and white-fleshed pitahaya [Hylocereus undatus (Haw.) Britton & Rose]. The explored factors (pH value and compounds) to compose the culture medium were essential to ensure the efficiency of pollen grains germination of each species. The presence of boric acid must be highlighted since this compound was fundamental to allowed the increase of pollen tube length, and possibly favored the pollen grains germination. The pollen grains viability and germination varied according to species and were influenced by composition of culture medium.
Acknowledgments
To CAPES for providing scholarship and the FAPEMIG, CNPq and MEC for the financial support.
Literature Cited
- Abdelgadir, H.A., S.D. Johnson, and J. Van Staden. 2012. Pollen viability, pollen germination and pollen tube growth in the biofuel seed crop Jatropha curcas (Euphorbiaceae). South Afr. J. Bot. 79:132–139. doi: https://doi.org/10.1016/j.sajb.2011.10.005.
- Abreu, W.C.D., C.D.O. Lopes, K.M. Pinto, L.A. Oliveira, G.B.M.D. Carvalho, and M.D.F.P. Barcelo. 2012. Características físico-químicas e atividade antioxidante total de pitaias vermelha e branca. Rev. Inst. Adolfo Lutz 71(4):656–661.
- Albuquerque Junior, C.L., F. Denardi, A.C. De Mesquita Dantas, and R.O. Nodari. 2010. Número de anteras por flor, grãos de pólen por antera e capacidade germinativa do pólen de diferentes cultivares de macieiras. Revista Brasileira De Fruticultura 32:1255–1260. doi: https://doi.org/10.1590/S0100-29452010005000129.
- Alva, O., R.N. Roa-Roco, R. Pérez-Díaz, M. Yáñez, J. Tapia, Y.M. Moreno, S. Ruiz-Lara, and E. González. 2015. Pollen morphology and boron concentration in floral tissues as factors triggering natural and GA-Induced parthenocarpic fruit development in grapevine. Plos One 10(10):e0139503. doi: https://doi.org/10.1371/journal.pone.0139503.
- Ateyyeh, A.F. 2005. Improving in vitro pollen germination of five species of fruit trees. Dirasat. Agric. Sci. 32(2):189–194.
- Chagas, E.A., R. Pio, P.C. Chagas, M. Pasqual, and J.E. Bettiol Neto. 2010. Composição do meio de cultura e condições ambientais para germinação de grãos de pólen de porta-enxertos de pereira. Ciência Rural 40:261–266. doi: https://doi.org/10.1590/S0103-84782010000200002.
- Chang, S.K., C. Alasalvar, and F. Shahidi. 2019. Superfruits: Phytochemicals, antioxidant efficacies, and health effects–A comprehensive review. Crit. Rev. Food Sci. Nutr. 59(10):1580–1604. doi: https://doi.org/10.1080/10408398.2017.1422111.
- Cuchiara, C.C., P.S. Justo, J.D. Schmitz, and V.L. Bobrowski. 2015. Pollen germination and viability of castor bean (Ricinus communis L.): Culture medium composition and environmental conditions. Científica 43:1–7. doi: https://doi.org/10.15361/1984-5529.2015v43n1p1-7.
- Dantas, A.C.D.M., M.L. Peixoto, R.O. Nodari, and M.P. Guerra. 2005. Viabilidade do pólen e desenvolvimento do tubo polínico em macieira (Malus spp.). Revista Brasileira De Fruticultura 27(3):356–359. doi: https://doi.org/10.1590/S0100-29452005000300005.
- De Jesus, L.D.G.A., R.N.O. Silva, M.F. Da Costa Gomes, S.E. Dos Santos Valente, R.L.F. Gomes, A.C. De Almeida Lopes, and M.F. Costa. 2018. Efficiency of colorimetric tests to determine pollen viability in peppers. Revista Brasileira De Agropecuária Sustentável (RBAS) 8(2):77–82.
- Einhardt, P.M., E.R. Correa, and M.D.C.B. Raseira. 2006. Comparação entre métodos para testar a viabilidade de pólen de pessegueiro. Revista Brasileira De Fruticultura 28(1):5–7. doi: https://doi.org/10.1590/S0100-29452006000100004.
- Ferreira, D.F. 2011. Sisvar: A computer statistical analysis system. Cienc Agrotec 35:1039–1042. doi: https://doi.org/10.1590/S1413-70542011000600001.
- Figueiredo, M.A., R. Pio, T.C. Silva, and K.N. Silva. 2013. Características florais e carpométricas e germinação in vitro de grãos de pólen de cultivares de amoreira-preta. Pesquisa Agropecuária Brasileira 48:731–740. doi: https://doi.org/10.1590/S0100-204X2013000700005.
- Fragallah, S.A.D.A., S. Lin, N. Li, E.J. Ligate, and Y. Chen. 2019. Effects of sucrose, boric acid, pH, and incubation time on in vitro germination of pollen and tube growth of chinese fir (Cunnighamial lanceolata L.). Forests 10(2):102. doi: https://doi.org/10.3390/f10020102.
- Franzon, R.C., and M.D.C.B. Raseira. 2006. Germinação in vitro e armazenamento do pólen de Eugenia involucrata DC (Myrtaceae). Revista Brasileira De Fruticultura 28:18–20. doi: https://doi.org/10.1590/S0100-29452006000100008.
- Gonçalves, C.X., A.R. Rufato, and J. Degenhart, (2008). Diferentes concentrações de ácido bórico e nitrato de cálcio acrescidas ao meio de cultura para germinação in vitro de grãos de pólen de genótipos de pereira. Paper presented at Congresso de Iniciação Científica, 17., e Encontro de Pós-Graduação da UFPel, 10 (Pelotas, Rio Grande do Sul).
- Hernandez, Y.D.O. 2000. Hacia el conocimiento y la conservación de la pitahaya, p. 124. Ipn-Sibej-Conacyt-Fmcn, Oaxaca.
- Metz, C., A. Nerd, and Y. Mizrahi. 2000. Viability of pollen of two fruit crop cacti of the genus Hylocereus is affected by temperature and duration of storage. HortScience 35(2):199–201. doi: https://doi.org/10.21273/HORTSCI.35.2.199.
- Nogueira, P.V., G. Coutinho, R. Pio, D.F.D. Silva, and C.R. Zambon. 2016. Establishment of growth medium and quantification of pollen grains and germination of pear tree cultivars. Revista Ciência Agronômica 47(2):380–386. doi: https://doi.org/10.5935/1806-6690.20160045.
- Nunes, R.D.C., F.D.O. Bustamante, V.H. Techio, and A. Mittelmann. 2012. Morphology and pollen viability of Lolium multiflorum Lam. Ciência E Agrotecnologia 36(2):180–188. doi: https://doi.org/10.1590/S1413-70542012000200006.
- Pasqual, M., D. Finotti, L. Dutra, E. Chagas, and L. Ribeiro. 2002. Cultivo in vitro de embriões imaturos de tangerineira ‘Poncã’em função do pH e da concentração de ágar. Curr. Agric. Sci. Technol. 8:3.
- Pio, L.A.S., J.D. Ramos, M. Pasqual, K.P. Junqueira, and A.B. Silva. 2006. Sacarose e pH na germinação in vitro de grãos de pólen de citros. Ciência E Agrotecnologia 30(1):170–174. doi: https://doi.org/10.1590/S1413-70542006000100025.
- Pio, L.A.S., F.C. Santos, J.C.M. Rufini, J.D. Ramos, and A.G. Araújo. 2004. Germinação in vitro de pólen de citros sob diferentes concentrações de cálcio e boro. Revista Brasileira De Agrociência 10(3):293–296.
- Ramos, J.D., M. Pasqual, L.A. Salles, E.A. Chagas, and R. Pio. 2008. Receptividade do estigma e ajuste de protocolo para germinação in vitro de grãos de pólen de citros. Interciencia 33:51–55.
- Santos, M.R.A., M.D.G.R. Ferreira, J.F. Da Rocha, and A.D.O. Correia. 2011. Estabelecimento de protocolo para germinação de pólen de Musa velutina H. Wendl. & Drude. Plant Cell Culture Micropropagation 7(1):22–29.
- Sharafi, Y. 2010. Suitable in vitro medium for studying pollen viability in some of the Iranian hawthorn genotypes. J. Med. Plants Res. 4(19):1967–1970. doi: https://doi.org/10.5897/JMPR10.419.
- Sharafi, Y. 2011. In vitro pollen germination in stone fruit tree of Rosaceae family. Afr. J. Agric. Res. 6(28):6021–6026.
- Sharafi, Y. 2019. Effects of zinc on pollen gamete penetration to pistils in some apple crosses assessed by fluorescence microscopy. Caryologia. Int. J. Cytol. Cytosystematics Cytogenet. 72(3):63–73.
- Sharafi, Y., Raina, M. Effect of Boron on Pollen Attributes in Different Cultivars of Malus domestica L.. Natl. Acad. Sci. Lett. (2020). https://doi.org/https://doi.org/10.1007/s40009-020-00986-0
- Sharafi, Y., S.F. Talebi, and D. Talei. 2017. Effects of heavy metals on male gametes of sweet cherry. Caryologia. Int. J. Cytol. Cytosystematics Cytogenet. 70(2):166–173.
- Silva, D.F.D., R. Pio, P.V. Nogueira, P.A.D.O. Silva, and A.L. Figueiredo. 2017. Viabilidade polínica e quantificação de grãos de pólen em espécies de fisális. Revista Ciência Agronômica 48(2):365–373.
- Soares, T.L., E.H.D. Souza, M.A.P.D.C. Costa, S.D.O. Silva, and J.A.D.S. Serejo. 2014. In vivo fertilization of banana. Ciencia rural 44(1):37–42. doi: https://doi.org/10.1590/S0103-84782013005000146.
- Tanaka, M., and T. Fujiwara. 2008. Physiological roles and transport mechanisms of boron: Perspectives from plants. Eur. J. Physiol. 456(4):671–677. doi: https://doi.org/10.1007/s00424-007-0370-8.
- Vilela, E.A., and M.A.P. Ramalho. 1979. Análise das temperaturas e precipitações pluviométricas de Lavras, Minas Gerais. Ciência E Prática 3(1):71–79.