ABSTRACT
Mango is the third most important fruit in the tropics due to its nutritional properties and delicious flavor. The fruit is exceptionally perishable due to its climacteric nature, which decreases the quality and shelf-life. Preserving fruit quality and preventing losses during postharvest is one of the critical solutions in sustaining human dietary demands. Postharvest treatments such as 1-Methylcyclopropene, edible coatings, and hot water treatment have shown to be effective in preserving fruit quality. However, developing environmental-friendly postharvest technologies that ensure the safety of consumers remains a challenge. Gaseous ozone, controlled atmosphere (CA), and pulsed electric field (PEF) are some of the emerging technologies with great potential for the mango fruit industry. The use of such technologies has been demonstrated to be effective in maintaining the sensory, nutritional, and physicochemical quality of the mango fruit. However, the mode of action of the emerging technologies is not yet understood. This review provides of an overview of various postharvest techniques used to preserve mango fruit quality. The potential of the emerging postharvest technologies to maintain mango fruit quality during storage and shelf-life is also discussed.
Introduction
Mango (Mangifera indica L.) is one of the most nutritional and commonly consumed fruit in tropical and subtropical agroclimatic regions (Aziz et al., Citation2012). As of 2017, global mango production was 47 133 thousand tones (Altendorf, Citation2017), with Asia leading the world mango production, followed by India and Africa (Altendorf, Citation2017). Mango fruit is a good source of polyphenols, ascorbic acid, carotenoids, vitamins, and carbohydrates (Singh et al., Citation2013). The nutritional properties of mango, especially antioxidants, are essential for human health as they are known to boost the immune system and also prevent cardiovascular diseases, cataracts and various types of cancer (Muhammad et al., Citation2014; Sivakumar et al., Citation2011). The fruit is susceptible to various postharvest diseases such as anthracnose and physiological disorders, including chilling injury, spongy tissue and lenticel spot. Unfortunately, these individual problems or their combination may result in postharvest losses as well as the loss of revenue for the producers and everyone involved in the postharvest value chain. A significant proportion of losses of mango occur during storage and transportation as a result of poor handling and improper facilities (Sivakumar et al., Citation2011).
Postharvest technologies such as chemical and non-chemical treatments are used to maintain fruit quality during storage. For instance, 1-methylcyclopropene (1-MCP) and nitric oxide (NO) have been demonstrated to be effective chemical treatments for preserving mango fruit quality (Faasema et al., Citation2012; Tran et al., Citation2015). Postharvest 1-MCP treatments inhibit ethylene biosynthesis, which retards the respiration rate, retain firmness and delay fruit ripening (Wang et al., Citation2009; Hong et al., Citation2014; (Razzaq et al., Citation2015). Nitric oxide, as a postharvest treatment, is known for prolonging the shelf-life by reducing the incidence of postharvest pathogens and chilling injury (Barman et al., Citation2014). The use of these treatments has shown to be a promising strategy to enhance fruit quality during storage and postharvest handling chain. However, there are growing concerns regarding the postharvest application of chemical treatments mainly because they do not only harm the environment but also pose various risks to human health. As a result, the focus of postharvest research for mangoes has recently shifted toward environmental-friendly and non-chemical treatments.
Edible coatings are biodegradable postharvest treatments applied to fruit and vegetables. They form a thin layer of material over the fruit surface, creating a protective barrier to oxygen, solute movement of food, and moisture (Baldwin et al., Citation1995; Bourtoom, Citation2008). The advantage with edible coatings is that they are natural, contain antioxidants and sometimes vitamins which are beneficial to the consumers. They also possess anti-browning and anti-microbial properties which maintain fruit quality (Ducamp-Collin et al., Citation2009; Gurjar et al., Citation2018). Natural edible coatings such as chitosan, Gum Arabic, and carboxymethyl cellulose (CMC) can control postharvest disorders and diseases such as anthracnose, stem-end rot, and black spot in mango (Gava et al., Citation2018; Zhu et al., Citation2008).
Heat treatment is another non-chemical technique that has proved to be effective in decreasing postharvest diseases, fruit softening and maintaining mango fruit color (Dautt-Castro et al., Citation2018; Le et al., Citation2010; Luria et al., Citation2014; Wang et al., Citation2016). Cell wall degrading enzymes, such as β-galactosidase and polygalacturonase (PG) are reportedly inhibited by heat treatments (Dautt-Castro et al., Citation2018). However, the response of mango fruit to heat treatment significantly depends on various factors including cultivar, temperature, and exposure time. For certain cultivars such as ‘Kent’ and ‘cat Hoa loc’, high temperature and increased exposure time can damage the fruit peel, leading to fruit softening and susceptibility to diseases.
presents a list of published review articles on postharvest treatments of mango fruit. Notably, these reviews have largely focused on the causes of quality loss and commercially adopted postharvest treatments of mango fruit. Non-chemical postharvest treatments, as well as innovative and environmental-friendly technologies, have received little attention from researchers. Therefore, the current review provides an extensive overview of different postharvest techniques currently used on mango fruit, focusing on non-chemical treatments. Anthracnose and chilling injury are the most commercially important postharvest challenges affecting the mango industry. This review further discusses the potential of emerging postharvest technologies to preserve fruit quality and control postharvest diseases. Research gaps, as well as prospects for future research of the postharvest research in mangoes, are also highlighted.
Table 1. Published literature reviews on postharvest technologies of mango fruit
Postharvest Chemical Treatments
The use of chemical treatments is quite an old postharvest management practice, especially for perishable horticultural crops. Chemical treatments such as nitric oxide, salicylic acid, 1-MCP and oxalic acid are commercially used by the mango industry (Ding et al., Citation2007; Hong et al., Citation2014; Junmatong et al., Citation2015; Razzaq et al., Citation2015). Their use has shown to be effective in maintaining fruit quality and extend shelf-life.
1-Methylcyclopropene
The 1-Methylcyclopropene, commercially known as SmartFresh®, is a well-known ethylene antagonist that is used in various fresh horticultural fruits and vegetables. Its efficacy as a mango postharvest treatment has been well researched and documented. For instance, recent studies have shown that 1-MCP (1 µL L−1 for 12 hours) reduced ethylene production and respiration rate in ‘Kensington Pride’ mango fruit after 16 days of storage at ambient temperature (Razzaq et al., Citation2015). Wang et al. (Citation2009) reported a low 1-aminocyclopropane-1-carboxylic acid (ACC) and 1-aminocyclopropane-1-carboxylate oxidase (ACO) concentrations in 1-MCP (1 µL L−1 for 24 hours) treated mango fruit (cv. ‘Tainong’) compared to the untreated control after 16 days of storage at 20°C. The 1-MCP inhibits the initial step in ethylene biosynthesis, leading to delayed ethylene production and fruit ripening.
Ripening in mango fruit is characterized by an increase in total soluble solids (TSS) and a decrease in titratable acidity. Previous research revealed that 1-MCP (652 µg L−1 for 5 minutes) reduced the accumulation of TSS in ‘Kent’ mangoes stored at 12°C (Osuna-Garcia et al., Citation2015); however, contrasting results have been observed in other varieties (). Further studies of 1-MCP (1 µL L−1) treatment of ‘Carabao’ mangoes stored at 5°C showed an increase in TSS content (Castillo-Israel et al., Citation2015). An increase in TSS designates the inability of 1-MCP to retard biochemical reactions associated with fruit ripening. Accumulation of TSS indicates an increase in fruit sweetness, as starch is hydrolyzed to the predominant soluble sugars (sucrose, fructose, and glucose) during ripening (Singh et al., Citation2013). The 1-MCP (1 µL L−1) treatment delayed the accumulation of sucrose and total sugars of ‘Kensington Pride’ mango (Razzaq et al., Citation2015).
Table 2. The effect of postharvest application of 1-MCP on physiochemical and biochemical attributes of mango fruit
Fruit texture is one of the critical fruit quality parameters. Textural properties include firmness, adhesiveness, springiness, cohesiveness, gumminess (Valente et al., Citation2011). The application of 1-MCP has been reported to have an enormous effect on mango fruit firmness. For example, Razzaq et al. (Citation2015) reported that 1-MCP treated fruit had high rheological properties such as springiness and stiffness. Fruit quality and rheological properties are affected by moisture loss. Previous studies revealed that 1-MCP (5 µL L−1 for 12 hours) treatment decreased electrical conductivity, thereby maintaining the membrane integrity of ‘Irwin’ mango fruit stored at10°C for twenty-five days (Wongmetha and Ke, Citation2013). Polysaccharides, hemicellulose, and pectin are depolymerized during mango ripening leading to fruit softening (Yashoda et al., Citation2006). The process of textural changes is due to enzyme activities, and the modification of cell wall polymers.
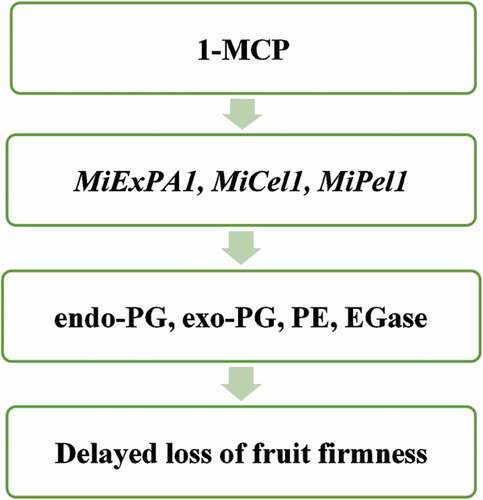
The 1-MCP treatment affects cell wall degrading enzymes in mango fruit (). For instance, Razzaq et al. (Citation2015) observed a reduced enzyme activity of endo-polygalacturonase (endo-PG), pectinesterase (PE), and endo-1,4-β-D-glucanase (EGase) in 1-MCP treated fruit. EGase gene MiCel1 was suppressed in100 µL L−1 1-MCP treated mango fruit (Chourasia et al., Citation2008). Endoglucanase is partially responsible for the depolymerization of cellulose and hemicellulose (Chourasia et al., Citation2008). The 1-MCP (100 µL L−1 for 12 hours) also delayed the accumulation of the MiPel1 gene in mango (Chourasia et al., Citation2006). Pectate lyases gene MiPel1 is related to ripening in ‘Dashehari’ mango and correlated to pectin solubilization (Chourasia et al., Citation2006). Accordingly, a delayed accumulation of MiPel1 gene causes a decrease in enzyme activities of pectate lyases. Total pectin decreases during fruit ripening resulting in cell wall degradation (Chourasia et al., Citation2006). Sane et al. (Citation2005) reported that 1-MCP (100 µL L−1 for 12 hours) treatment reduced the levels of MiExPA1. The MiExPA1 expansion gene is ripening-and-ethylene related, it is also strongly linked to the late stages of mango fruit softening (Sane et al., Citation2005). Clearly, there is enough empirical evidence to conclude that the postharvest application of 1-MCP inhibits the accumulation of genes and enzyme activities involved in cell wall modification, thus maintaining the firmness and delaying ripening. Therefore, the manipulation of these genes could play a vital role in developing mango cultivars that can retain fruit firmness during long-term storage and shipping to distant overseas markets.
Nitric Oxide
Nitric oxide is a free radical gas that is highly reactive. It is a signaling molecule with a crucial role in various physiological and biochemical processes, especially during fruit ripening (Freschi, Citation2013; Romero-Puertas et al., Citation2004). The NO treatment is strongly linked with inhibiting ethylene biosynthesis during postharvest handling (Tran et al., Citation2015). A study by Hong et al. (Citation2014) showed that treating ‘Zill’ mango fruit with NO (100 µM) for 30 minutes significantly reduced ethylene production and delayed climacteric peak. Similarly, a reduced respiration rate has been reported in ‘Kensington Pride’ mangoes fumigated with NO (20 µL L−1 for 2 hours) after seven days of storage at 13°C (Zaharah and Singh, Citation2011a). The NO mechanism of action is linked to its ability to bind ACO thereby forming an ACO-NO binary complex (Zaharah and Singh, Citation2011a). Interestingly, biochemical studies have demonstrated that, the ACC-ACO-NO trinomial complex, a product of ACO-NO chelation by ACC, reduces ethylene production (Freschi, Citation2013; Hong et al., Citation2014). There is a growing body of knowledge suggesting that ethylene biosynthesis genes are affected by NO treatment. For instance, Hong et al. (Citation2014) reported a lower expression of MiACO mRNA gene after NO treatment in mango fruit. These researchers also noted that MiETR1 mRNA, a well-known ethylene receptor gene, was upregulated while MiERS1 mRNA was suppressed in mango peel during storage at 25°C. Ethylene receptors act as negative regulators, suppressing the ethylene signaling pathway (Wang et al., Citation2002). It is can be hypothesized that ethylene production is inhibited by the suppression of ethylene-related genes and enzymes.
The use of NO treatment has also been reported to affect the physicochemical attributes of mango fruit. For example, postharvest application of NO (1 mM) aqueous solution minimized weight loss and maintained firmness in ‘Nam Dok Mai Si Thong’ mango fruit after seven days of storage at 22°C (Tran et al., Citation2015). Change in fruit texture is due to water loss, rupture, and cell wall weakening (Vázquez-Celestino et al., Citation2016; Zerbini et al., Citation2015). Modification of fruit firmness is associated with ripening, softening, and senescence. Notably, Zaharah and Singh (Citation2011b) and Zaharah and Singh (Citation2013) reported that mango mesocarp tissue fumigated with 20 μL L−1 or 40 μL L−1 NO had high adhesiveness, firmness, chewiness, stiffness, and springiness. The high firmness retention is partly due to the fact that fruit exposure to NO reduces cell wall linked enzyme activities such as polygalacturonase (exo-PG) and endo-1,4-β-D-glucanase (EGase) (Zaharah and Singh, Citation2011a). A high PE enzyme activity in NO fumigated mango fruit has also been reported. Pectinesterase has various roles during postharvest biochemical processes, including the depolymerization of pectin molecules into soluble pectate and methanol. Thus, the released pectate interacts with calcium, leading to higher PE and improved cell wall integrity (Zaharah and Singh, Citation2011a). Therefore, it can be deduced that the decrease in these enzyme activities reduces cell wall breakdown, thereby maintaining fruit firmness.
Nitric oxide is effective in controlling postharvest diseases such as anthracnose in mango fruit. For instance, Hu et al. (Citation2014) reported that NO (0.1 mM sodium nitroprusside (SNP) treatment reduced the natural disease incidence and lesion diameter in ‘Guifei’ mango stored at ambient temperature for ten days. NO increased the defense-related enzyme activities such as phenylalanine ammonia-lyase (PAL), cinnamate-hydroxylase (C4H), peroxide (POD), Chitinase (CHI) and β-1,3-Glucanase (GLU) (Hu et al., Citation2014). Recently, Zheng et al. (Citation2017) reported that NO (0.2 mM SNP aqueous solution,10 min at 25°C) upregulated gene expression of POD, CHI and PAL in kiwifruit during storage at ambient temperature for thirteen days. The increased enzyme activity initiates the biosynthesis of anti-fungal metabolites such as flavonoids, phenolics, phytoalexins, and tannins (Hu et al., Citation2014; Zheng et al., Citation2017). These secondary metabolites form a protective barrier against pathogen infection, thus inhibiting infection and pathogen growth on the fruit (Zheng et al., Citation2017). The mode of action of NO in inducing defense against postharvest pathogens is through the activation of pathogenesis-related proteins and phenylpropanoid metabolism (Hu et al., Citation2014). The resistance of mango fruit to anthracnose is through the increased enzyme activities and accumulation of secondary metabolites, causing hypersensitive response cell death, strengthening fruit immunity (Hu et al., Citation2014; Scheler et al., Citation2013; Zheng et al., Citation2017).
Salicylic Acid
Salicylic acid (SA) is a plant hormone that regulates various physiological processes in plants (He et al., Citation2016). Such physiological processes include fruit ripening, tolerance to chilling injury (CI), and resistance to postharvest diseases (Ding et al., Citation2007; Zainuri et al., Citation2001). Chilling injury occurs in mango fruit when exposed to temperatures below 13°C, depending on canopy position and cultivars (Barman and Asrey, Citation2014; Sudheeran et al., Citation2018). Sudheeran et al. (Citation2018) reported that mango fruit (cv. ‘Shelly’) from the outside canopy had less CI than the inside canopy stored at 5°C for twenty-one days. Phakawatmongkol et al. (Citation2004) reported that cultivars such as ‘Nam Dok Mai’ and ‘Okrong’ were more and least susceptible to CI, respectively. The CI symptoms include sunken lesions, shriveling, pitting, discoloration of the peel, susceptibility to decay, and uneven ripening (Li et al., Citation2015). Severe CI symptoms have been reported in fruit at ambient temperature during shelf-life after cold storage (Ntsoane et al., Citation2019b). Mango fruit induce CI resistance through the accumulation of anthocyanin and flavonoids (Ntsoane et al., Citation2019b; Sivankalyani et al., Citation2016).
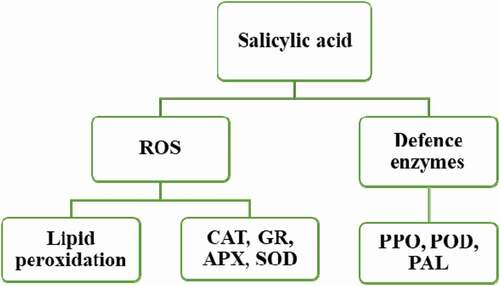
Storing mango fruit at low temperature induces free radicals such as hydrogen peroxide (H2O2) and superoxide radicals (O2⁻), causing oxidative stress and CI (Junmatong et al., Citation2015). Increased ROS levels can cause lipid peroxidation leading to reduced membrane integrity and fruit firmness. Junmatong et al. (Citation2015) reported that SA (1 mM) inhibited the accumulation of H2O2 and O2⁻in ‘Nam Dok Mai No. 4ʹ mangoes stored at 5°C for forty-two days. These authors further found that the SA treatment increased the activities of CAT, ascorbate peroxidase (APX), and SOD. SA is a signaling molecule that activates the gene expression of CAT, SOD, and APX at cold temperatures (). The SOD dismutate O2⁻ into H2O2, which is detoxified by APX and CAT (Ding et al., Citation2007). The enzyme CAT, APX, and SOD can scavenge ROS, leading to fruit adapting to cold temperatures and reducing CI.
Figure 2. Salicylic acid induces resistance to CI in the fruit (Modified from Asghari and Aghdam, Citation2010). Application of SA in fruit prior to chilling temperatures induces ROS scavenging and avoidance genes such as APX, SOD and CAT. The increased antioxidant capacity of the cells leads to fruit adapting to cold temperatures, thereby reducing the incidence of postharvest disorders such as chilling injury
SA is known to control anthracnose caused by Colletotrichum gloeosporioides in mango fruit. Zeng et al. (Citation2006) reported that treating ‘Matisu’ mango fruit with SA (1 mmolL−1) reduced the disease incidence and lesion diameter of Colletotrichum gloeosporioides. In their in vitro study, He et al. (Citation2017) revealed that mycelial growth was significantly reduced by SA (2 and 5 mM) treatment in ‘Tainong’ mangoes. These researchers also reported that SA (2 mM) increased the enzyme activities of CHI and GLU. These enzymes are involved in inducing resistance against diseases. The GLU is reported to cause disease resistance at the early stages of fruit ripening (Zeng et al., Citation2006). Mango fruit treated with SA has been shown to accumulate more polyphenoloxidase (PPO), POD and record low phenolic content (Zeng et al., Citation2006). The enzyme PPO plays a vital role in the defense against diseases as it catalyzes phenolics into quinines. Thus, it can be concluded that SA induces resistance against anthracnose by stimulating CHI, GLU and PPO activities during postharvest handling.
Edible coatings
Edible coatings are semipermeable membrane on the fruit skin, creating a modified internal atmosphere, decreasing moisture loss, and respiration rate (Bourtoom, Citation2008). They are composed of proteins, polysaccharides, lipids, and resins (Baldwin et al., Citation1995). The efficacy of edible coatings as a postharvest treatment in horticultural crops has extensively been evaluated (). Among many benefits, edible coatings preserve antioxidant activity, prolong shelf-life, reduce mass loss, respiration rate, maintain firmness and color in treated fruits.
Table 3. The effect of edible coatings on postharvest quality of mango fruit
Chitosan
Chitosan is derived from the deacetylation of beta 1, 4-D-glucosamine, a natural polymer (Gurjar et al., Citation2018). The coating is biodegradable, nontoxic, and characterized by anti-microbial properties. Chitosan is effective in inhibiting postharvest diseases such as Colletotrichum gloeosporioides, Alternaria alternate, and Dothoriella spp. Recent research by Gurjar et al. (Citation2018) revealed that chitosan coating is effective in suppressing microbial growth in processed ‘Mallika’ mangoes. Similarly, chitosan coating at 2% suppressed the incidence and reduced the lesion diameter of Colletotrichum gloeosporioides associated with anthracnose in ‘Tainong’ mango fruit (Zhu et al., Citation2008). Lower incidences of stem-end rot incidence caused by Dothoriella spp. (Wang et al., Citation2007) as well as reduced germination and mycelia growth of Alternaria alternata associated with black spot (López-Mora et al., Citation2013) have been reported in chitosan-coated mangoes. Chitosan is positively charged and thus interacts with the negatively charged cell membrane, therefore affecting the permeability of the cell (Cissé et al., Citation2015). The mechanism of chitosan antibacterial activity is associated with low pH and attributes of the cell surface. It is speculated that chitosan interacts with the outer membrane of the pathogen cell surface, causing leakage of the intracellular substances leading to cell death.
Except for reducing postharvest diseases, chitosan also regulates various physiological and biochemical processes that have an enormous effect on mango fruit quality. For example, Cosme Silva et al. (Citation2017) observed a delayed climacteric peak and decreased respiration rate in ‘Palmer’ mango treated with 3% chitosan. Coating ‘Tainong’ mango with a 2% chitosan reduced the respiration rate (Wang et al., Citation2007). Chitosan coating is reported to be selective to CO2 permeability than O2. For instance, Cissé et al. (Citation2015) observed decreased O2 consumption and increased CO2 production in ‘Kent’ mango fruit coated with 1 or 1.5% chitosan after eight days of storage at ambient temperature. The increased CO2 could enhance the succinic acid and inhibit succinic dehydrogenase activity, resulting in decreased respiration rate (Deng et al., Citation2006; Mathooko, Citation1996). Respiration plays a crucial role in fruit metabolic activity and affects shelf-life. Chitosan forms a permeable barrier against carbon dioxide, moisture, and oxygen, resulting in reduced water loss, respiration, and oxidation reaction rate. However, the protective barrier formed by chitosan in fruit can lead to off-flavors. For example, Wang et al. (Citation2007) reported that chitosan (2%) resulted in poor taste in ‘Tainong’ mango after thirty-five days of storage at 15°C. The off flavors could be attributed to the anaerobic respiration caused by the coating (Sothornvit and Rodsamran, Citation2008). Alcohol and acetaldehyde are produced during anaerobic respiration resulting in bad odor and off flavor (Sothornvit and Rodsamran, Citation2008).
When detached from the tree, fruits continue to respire, consuming all the oxygen inside the fruit. Continuous respiration and loss of water through transpiration lead to the weight loss of the fruit. Fortunately, edible coatings such as chitosan are effective in reducing both water loss as well as fruit weight loss. For instance, 3% chitosan reduced weight loss in processed mango fruit (cv. ‘Mallika’) during storage at 8°C for eight days (Gurjar et al., Citation2018). Fruit weight loss leads to shriveling and decreased esthetic quality. Consumers buy fruit based on visual appearance, and weight loss can affect the acceptability of the fruit because severe mass loss results in shriveling. Furthermore, like other fruits, mango is sold on a weight basis, therefore losing more weight could result in the loss of profit.
Chitosan coating is effective in maintaining firmness in mango during storage. Cissé et al. (Citation2015) reported that chitosan coating retained firmness in ‘Kent’ mango fruit. The loss of membrane integrity is an indicator of fruit ripening and senescence. Chitosan coating retarded the increase of malondialdehyde (MDA) content and maintained membrane integrity (Khaliq et al., Citation2017). The MDA is a secondary end-product resulting from free radicals damaging lipid peroxidation. Lipid degradation alters cellular membrane structure and function. The protective barrier formed by the coating reduces lipid peroxidation leading to decreased electrolyte leakage, thus retaining fruit firmness and delaying senescence.
Carboxymethyl Cellulose
Carboxymethyl cellulose (CMC) is derived from cellulose and composed of linear chains of β (1–4) glucosidic units with carboxyl substituent, hydroxypropyl, and methyl (Salinas-Roca et al., Citation2018). CMC coating can form a semipermeable barrier, thereby modifying the internal fruit atmosphere, limiting the exchange of gases into and out of fruit, and hence reducing respiration rate and delaying senescence. There is a considerable amount of published research illustrating the potential of CMC as the postharvest treatment of mango fruit. For instance, Phaiphan and Rattanapanone (Citation2008) reported that mango fruit coated with 1% CMC had a lower respiration rate compared to their uncoated counterparts. The efficacy of CMC is partly linked to its ability to modify the biochemical processes causing partial anaerobic respiration. A decline in respiration rate and shift of climacteric peak leads to delayed fruit senescence. An increase in respiration rate is associated with fruit color changes from green to yellow, indicating fruit ripening. Coating mango fruit with 2% CMC is effective in maintaining fruit color during storage (Abbasi et al., Citation2011). Plotto et al. (Citation2004) reported delayed color changes in CMC-treated mango fruit.
The use of CMC as a standalone treatment has been reported to be ineffective for some cultivars and postharvest handling conditions. For instance, Salinas-Roca et al. (Citation2018) reported that 2% CMC failed to reduce mold, yeast, and psychrophilic bacteria in fresh-cut ‘Tommy Atkins’ mango fruit stored at 4°C for ten days. Interestingly, the researchers found that incorporating CMC with gum rabic coating proved to be effective in reducing microbial growth in ‘Button’ mushrooms stored at 4°C for twelve days (Srivastava and Bala, Citation2016). The anti-microbial effect of CMC is complex and not well understood. The microbial count was reduced in ‘Tommy Atkins’ mango fruit coated with CMC (5 g kg−1) after twenty-one days of storage at 5°C (Plotto et al., Citation2010).
Recent attempts to improve the efficacy of edible coatings have focused on combining two or more coating agents to improve the anti-fungal and physical properties. The use of plant extracts such as moringa, aloe vera gel with CMC, or chitosan has been shown to be effective in various horticultural fresh vegetables and fruits (Tesfay and Magwaza, Citation2017). Ali et al. (Citation2012) reported that treatment combination of Gum Arabic (GA)10% + chitosan 1% effectively decreased the disease index and mycelia growth of Colletotrichum gloeosporioides in ‘Eksotika II’ papaya stored at 12°C for twenty-eight days. The combination of GA that contains no antifungal properties with chitosan, which has antimicrobial properties, augmented the coating, thus decreasing anthracnose in the fruit (Ali et al., Citation2012; Siddiqui and Asgar, Citation2014). Edible coatings that contain natural antimicrobial and antioxidants have shown high resistance against microorganisms (Ali et al., Citation2017). However, so far, very little research has focused on the combinational use of plant extracts with edible coatings as postharvest treatment of mango fruit. Thus, postharvest research assessing the effect of plant extracts and the commercially used edible coatings is warranted.
Gum Arabic
Gum Arabic (GA) is a natural polysaccharide obtained from the branches and stem of Acacia species (Ali et al., Citation2010). Although it is commonly used as a stabilizer and thickener by the food industry, recent studies have demonstrated its potential as an edible coating (Khaliq et al., Citation2016b). Gum arabic does not only affect the physical attributes of the fruit, the biochemical and nutritional quality is also influenced by the treatment. Vitamin C is one of the prominent nutritional attributes in mango fruit, it is water-soluble and highly beneficial for human health (Mditshwa et al., Citation2017; Muhammad et al., Citation2014). A recent study by Daisy et al. (Citation2020) demonstrated that 15% GA coating effectively maintained the AA in ‘Apple’ mango stored at 23°C for fifteen days. The AA is a powerful antioxidant that reduces oxidative stress caused by ROS (Khaliq et al., Citation2015). Khaliq et al. (Citation2016a) reported that GA (10%) coating reduced H2O2 and O2⁻ in ‘Choke Anan’ mango fruit during storage at 6°C for twenty-eight days. The GA coating improves the antioxidant pool (Khaliq et al., Citation2016a), which scavenges excess ROS, thus decreasing oxidative damage to the fruit. Additionally, the high content of vitamin C in GA treated mangoes improves nutritional fruit quality. The consumption of vitamin C-rich foods is known for boosting the immune system and reducing the risk of cardiovascular diseases and various types of cancer (Muhammad et al., Citation2014).
Ethylene production is a well-known indicator of the metabolic activity and has tremendous influence on shelf-life and quality of mango fruit. During fruit ripening, there is an upsurge in ethylene production, modulating biochemical changes such as aroma, texture, and color changes (Daisy et al., Citation2020; Khaliq et al., Citation2015). Khaliq et al. (Citation2015) reported that GA (10%) delayed the ethylene climacteric peak for twenty-one days in ‘Choke Anan’ mango stored at 6°C. Lawson et al. (Citation2019) reported that ethylene is negatively correlated to firmness during fruit ripening. The decreased ethylene production slows down the rate of fruit ripening and softening. Khaliq et al. (Citation2016b) reported that GA (10%) coating maintained firmness in ‘Choke Anan’ mango during storage at 13°C for twenty-eight days. The loss of fruit firmness in mango is due to the changes in cell wall composition and structure (Khaliq et al., Citation2015). The barrier formed by the GA coating could decrease the cell wall degrading enzyme activity, thus delaying loss of fruit firmness.
Non-chemical treatments
Non-chemical treatments such as ultraviolet irradiation and heat treatment have shown to be effective in maintaining the postharvest quality of mango (). These treatments have been used successfully for controlling postharvest diseases and extending the shelf-life. Among these treatments, heat treatment is used commercially by the mango industry, as it is cost-effective and easily adopted by mango producers (Sivakumar et al., Citation2011).
Table 4. Mango fruit quality as influenced by heat treatment and UV-C irradiation
Heat treatment
Hot Water Treatment
Postharvest heat technology has been used in horticultural crops to sanitize and extend shelf-life. Hot air (HAT) and hot water treatment (HWT) are some of the cheap and commonly used heat treatments. The use of heat as a postharvest treatment of mango fruit has been well-researched and documented. For instance, HWT at 55°C for ten-minutes suppressed respiration in ‘Tainong’ mango fruit during storage at 20°C for six days (Zhang et al., Citation2012). Similarly, studies on ‘Ivory’ mango revealed that hot water treatment at 60°C for one-minute inhibited ethylene production and respiration rate (Wang et al., Citation2016). The efficacy of HWT is strongly linked to its potential to regulate key enzymatic activities affecting quality attributes of fresh horticultural produce. The activities of ACC oxidase have been demonstrated to inhibit in hot water treated (46°C, 90-minutes) ‘Keitt’ mango (Bender et al., Citation2003). ACC oxidase regulates ethylene biosynthesis; therefore, inhibition of this enzyme delays production of ethylene. Ethylene production activates the physical and biochemical processes involved in fruit softening (Khaliq et al., Citation2015).
Increased firmness retention in fruit subjected to HWT before long-term cold storage has been reported (Ding and Mijin, Citation2013). Notably, heat treatment may cause stress resistance by stimulating the antioxidant activities and protective enzymes of the treated fruit. Enzymes such as PG, β-galactosidase, α-mannosidase, and β-hexosaminidase are involved in cell wall modification and softening in mango fruit (Abu-Sarra and Abu-Goukhi, Citation1992; Hossain et al., Citation2014). HWT (60°C for 1 minute) inhibits the cell wall degrading enzyme PG after ten days of storage at 25°C (Wang et al., Citation2016). Previous studies by Ketsa et al. (Citation1998) revealed that HWT at 33°C for three days increased the activity of β-galactosidase caused in ‘Nam Dokmai’ mango after eight days of storage at 25°C. This suggests that β-galactosidase might play a prominent role in mango fruit softening than PG. Sripong et al. (Citation2015) reported a rapid fruit softening in ‘Chok-Anan’ mango dipped in 55°C for five minutes. Dautt-Castro et al. (Citation2018) indicated that HWT (47°C for 5 minutes) upregulates cell wall genes of β-galactosidase (MiBGAL c23904), pectate lysase (MiPL c20761), polygalacturonases (MiPG c21885), ram-nogalaturonase (MiRGL c23797) and small heat shock proteins (MiHSP20 c12121). Ram-nogalaturonase MiRGL c23797 gene is involved in rhamnogalacturonan degradation and has a physiological role in abiotic stress (Dautt-Castro et al., Citation2018). The upregulation of these genes causes an increase in their related enzymes, leading to rapid fruit softening and ripening. The variation in temperature and HWT time could trigger different reactions with regard to gene expression and enzyme activities.
HWT has an enormous effect on the organoleptic and physicochemical quality attributes of mangoes. A recent study by Dautt-Castro et al. (Citation2018) demonstrated that HWT (47°C for 5 minutes) increased TSS accumulation in ‘Ataulfo’ mango during storage at 20°C for eight days. Hot water is known to upregulate beta-amylase gene MiBAM c23077 involved in starch hydrolyzes (Dautt-Castro et al., Citation2018). Sucrose synthase gene MiSS, c10928, is upregulated by HWT (Dautt-Castro et al., Citation2018). The upregulation of these genes could lead to rapid starch degradation, an increase in TSS accumulation, thus enhanced fruit ripening. It should, however, be noted that the effect of heat treatments on some quality attributes is cultivar dependent. Le et al. (Citation2010) reported vapor heat treatment at 46.5°C for 40 minutes did not affect TSS accumulation in ‘Tuu Shien’ mango stored at 12°C for three weeks. Thus, it is critical to design an appropriate post-harvest protocol for each mango cultivar.
Research has also shown that HWT maintains color and appearance of the fruit peel. For instance, ‘Tuu Shien’ mango fruit treated with hot water at 50°C for ten minutes retained the green color during storage (Le et al., Citation2010). Dautt-Castro et al. (Citation2018) reported that hot water down-regulates chloroplastic-like (LHCIIb) (EC4.99.1.1) genes involved in chlorophyll biosynthesis. These researchers found that HWT increased gene expression of anthocyanin 5-aromatic (anthocyanin5a) (EC:2.3.1.144) and UD-Pglycosyltransferase 85a2-like (85A2) (EC:2.4.1.115) which are involved in anthocyanin accumulation. An increased chlorophyll degradation and increased production of anthocyanin resulted in homogenous color development of mango fruit.
Hot water treatment is effective in suppressing the severity of CI in mango fruit. Zang et al. (Citation2012) reported that HWT (55°C for10 minutes) reduced the CI in ‘Tainong 1ʹ mango fruit during storage (21 days, 5°C and 5 days, 20°C). The HWT activates lipid-related metabolism in mango fruit during low-temperature storage. Vega-Alvarez et al. (Citation2020) observed high levels of linolenic acid in ‘Keitt’ mango treated with hot water (46.1°C for 90 minutes) and stored at 5°C for twenty-one days followed by seven days at 21°C. Similarly, Yimyong et al. (Citation2011) observed high levels of lipoxygenase (LOX) protein in ‘Okrong’ mango treated with hot water at 50°C for ten minutes and stored at 8°C for fifteen days. The increased levels of LOX and fatty acids could induce the CI tolerance in mango fruit.
Hot Air Treatment
Hot air treatments (HAT) influence postharvest pathological disorders and diseases. For examples, studies have shown that a combined treatment of heat vapor at 46.5°C for 40 minutes and hot water at 55°C for three minutes decreased the incidence of anthracnose caused by Colletotrichum gloeosporioides and Alternaria alternate associated with black spot in ‘Tuu Shien’ mango (Le et al., Citation2010). Further studies on heat treatment indicated that hot water vapor at 55°C for 15–20 seconds decreased the incidence of black spot caused by Alternaria alternate in ‘Shelly’ mango stored at 12°C for twenty-one days (Luria et al., Citation2014). Defense-related genes associated with salicylic acid and jasmonic acid such as Syntaxin-121-like (Syn121), glutaredoxin (EC 1.20.4.1), and Allene oxide synthase (AOS) were upregulated by heat treatment in mango fruit (Luria et al., Citation2014). Syntaxin is a plant defense protein that causes resistance against disease and pathogen penetration (Shukla et al., Citation2010). This suggests that heat treatment regulates cellular defense genes inducing resistance against pathogens, thus reducing decay of mango fruit during storage.
It is crucial to note that the various factors affecting the efficacy of heat treatments. These factors include the maturity stage, cultivars, exposure time, and temperature. Fruit size is another critical factor that has to be considered when applying heat treatments. It is well known that small fruit are easily damaged compared to larger fruit (Sivakumar and Fallik, Citation2013). Moreover, immature fruit are less heat tolerant than mature fruit; due to internal breakdown that can occur when they are exposed to heat (Sivakumar and Fallik, Citation2013; Sivakumar et al., Citation2011). Mechanical damage and poor quality has been reported following heat treatment. For instance, Osuna-Garcia et al. (Citation2015) observed lenticel damage and dark browning spots in ‘Kent’ mango treated with 46.1°C for 90 minutes. Increasing the temperature or exposure time can cause heat-induced injury, firmness, and weight loss leading to rapid fruit decay. Thus, it is important to consider all these factors when heat is used as the treatment for fresh mango fruit.
Ultraviolet-C (UV-C) radiation
Short-wave ultraviolet is a non-thermal technology with a wavelength of 190–280 nm (Mohamed et al., Citation2017). UV-C irradiation is used as a postharvest treatment to enhance fruit quality and extend the shelf-life of fresh fruits and vegetables. The use of UV-C as a postharvest treatment has been reported in mangoes. For example, George et al. (Citation2015) reported that UV-C treatment at 254 nm preserved quality and increased the shelf-life of fresh-cut mango (cv. ‘Chokanan’) up to fifteen days. UV-C treatment (250 nm for 15 minutes) preserved sensory attributes of ‘Chokanan’ mangoes stored at 4°C for fifteen days. The taste and aroma are closely correlated and influence how the consumer perceives the fruit. Loss of either one of these attributes may result in the fruit being rejected by the consumers.
Antioxidants are an important quality attribute in mangoes as they have a prominent role in human health. There is growing literature evidence demonstrating that UV-C treatment does affect the accumulation of phytochemicals, such as antioxidants. For instance, UV-C irradiation (250–280 nm for10 minutes) of ‘Tommy Atkin’ mango fruit increased the total phenolic and flavonoid content after fifteen days of storage at 5°C (González-Aguilar et al., Citation2007). The high antioxidant activity in mangoes provides a much desired health benefit to the consumers.
Various physiological and postharvest quality-linked enzymatic activities are also influenced by irradiation. For example, Safitri et al. (Citation2015) demonstrated that UV-C irradiation at 4.93 kJ/m2 reduced respiration rate in ‘Nam Dok Mai Si Thong’ mango fruit stored at 14°C for twenty days. The expression of 1-aminocyclopropane carboxylate synthase (ACS) and ACO was significantly inhibited in UV-C treated ‘Chikanan’ mangoes (George et al., Citation2016). As above mentioned, ACS and ACO are strongly involved in ethylene biosynthesis; therefore, their reduction may delay ripening, minimize fruit decay and prolong shelf-life.
Experimental studies have also demonstrated that irradiation has the potential of inhibiting various postharvest diseases and disorders. A recent in vitro study by Terao et al. (Citation2015) showed that UV-C treatment at 20 kJ m−1 reduced the mycelia growth of Colletotrichum gloeosporioides and Botryosphaeria dothidea. Romero et al. (Citation2017) reported that UV-C treatment (2.064 kJ/m2 for 5 minutes) decreased Escherichia coli and Listeria innocua growth in ‘Tommy Atkins’ sliced mango stored at 4°C for fifteen days. Biochemically, UV-C irradiation induces defense-related enzyme activities of GLU, POD, PAL and CHI (Sripong et al., Citation2015). The increased expression of GLU and CHI in UV-C treated fruit could be an indication of their involvement in degrading the cell wall of the postharvest pathogens, while PAL creates an unconducive environment for pathogen growth and development.
Storage technologies
Preservation of mango fruit quality using techniques such as controlled atmosphere (CA) and modified atmosphere packaging (MAP) has been used over the past decade (). In these storage conditions, the oxygen (O2) concentration is reduced while carbon dioxide (CO2) is increased to extend fruit shelf-life. The benefits of decreasing O2 and increasing CO2 levels include the reduction of respiration rate, which slows down the metabolic processes, resulting in reduced fruit senescence (Costa et al., Citation2018; Ntsoane et al., Citation2019a).
Table 5. Effect of MAP, CA and LOS on mango fruit quality
Controlled Atmosphere
Controlled atmosphere (CA) incorporated with optimum low temperatures has been used to maintain the quality of mango fruit during storage (Sumual et al., Citation2017). The adequate level of CO2 and O2 are important factors affecting fruit quality. Ullah et al. (Citation2010) reported that CA treatment of 3% O2 and 6% CO2 retained sweetness and flavor in ‘Alphonso’ mangoes stored at10°C for twenty-one days. However, CA storage has been reported to compromise fruit aroma during storage. A study by Rattanapanone et al. (Citation2001) reported that aroma diminished in ‘Tommy Atkins’ mango fruit stored at 4 kPa O2 and10 kPa CO2 for eight days at10°C. The loss of aroma in mango fruit could be linked to drastic changes in the accumulation of volatile compounds under low oxygen and high carbon dioxide storage conditions. A reduction of aroma volatile compounds such as esters, ketones and aldehydes has been reported in ‘Kensington Pride’ mangoes stored at CA of 2% O2 and 9% CO2 at 13°C for thirty-five days (Lalel et al., Citation2003). High CO2 concentrations in the storage chambers can easily trigger anaerobic respiration. Thus, an appropriate gas composition is critical for ensuring the superior nutritional and physicochemical quality of mangoes stored in CA.
Modified Atmosphere Packaging
Modified atmosphere packaging (MAP) is used to increase shelf-life and maintain the postharvest quality of horticultural crops. MAP has been shown to be effective in combination with other treatments such as HWT and coatings. Ramayya et al. (Citation2012) reported that unperforated oriented polypropylene bags decreased weight loss in ‘Alphanso’ mangoes stored at10°C for twenty-one days. The storage temperature may determine the success of MAP storage. Increased weight loss has been reported in ‘Tommy Atkins’ mangoes wrapped in flexible Xtend® and stored at 25°C compared to those at 12°C for twenty-one days (Costa et al., Citation2018). These studies highlight the importance of cold storage in decreasing weight loss and fruit spoilage in MAP. Preserving fruit quality is dependent on optimum treatment combination of MAP and cold storage. The combination of MAP with low temperatures is crucial for reducing respiration rate and other metabolic processes that may compromise fruit quality during postharvest storage and shelf-life.
Low Oxygen Storage
Low oxygen storage (LOS) is an emerging technology that allows fresh horticultural produce to be stored under extremely low O2 levels (Wright et al., Citation2015). Firmness retention and reduced respiration rate are some of the benefits associated with LOS storage. A recent study by Ntsoane et al. (Citation2019a) reported that combing CA storage with 1% O2 reduced the respiration rate in ‘Shelly’ mango during storage at 13°C for twenty-one days. An earlier experiment by De Almeida Teixeira and Durigan (Citation2011) also revealed that storing ‘Palmer’ mangoes in CA + 1 or 5% O2 notably retarded respiration rate for twenty-eight days. The reduced respiration rate could be attributed to low levels of O2 concentration, which also inhibits ethylene biosynthesis.
Although LOS offers many benefits to mango producers, it should be noted that it may cause internal browning, off-flavors as well as peel discolouration (Wright et al., Citation2015). In fact, Ntsoane et al. (Citation2019a) reported that consumers rejected ‘Shelly’ mangoes stored at 1% O2 for twenty-one days at 13°C due to off-flavor and bad odor. Interestingly, these researchers also found that elevating O2 concentration to10% eliminated off-flavors. This suggests that storing mangoes below their oxygen limit can result in anaerobic conditions as well as accumulation of undesired volatile compounds, resulting in poor sensory quality and marketability. Thus, proper LOS protocols must be developed for each mango cultivar as varieties might have a different response. Moreover, the interaction between LOS and maturity must be investigated as the mango fruit might respond to low oxygen levels might be influenced by the physiological and biochemical status at harvest.
Emerging Postharvest Technologies
Postharvest technologies such as ozone and pulsed electric field are gaining the attention of researchers. More research is done to explore the potential of these technologies to preserve fruit quality. However, limited data is available on the use of such technologies as postharvest treatments of mango fruit
Ozone
Ozone (O3) is a triatomic oxygen molecule with a high oxidative reduction potential (Sandhu et al., Citation2011). It is highly unstable and when it decomposes, it forms radicals such as carbon dioxide, hydrogen, carbon monoxide as well as water (Anglada et al., Citation1999). The use of O3 to maintain the quality and extend the shelf-life of horticultural crops has been documented (Shezi et al., Citation2020a). Emerging evidence indicates that O3 is effective in preserving firmness, decreasing weight loss and shelf-life in fruits (Minas et al., Citation2014; Shezi et al., Citation2020b; Tran et al., Citation2013).
The use of ozone as a postharvest treatment has been evaluated on mangoes, even though the research in this area remains very insignificant. Tran et al. (Citation2013) observed a reduced respiration rate in mango fumigated with10 μL L−1 O3 for10 minutes during storage at 25°C for six days. Although the mechanism of action is not yet fully understood, ozone is reported to inhibit ethylene biosynthesis by reducing the ACC levels in fruit cell walls (Minas et al., Citation2014). Additionally, ACO protein and ACO1, an enzyme responsible for ethylene production, are suppressed and down-regulated, respectively, by postharvest ozone treatments (Tzortzakis et al., Citation2013).
Due to its anti-microbial activity, ozone seems to be effective against various postharvest pathogens. Barbosa-Martínez et al. (Citation2002) reported that ozone inhibited the spore germination of various pathogens, including Fusarium oxysporum and Colletotrichum gloeosporioides. Recent studies by Da Silva Neto et al. (Citation2019) has shown that O3 at 3.3 ppm reduced the disease incidence and severity of anthracnose in papaya fruit stored at room temperature for twelve days. In an invitro study, Ong and Ali (Citation2015) reported that O3 (3.5 µL/L or 5 µL/L for twenty-four hours) treatment generates ROS, which degrades mitochondria of Colletotrichum gloeosporioides spores. ROS causes oxidative stress in fungi, which modifies the endoplasmic reticula and mitochondria structure (Ong and Ali, Citation2015). Ozone inhibits the defense-related genes Chi3a, Chi9b, Gluac, Glubs and plant defensin gene Pdf1.2 (Tzortzakis et al., Citation2011). The possible mechanism of O3 in inducing disease resistance in fruit is through the accumulation of phenolic compounds and downregulation of defense-related genes (Minas et al., Citation2010; Tzortzakis et al., Citation2011).
The effectiveness of O3 against pathogens is influenced by storage conditions, particularly relative humidity, temperature (Batakliev et al., Citation2014; Egorova et al., Citation2015; Miller et al., Citation2013). While the potential of ozone has extensively been evaluated for other fresh horticultural products, it has received little attention from the mango industry. For instance, the best ozone concentration and exposure time for mangoes are currently not known. Moreover, the relationship between harvest maturity and ozone concentration has never been determined. Thus, concerted research efforts must be made to ascertain the possible role of ozone as a postharvest treatment of mangoes.
Pulsed Electric Field
Pulsed electric field (PEF) is a non-thermal technology used for microbial inactivation of food items. The use of PEF is linked with reduced respiration rate as well as retention of nutritional quality attributes such as ascorbic acid and antioxidants (González-Casado et al., Citation2018). Although the effect of PEF on fresh mangoes has not yet been reported, treating ‘Mallika’ mango nectar with PEF has been shown to inactivate microflora and maintain sensory quality attributes (Kumar et al., Citation2015). Ascorbic acid and alpha-tocopherol are some of the key antioxidants that prevent off-flavor development mango fruit (Kaur et al., Citation2020). Similarly, a recent report by Kumar et al. (Citation2019) demonstrated that PEF (70–120 Hz pulse frequency, 15–24 µs pulse width) treatment significantly prolonged the shelf-life of mango nectar stored at 5°C for ninety days. The authors linked the prolonged shelf-life with the ability of PEF to kill pathogens without compromising food quality. The mechanism of PEF on the inactivation of microorganisms is not well understood. Moreover, there is limited data available on PEF as a postharvest treatment of mango fruit. Considering that PEF is non-thermal and could be cost-effective compared to the currently used postharvest treatments, research assessing its potential for the mango fruit industry is warranted.
Conclusion and Research Prospects
The literature provides exciting findings on emerging technologies such as PEF and O3. While the use of such postharvest treatment has yielded impressive results, they are yet to be commercially adopted. Obtaining a balance between fruit quality and consumer safety is required for the product to be accepted by consumers and industry. Further research is necessary to gain understanding and explore the potential use of these technologies on the quality of fresh produce. The ozone decomposition mechanism is intricate, and research should aim at enhancing the efficacy of ozone and explore different exposure times suitable for each harvest maturity and mango cultivar. The potential of O3 in combination with other treatments such as edible coatings to decrease postharvest diseases and enhance fruit quality requires further investigation. There is a gap of knowledge on postharvest use of O3 incorporated with edible coatings such as CMC and moringa in mango fruit. Moreover, further research is needed to develop useful cellulose-based coatings to prolong shelf-life. Future research should focus on understanding the mode of action of O3 incorporated with other postharvest treatments and commercializing these postharvest technologies
Declaration of Interest Statement
There is no potential conflict of interest declared by the author(s).
Acknowledgments
The authors are grateful for the financial from the National Research Foundation’s competitive support for unrated researchers grant (CSUR:105978).
Additional information
Funding
References
- Abbasi, K.S., N. Anjum, S. Sammi, T. Masud, and S. Ali. 2011. Effect of coatings and packaging material on the keeping quality of mangoes (Mangifera indica L.) stored at low temperature. Pakistan J. Nutr. 10(2):129–138. doi: https://doi.org/10.3923/pjn.2011.129.138.
- Abd El-Gawad, M.G., Z.A. Zaki, and Z.A. Ekbal. 2019. Effect of some postharvest treatments on quality of “ Alphonse” mango fruits during cold storage. Middle East J 8(4):1067–1079.
- Abu-Sarra, A.F., and A.A. Abu-Goukhi. 1992. Changes in pectinesterase, polygalacturonse and cellulase activity during mango fruit ripening. J. Hortic. Sci. 67(4):561–568. doi: https://doi.org/10.1080/00221589.1992.11516284.
- Ali, A., M. Maqbool, P.G. Alderson, and N. Zahid. 2012. Efficacy of biodegradable novel edible coatings to control postharvest anthracnose and maintain quality of fresh horticultural produce. Acta Hortic. 945:39–44. doi: https://doi.org/10.17660/ActaHortic.2012.945.3.
- Ali, A., M. Maqbool, S. Ramachandran, and P.G. Alderson. 2010. Gum arabic as a novel edible coating for enhancing shelf-life and improving postharvest quality of tomato (Solanum lycopersicum L.) fruit. Postharvest Biol. Tech. 58:42–47. doi: https://doi.org/10.1016/j.postharvbio.2010.05.005.
- Ali, A., W.K. Yeoh, C. Forney, and M.W. Siddiqui. 2017. Advances in postharvest technologies to extend the storage life of minimally processed fruits and vegetables. Crit. Rev. Food Sci. Nutr. doi: https://doi.org/10.1080/10408398.2017.1339180.
- Altendorf, S. 2017. Global prospects for major tropical fruits. Short-term outlook, challenges and opportunities in a vibrant global marketplace. Food outlook. http://www.fao.org/fileadmin/templates/est/COMM_MARKETS_MONITORING/Tropical_Fruits/Documents/Tropical_Fruits_Special_Feature.pdf
- Alvindia, D.G., and M.A. Acda. 2015. Revisiting the efficacy of hot water treatment in managing anthracnose and stem-end rot diseases of mango cv. ‘Carabao’. Crop Prot. 67:96–101. doi: https://doi.org/10.1016/j.cropro.2014.09.016.
- Anglada, J.M., R. Crehuet, and J.M. Bofill. 1999. The ozonolysis of ethylene: A theoretical study of the gas-phase reaction mechanism. Chem. Eur. J. 5(6):1809–1822. doi: https://doi.org/10.1002/(SICI)1521-3765(19990604)5:6<809::aidchem1809>3.0.CO;2-N.
- Asghari, M., and M.S. Aghdam. 2010. Impact of salicylic acid on post-harvest physiology of horticultural crops. Trends in Food Sci Technol. 21:502–509. doi: https://doi.org/10.1016/j.tifs.2010.07.009.
- Asio, L.G., and F.D. Cuaresma. 2016. A review of postharvest treatments to maintain mango (Mangifera Indica L.) quality. Annals of Trop. Res. 38(1):81–93. doi: https://doi.org/10.32945/atr3817.2016.
- Awad, M.A., A.D. Al-Qurashi, S.A. Mohamed, and R.M. El-Shishtawy. 2017. Quality and biochemical changes of ‘Hindi-Besennara’ mangoes during shelf life as affected by chitosan, gallic acid and chitosan gallate. J. Food Sci. Technol. 54(13):4139–4148. doi: https://doi.org/10.1007/s13197-017-2762-x.
- Aziz, N.A.A., L.M. Wong, R. Bhat, and L.H. Cheng. 2012. Evaluation of processed green and ripe mango peel and pulp flours (Mangifera indica var. Chokanan) in terms of chemical composition, antioxidant compounds and functional properties. J. Sci. Food Agric. 92:557–563. doi: https://doi.org/10.1002/jsfa.4606.
- Baldwin, E.A., M.O. Nisperos-Carriedo, and R.A. Baker. 1995. Edible coatings for lightly processed fruits and vegetables. Hort. Sci. 30(1):35–38.
- Barbosa-Martínez, C., L. Ponce De León-garcía, and J. Sepúlveda-Sánchez. 2002. Effects of ozone, iodine and chlorine on spore germination of fungi isolated from mango fruits. Revista Mexicana de Fitopatología. 20(1):60–65.
- Barman, K., and R. Asrey. 2014. Salicylic acid pre-treatment alleviates chilling injury preserve bioactive compounds and enhances shelf life of mango fruit during cold storage. J. Sci. Ind. Resh. 73:713–718.
- Barman, K., R. Asrey, R.K. Pal, S.K. Jha, and K. Bhatia. 2014. Post-harvest nitric oxide treatment reduces chilling injury and enhances the shelf-life of mango (Mangifera indica L.) fruit during low-temperature storage. J. Hort. Sci. Biotech. 89(3):253–260. doi: https://doi.org/10.1080/14620316.2014.11513076.
- Batakliev, T., V. Georgiev, M. Anachkov, S. Rakovsky, and G.E. Zaikov. 2014. Ozone decomposition. Interdisc. Toxicol. 7(2):47–59. doi: https://doi.org/10.2478/intox-2014-0008.
- Bender, R.J., E. Seibert, and J.K. Brecht. 2003. Heat treatment effects on ACC oxidase activity of ‘Keitt’ mangoes. Braz. J. Plant Physiol. 15(3):145–148. doi: https://doi.org/10.1590/S1677-04202003000300003.
- Bourtoom, T. 2008. Edible films and coatings: Characteristics and properties. Int. Food Res. J. 15(3):237–248.
- Castillo-Israel, K.A.T., J.B.L. Gandia, A.C.G. Velez, W.L. Absulio, and L.C. Bainto. 2015. Storage quality of fresh-cut Philippine ‘Carabao’ mango (Mangifera indica L. cv. ‘Carabao’) fruits with 1-Methylcyclopropene (1-MCP) post-cutting treatment. Int. Food Res. J. 22(6):2196–2202.
- Chourasia, A., V.A. Sane, and P. Nath. 2006. Differential expression of pectate lyase during ethylene-induced postharvest softening of mango (Mangifera indica var. Dashehari). Physiol. Plant. 128:546–555. doi: https://doi.org/10.1111/j.1399-3054.2006.00752.x.
- Chourasia, A., V.A. Sane, R.K. Singh, and P. Nath. 2008. Isolation and characterization of the MiCel1 gene from mango: Ripening related expression and enhanced endoglucanase activity during softening. Plant Growth Regul. 56:117–127. doi: https://doi.org/10.1007/s10725-008-9292-5.
- Cissé, M., J. Polidori, D. Montet, G. Loiseau, and M.N. Ducamp-Collin. 2015. Preservation of mango quality by using functional chitosan lactoperoxidase systems coatings. Postharvest Biol. Tec. 101:10–14. doi: https://doi.org/10.1016/j.postharvbio.2014.11.003.
- Cosme Silva, G.M., W.B. Silva, D.B. Medeiros, A.R. Salvador, M.H.M. Cordeiro, N.M. Da Silva, D.B. Santana, and G.P. Mizobutsi. 2017. The chitosan affects severely the carbon metabolism in mango (Mangifera indica L. cv. Palmer) fruit during storage. Food Chem. 237:372–378. doi: https://doi.org/10.1016/j.foodchem.2017.05.123.
- Costa, J.D.D.S., A. Figueiredo Neto, F.D.A.C. Almeida, and M.D.S. Costa. 2018. Conservation of ‘Tommy Atkins’ mangoes stored under passive modified atmosphere. Rev. Caatinga. 31(1):117–125. doi: https://doi.org/10.1590/1983-21252018v31n114rc.
- Da Silva Neto, O.P., E.V. Da Silva Pinto, M.A. Ootani, J.L. Da Silva Junior, J.L. Da Silva Bentes Lima, and A.E.D. De Sousa. 2019. Ozone slows down anthracnose and increases shelf life of papaya fruits. Rev. Bras. Frutic.Jaboticabal. 41(5):e–439. doi: https://doi.org/10.1590/0100-29452019439.
- Daisy, L.L., J.M. Nduko, W.M. Joseph, and S.M. Richard. 2020. Effect of edible gum Arabic coating on the shelf life and quality of mangoes (Mangifera indica) during storage. J. Food Sci. Tech. 57(1):79–85. doi: https://doi.org/10.1007/s13197-019-04032-w.
- Dautt-Castro, M., A. Ochoa-Leyva, C.A. Contreras-Vergara, A. Muhlia-Almazán, M. Rivera-Domínguez, S. Casas-Flores, M.A. Martinez-Tellez, A. Sañudo-Barajas, T. Osuna-Enciso, M.A. Baez-Sañudo, et al. 2018. Mesocarp RNA-Seq analysis of mango (Mangifera indica L.) identify quarantine postharvest treatment effects on gene expression. Sci. Hortic. 227:146–153. doi: https://doi.org/10.1016/j.scienta.2017.09.031.
- De Almeida Teixeira, G.H., and J.F. Durigan. 2011. Storage of ‘Palmer’mangoes in low-oxygen atmospheres. Fruits. 66(4):279–289. doi: https://doi.org/10.1051/fruits/2011037.
- De Almeida Teixeira, G.H., L.O. Santos, L.C.C. Júnior, and J.F. Durigan. 2018. Effect of carbon dioxide (CO2) and oxygen (O2) levels on quality of ‘Palmer’ mangoes under controlled atmosphere storage. J. Food Sci. Tech. 55(1):145–156. doi: https://doi.org/10.1007/s13197-017-2873-4.
- Denga, Y., Y. Wu, and Y. Li. 2006. Physiological responses and quality attributes of ‘Kyoho’ grapes to controlled atmosphere storage. LWT. 39:584–590. doi: https://doi.org/10.1016/j.lwt.2005.05.001.
- Ding, P., and S. Mijin. 2013. Physico-chemical characteristics of Chok Anan Mango fruit after hot water treatment. Pertanika J. Trop. Agric. Sci. 36(4):359–372.
- Ding, Z.S., S.P. Tian, X.L. Zheng, Z.W. Zhou, and Y. Xu. 2007. Responses of reactive oxygen metabolism and quality in mango fruit to exogenous oxalic acid or salicylic acid under chilling temperature stress. Physiol. Plant. 130:112–121. doi: https://doi.org/10.1111/j.1399-3054.2007.00893.x.
- Ducamp-Collin, M.N., Reynes, M., Lebrun, M. and Freire Jr., M. (2009). Fresh cut mango fruits: Evaluation of edible coatings. Acta Hortic. 820, 761-768.
- Egorova, G.V., V.A. Voblikova, L.V. Sabitova, I.S. Tkachenko, S.N. Tkachenko, and V.V. Lunin. 2015. Ozone solubility in water. Vestn. Mosk. Univ. Khimiya. 5:261–265. doi: https://doi.org/10.3103/S0027131415050053.
- Faasema, J., J.S. Alakali, and J.O. Abu. 2012. Effects of storage temperature on 1-methylcyclopropene-treated mango (Mangnifera indica) fruit varieties. J. Food Process. Pres. 38:289–295. doi: https://doi.org/10.1111/j.1745-4549.2012.00775.x.
- Freschi, L. 2013. Nitric oxide and phytohormone interactions: Current status and perspectives. Front. Plant Sci. 4. doi: https://doi.org/10.3389/fpls.2013.00398.
- Gava, C.A.T., A.P.C. De Castro, C.A. Pereira, and P.I. Fernandes-Júnior. 2018. Isolation of fruit colonizer yeasts and screening against mango decay caused by multiple pathogens. Biol. Control. 117:137–146. doi: https://doi.org/10.1016/j.biocontrol.2017.11.005.
- George, D.S., Z. Razali, V. Santhirasegaram, and C. Somasundram. 2015. Effects of ultraviolet light (UV-C) and heat treatment on the quality of fresh-cut Chokanan mango and Josephine pineapple. J. Food Sci. 80(2):426–434. doi: https://doi.org/10.1111/1750-3841.12762.
- George, D.S., Z. Razali, V. Santhirasegaram, and C. Somasundram. 2016. Effect of postharvest ultraviolet-C treatment on the proteome changes in fresh cut mango (Mangifera indica L. cv. Chokanan). J. Sci. Food Agric. 96:2851–2860. doi: https://doi.org/10.1002/jsfa.7454.
- González-Aguilar, G.A., C.Y. Wang, J.G. Buta, and D.T. Krizek. 2001. Use of UV-C irradiation to prevent decay and maintain postharvest quality of ripe `Tommy Atkins’ mangoes. Int. J. Food Sci. Tech. 36:767–773. doi: https://doi.org/10.1111/j.1365-2621.2001.00522.x.
- González-Aguilar, G.A., M.A. Villegas-ochoa, M.A. Martínez-Téllez, A.A. Gardea, and J.F. Ayala-Zavala. 2007. Improving antioxidant capacity of fresh-cut mangoes treated with UV-C. J. Food Sci.: Sens. Nutr. Qual. Food. 72(3):197–202. doi: https://doi.org/10.1111/j.1750-3841.2007.00295.x.
- González-Casado, S., O. Martín-Belloso, P. Elez-Martínez, and R. Soliva-Fortuny. 2018. Enhancing the carotenoid content of tomato fruit with pulsed electric field treatments: Effects on respiratory activity and quality attributes. Postharvest Biol. Tech. 137:113–118. doi: https://doi.org/10.1016/j.postharvbio.2017.11.017.
- Gurjar, P.S., N. Garg, K.K. Yadav, J. Lenka, and D.K. Shukla. 2018. Effect of chitosan on biochemical and microbial quality of minimally processed mango (Mangifera indica L.) cubes during storage. Appl. Biol. Res. 20(1):98–103. doi: https://doi.org/10.5958/0974-0112.2018.00022.1.
- Hafeez, O., U. Malik, M.S. AKhalid, M. Amin, S. Khalid, and M. Umar. 2016. Effect of modified atmosphere packaging on postharvest quality of Mango cv. Sindhri and Sufaid Chaunsa during storage. Turk. J. Agric.-Food Sci. Tech 4(12):1104–1111.
- He, J., Ren, Y., Chen, C., Liu, J., Liu, H., & Pei, Y. (2017) Defense responses of salicylic acid in mango fruit against postharvest anthracnose, caused by Colletotrichum gloeosporioides and its possible mechanism. Journal of Food Safety, 37(1), e12294..
- He, J., Y. Ren, C. Chen, J. Liu, H. Liu, and Y. Pei. 2016. Defense responses of salicylic acid in mango fruit against postharvest anthracnose, caused by Colletotrichum gloeosporioides and its possible mechanism. J. Food Saf. 37. doi: https://doi.org/10.1111/jfs.12294.
- Hoa, T.T., G. Self, and M.N. Ducamp. 2010. Effects of hot air treatment on postharvest quality of ‘cat Hoa loc’ mangoes. Fruits. 65(4):237–244. doi: https://doi.org/10.1051/fruits/2010019.
- Hong, K., D. Gong, H.S. Xu, S. Wang, Z. Jia, J.L. Chen, and L. Zhang. 2014. Effects of salicylic acid and nitric oxide pretreatment on the expression of genes involved in the ethylene signaling pathway and the quality of postharvest mango fruit. N. Z. J. Crop Hortic. Sci. 42(3):205–216. doi: https://doi.org/10.1080/01140671.2014.892012.
- Hossain, M.A., M.M. Rana, Y. Kimura, and H.A. Roslan. 2014. Changes in biochemical characteristics and activities of ripening associated enzymes in mango fruit during the storage at different temperatures. Hindawi Publishing Corporation BioMed Res. Int. 2014:1–11. ID 232969. doi: https://doi.org/10.1155/2014/232969.
- Hu, M., D. Yanga, D.J. Huber, Y. Jiang, M. Lia, Z. Gao, and Z. Zhang. 2014. Reduction of postharvest anthracnose and enhancement of disease resistance in ripening mango fruit by nitric oxide treatment. Postharvest Biol. Technol. 97:115–122. doi: https://doi.org/10.1016/j.postharvbio.2014.06.013.
- Jitareerat, P., S. Paumchai, S. Kanlayanarat, and S. Sangchote. 2007. Effect of chitosan on ripening, enzymatic activity, and disease development in mango (Mangifera indica) fruit. N. Z. J. Crop Hortic. Sci. 35:211–218. doi: https://doi.org/10.1080/01140670709510187.
- Junmatong, C., B. Faiyue, S. Rotarayanont, J. Uthaibutra, D. Boonyakiat, and K. Saengnil. 2015. Cold storage in salicylic acid increases enzymatic and non-enzymatic antioxidants of Nam Dok Mai No. 4 mango fruit. Sci. Asia. 41:12–21. doi: https://doi.org/10.2306/scienceasia1513-1874.2015.41.012.
- Kaur, K., G. Kaur, and J.S. Brar. 2020. Pre-harvest application of hexanal formulations for improving post-harvest life and quality of mango (Mangifera indica L.) cv. Dashehari. J Food Sci Technol. 57:4257–4264. doi: https://doi.org/10.1007/s13197-020-04464-9.
- Ketsa, S., S. Chidtragool, J.D. Klein, and S. Lurie. 1998. Effect of heat treatment on changes in softening, pectic substances and activities of polygalacturonase, pectinesterase and β-galactosidase of ripening mango. J. Plant Physiol. 153:457–461. doi: https://doi.org/10.1016/S0176-1617(98)80174-0.
- Khaliq, G., M.T.M. Mohamed, A. Ali, P. Ding, and H.M. Ghazali. 2015. Effect of gum arabic coating combined with calcium chloride on physico-chemical and qualitative properties of mango (Mangifera indica L.) fruit during low temperature storage. Sci. Hortic. 190:187–194. doi: https://doi.org/10.1016/j.scienta.2015.04.020.
- Khaliq, G., M.T.M. Mohamed, H.M. Ghazali, P. Ding, and A. Ali. 2016a. Influence of gum arabic coating enriched with calcium chloride on physiological, biochemical and quality responses of mango (Mangifera indica L.) fruit stored under low temperature stress. Postharvest Biol. Tech. 111:362–369. doi: https://doi.org/10.1016/j.postharvbio.2015.09.029.
- Khaliq, G., M.T.M. Mohamed, P. Ding, H.M. Ghazali, and A. Ali. 2016b. Storage behaviour and quality responses of mango (Mangifera indica L.) fruit treated with chitosan and gum arabic coatings during cold storage conditions. Int. Food Res. J. 23(Suppl):S141–S148.
- Khaliq, K., U.N. Mehar, R. Muhammad, and K. Naimatullah. 2017. Textural properties and enzyme activity of mango (Mangifera indica L.) fruit coated with chitosan during storage. J. Agric. Stud. 5(2). doi: https://doi.org/10.5296/jas.v5i2.10946.
- Kumar, R., A.S. Bawa, T. Kathiravan, and S. Nadanasabapathi. 2015. Optimization of pulsed electric field parameters for mango nectar processing using response surface methodology. Int. Food Res. J. 22(4):1353–1360.
- Kumar, R., S. Vijayalakshmi, R. Rajeshwara, K. Sunny, and S. Nadanasabapathi. 2019. Effect of storage on thermal, pulsed electric field and combination processed mango nectar. J. Food Meas. Charact. 13(1):131–143. doi: https://doi.org/10.1007/s11694-018-9926-x.
- Lalel, H.J.D., Z. Singh, and S.C. Tan. 2003.Elevated levels of CO2 in controlled atmosphere storage affects shelf life, fruit quality and aroma volatiles of mango. Acta Hortic. 628, 407–413
- Lalel, H.J.D., and Z. Singh. 2004. Biosynthesis of aroma volatile compounds and fatty acids in ‘Kensington Pride’mangoes after storage in a controlled atmosphere at different oxygen and carbon dioxide concentrations. J. Hortic. Sci. Biotech 79(3):343–353. doi: https://doi.org/10.1080/14620316.2004.11511771.
- Lawson, T., G.W. Lycett, A. Ali, and C.F. Chin. 2019. Characterization of Southeast Asia mangoes (Mangifera indica L) according to their physicochemical attributes. Sci. Hortic. 243:189–196. doi: https://doi.org/10.1016/j.scienta.2018.08.014.
- Le, T., C. Shiesh, and H. Lin. 2010. Effect of vapor heat and hot water treatments on disease incidence and quality of Taiwan native strain mango fruits. Int. J. Agric. Biol. 12:673–678.
- Li, P., X. Zheng, M.G.F. Chowdhury, K. Cordasco, and J.K. Brecht. 2015. Prestorage application of oxalic acid to alleviate chilling injury in mango fruit. HortScience. 50(12):1795–1800. doi: https://doi.org/10.21273/HORTSCI.50.12.1795.
- Liu, X., Y. Fu, P. Guo, and W. Xu. 2018. Modified atmosphere packaging and postharvest treatments on mango preservation: A review, p. 511–516. In: P. Zhao, Y. Ouyang, M. Xu, L. Yang and Y. Ren (Eds). Applied Sciences in Graphic Communication and Packaging. Singapore, Springer
- López-López, M.E., J.A. López-Valenzuela, F. Delgado-Vargas, G. López-Angulo, A. Carrillo-López, L.E. Ayón-Reyna, and M.O. Vega-Garcí. 2018. A treatment combining hot water with calcium lactate improves the chilling injury tolerance of mango fruit. HortScience. 53(2):217–223. doi: https://doi.org/10.21273/HORTSCI12575-17.
- López-Mora, L.I., P. Gutiérrez-Martínez, S. Bautista-Baños, L.F. Jiménez-García, and H.A. Zavaleta-Mancera. 2013. Evaluation of antifungal activity of chitosan in Alternaria alternata and in the quality of ‘Tommy Atkins’ mango during storage. Rev. Chapingo Ser. Hortic. 19(3):315–331. doi: https://doi.org/10.5154/r.rchsh.2012.07.038.
- Luria, N., N. Sela, M. Yaari, O. Feygenberg, I. Kobiler, A. Lers, and D. Prusky. 2014. De-novo assembly of mango fruit peel transcriptome reveals mechanisms of mango response to hot water treatment. BMC Genomics. 15: 957. http://www.biomedcentral.com/1471-2164/15/957
- Mathooko, F.M. 1996. Regulation of respiratory metabolism in fruits and vegetables by carbon dioxide. Postharvest Biol. Technol. 9:247–264. doi: https://doi.org/10.1016/S0925-5214(96)00019-1.
- Mditshwa, A., L.S. Magwaza, S.Z. Tesfay, and U.L. Opara. 2017. Postharvest factors affecting vitamin C content of citrus fruits: A review. Sci. Hortic. 218:95–104. doi: https://doi.org/10.1016/j.scienta.2017.02.024.
- Miller, F.A., C.L.M. Silva, and T.R.S. Brandăo. 2013. A review on ozone-based treatments for fruit and vegetables preservation. Food Eng. Rev. 5:77–106. doi: https://doi.org/10.1007/s12393-013-9064-5.
- Minas, I.S., A.R. Vicente, A.P. Dhanapal, G.A. Manganaris, V. Goulas, M. Vasilakakis, C.H. Crisosto, and A. Molassiotis. 2014. Ozone-induced kiwifruit ripening delay is mediated by ethylene biosynthesis inhibition and cell wall dismantling regulation. Plant Sci. 229:76–85. doi: https://doi.org/10.1016/j.plantsci.2014.08.016.
- Minas, I.S., G.S. Karaoglanidis, G.A. Manganaris, and M. Vasilakakis. 2010. Effect of ozone application during cold storage of kiwifruit on the development of stem-end rot caused by Botrytis cinerea. Postharvest Biol. Technol. 58:203–210. doi: https://doi.org/10.1016/j.postharvbio.2010.07.002.
- Mohamed, N.T.S., P. Ding, J. Kadir, and H.M. Ghazali. 2017. Potential of UVC germicidal irradiation in suppressing crown rot disease, retaining postharvest quality and antioxidant capacity of Musa AAA “Berangan” during fruit ripening. Food Sci. Nutr. 5:967–980. doi: https://doi.org/10.1002/fsn3.482.
- Muhammad, I., S. Ashiru, A.I. Kanoma, I. Sani, and S. Garba. 2014. Effect of ripening stage on vitamin C content in selected fruits. Int. J. Agric. Fores. Fish. 2(3):60.
- Ngamchuachit, P., D.M. Barrett, and E.J. Mitcham. 2014. Effects of 1-Methylcyclopropene and hot water quarantine treatment on quality of “Keitt” mangos. J. Food Sci. 79:4. doi: https://doi.org/10.1111/1750-3841.12380..
- Nongtaodum, S., and A. Jangchud. 2009. Effects of edible chitosan coating on quality of fresh-cut mangoes (Fa-lun) during storage. Kasetsart J. (Nat. Sci.). 43:282–289. https://www.researchgate.net/publication/266466570
- Ntsoane, M.L., A. Luca, M. Zude-Sasse, D. Sivakumar, and P.V. Mahajan. 2019a. Impact of low oxygen storage on quality attributes including pigments and volatile compounds in ‘Shelly’ mango. Sci. Hortic. 250:174–183. doi: https://doi.org/10.1016/j.scienta.2019.02.041.
- Ntsoane, M.L., M. Zude-Sasse, P. Mahajan, and D. Sivakumar. 2019b. Quality assesment and postharvest technology of mango: A review of its current status and future perspectives. Sci. Hortic. 249:77–85. doi: https://doi.org/10.1016/j.scienta.2019.01.033.
- Ong, M.K., and A. Alia. 2015. Antifungal action of ozone against Colletotrichum gloeosporioides and control of papaya anthracnose. Postharvest Biol. Technol. 100:113–119. doi: https://doi.org/10.1016/j.postharvbio.2014.09.023.
- Osuna-García, J.A., M.H. Pérez-Barraza., J.A. Beltran, and V. Vázquez-Valdivia. 2009. Methylcyclopropene (1-MCP), a new approach for exporting ‘Kent’ mangos to Europe and Japan. Acta Hortic. 820, 721–724
- Osuna-Garcia, J.A., J.K. Brecht, D.J. Huber, and Y. Nolasco-Gonzalez. 2015. Aqueous 1-Methylcyclopropene to delay ripening of ‘Kent’ mango with or without quarantine hot water treatment. HortTechnology. 25(3):349–357. https://www.researchgate.net/publication/282265613
- Phaiphan, A., and N. Rattanapanone. 2008. Effect of edible coatings on quality of Mango Fruit (Mangifera indica) ‘Chok-Anan’ during storage. Acta Hortic. 773:227–232. doi: https://doi.org/10.17660/ActaHortic.2008.773.33.
- Phakawatmongkol, W., S. Ketsa, and W.G. Van Doorn. 2004. Variation in fruit chilling injury among mango cultivars. Postharvest Biol. Tec. 32:115–118. doi: https://doi.org/10.1016/j.postharvbio.2003.11.011.
- Plotto, A., J.A. Narciso, N. Rattanapanone, and E.A. Baldwin. 2010. Surface treatments and coatings to maintain fresh-cut mango quality in storage. J. Sci. Food Agric. 90:2333–2341. doi: https://doi.org/10.1002/jsfa.4095.
- Plotto, A., K.L. Goodner, E.A. Baldwin, J. Bai, and N. Rattanapanone. 2004. Effect of polysaccharide coating on quality of fresh cut mangoes (Mangifera indica). Proc. Fla. State Hort. Soc. 117:382–388.
- Ramayya, N., K. Niranjan, and E. Duncan. 2012. Effects of modified atmosphere packaging on quality of ‘Alphonso’ mangoes. J. Food Sci. Tech. 49(6):721–728. doi: https://doi.org/10.1007/s13197-010-0215-x.
- Rattanapanone, N., Y. Lee, T. Wu, and A.E. Watada. 2001. Quality and microbial changes of fresh-cut mango cubes held in controlled atmosphere. HortScience. 36(6):1091–1095. doi: https://doi.org/10.21273/HORTSCI.36.6.1091.
- Razzaq, K., Z. Singh, A.S. Khan, S.A.K.U. Khan, and S. Ullah. 2015. Role of 1-MCP in regulating ‘Kensington Pride’ mango fruit softening and ripening. Plant Growth Regul. doi: https://doi.org/10.1007/s10725-015-0101-7..
- Romero, L., J. Colivet, N.M. Aron, and A. Ramosvillarroel. 2017. Impact of ultraviolet light on quality attributes of stored fresh-cut mango. The annals of the University of Dunarea de Jos of Galati. Fascicle VI. Food Technol 41(1):62–80.
- Romero-Puertas, M.C., M. Perazzolli, E.D. Zago, and M. Delledonne. 2004. Nitric oxide signalling functions in plant–pathogen interactions. Cell. Microbiol. 6(9):795–803. doi: https://doi.org/10.1111/j.1462-5822.2004.00428.x.
- Safitri, A., T. Theppakorn, M. Naradisorn, and S. Setha. 2015. Effects of UV-C irradiation on ripening quality and antioxidant capacity of mango fruit cv. Nam Dok Mai Si Thong. J. Food Sci. Agric. Tech. 1(1):164–170. http://jfat.mfu.ac.th
- Sakhale, B.K., S.S. Gaikwad, and R.F. Chavan. 2018. Application of 1-methylcyclopropene on mango fruit (Cv. Kesar): Potential for shelf life enhancement and retention of quality. J. Food Sci. Technol. 55(2):776–781. doi: https://doi.org/10.1007/s13197-017-2990-0.
- Salinas-Roca, B., A. Guerreiro, J. Welti-Chanes, M.D.C. Antunes, and O. Martín-Belloso. 2018. Improving quality of fresh-cut mango using polysaccharide-based edible coatings. Int. J. Food Sci. Tech. 53:938–945. doi: https://doi.org/10.1111/ijfs.13666.
- Sandhu, H.P.S., F.A. Manthey, and S. Simsek. 2011. Quality of bread made from ozonated wheat (Triticum aestivum L.) flour. J. Sci Food Agric. 1(91):1576–1584. doi: https://doi.org/10.1002/jsfa.4350.
- Sane, V.A., A. Chourasia, and P. Nath. 2005. Softening in mango (Mangifera indica cv. Dashehari) is correlated with the expression of an early ethylene responsive, ripening related expansin gene, MiExpA1. Postharvest Biol. Tech. 38:223–230. doi: https://doi.org/10.1016/j.postharvbio.2005.07.008.
- Scheler, C., J. Durner, and J. Astier. 2013. Nitric oxide and reactive oxygen species in plant biotic interactions. Curr. Opin. Plant Biol. 16:534–539. doi: https://doi.org/10.1016/j.pbi.2013.06.020.
- Shezi, S., L.S. Magwaza, A. Mditshwa, and S.Z. Tesfay. 2020a. Changes in biochemistry of fresh produce in response to ozone postharvest treatment. Sci. Hortic. 269:109397. doi: https://doi.org/10.1016/j.scienta.2020.109397.
- Shezi, S., S.Z. Tesfay, L.S. Magwaza, A. Mditshwa, and N.M. Motsa. 2020b. Gaseous ozone and plant extract-based edible coatings as postharvest treatments for ‘Hass’ avocado (Persea americana Mill.) fruit. Acta Hortic. 1275:53–60. doi: https://doi.org/10.17660/ActaHortic.2020.1275.8.
- Shukla, S., P. Srivastava, S.K. Choubey, and V.S. Gomase. 2010. Comparative analysis and structure elucidation of Syntaxin- A novel component tends to defense mechanism in plant proteomics. J. Plant Genomics. 1(1):01–08.
- Siddiqui, Y., and A. Asgar. 2014. Colletotrichum gloeosporioides (Anthracnose). Academic Press, Postharvest Decay. doi:https://doi.org/10.1016/B978-0-12-411552-1.00011-9
- Singh, Z., R.K. Singh, V.A. Sane, and P. Nath. 2013. Mango - postharvest biology and biotechnology. Crit. Rev. Plant Sci. 32:217–236. doi: https://doi.org/10.1080/07352689.2012.743399.
- Sivakumar, D., and E. Fallik. 2013. Influence of heat treatments on quality retention of fresh and fresh-cut produce. Food Rev. Int. 29:294–320. doi: https://doi.org/10.1080/87559129.2013.790048.
- Sivakumar, D., Y. Jiang, and E.M. Yahia. 2011. Maintaining mango (Mangifera indica L.) fruit quality during the export chain. Food Res. Int. 44:1254–1263. doi: https://doi.org/10.1016/j.foodres.2010.11.022.
- Sivankalyani, V., O. Feygenberg, S. Diskin, B. Wright, and N. Alkan. 2016. Increased anthocyanin and flavonoids in mango fruit peel are associated with cold and pathogen resistance. Postharvest Biol. Tech. 111:132–139. doi: https://doi.org/10.1016/j.postharvbio.2015.08.001.
- Sothornvit, R., and P. Rodsamran. 2008. Effect of a mango film on quality of whole and minimally processed mangoes. Postharvest Biol. Tech. 47(3):407–415. doi: https://doi.org/10.1016/j.postharvbio.2007.08.005.
- Sothornvit, R., and P. Rodsamran. 2010. Mango film coated for fresh‐cut mango in modified atmosphere packaging. Int. J. Food Sci. Tech. 45(8):1689–1695. doi: https://doi.org/10.1111/j.1365-2621.2010.02316.x.
- Sripong, K., P. Jitareerat, S. Tsuyumu, A. Uthairatanakij, V. Srilaong, C. Wongs-Aree, G. Ma, L. Zhang, and M. Kato. 2015. Combined treatment with hot water and UV-C elicits disease resistance against anthracnose and improves the quality of harvested mangoes. Crop Prot. 77:1–8. doi: https://doi.org/10.1016/j.cropro.2015.07.004.
- Srivastava, S., and K.L. Bala. 2016. Effect of arabic gum-carboxymethylcellulose edible coatings on shelf life of button mushroom (Agaricus bisporus). Int. J. Res. Eng. Technol. 5(6):484–494. http://ijret.esatjournals.org
- Sudheeran, P.K., O. Feygenberg, D. Maurer, and N. Alkan. 2018. Improved cold tolerance of mango fruit with enhanced anthocyanin and flavonoid contents. Molecules. 23:1832. doi: https://doi.org/10.3390/molecules23071832.
- Sumual, M.F., Z. Singh, S.P. Singh, and S.C. Tan. 2017. Fruit ripening and quality of ‘Kensington Pride’ mangoes following the controlled atmosphere storage. Jurnal Teknologi Pertanian (Agricultural Technology Journal). 8:1.
- Terao, D., J.S. De Carvalho Campos, E.A. Benato, and J.M. Hashimoto. 2015. Alternative strategy on control of postharvest diseases of mango (Mangifera indica L.) by use of low dose of ultraviolet-c irradiation. Food Eng. Rev. 7:171–175. doi: https://doi.org/10.1007/s12393-014-9089-4.
- Tesfay, S.Z., and L.S. Magwaza. 2017. Evaluating the efficacy of moringa leaf extract, chitosan and carboxymethyl cellulose as edible coatings for enhancing quality and extending postharvest life of avocado (Persea americana Mill.) fruit. Food Packag. Shelf. 11:40–48. doi: https://doi.org/10.1016/j.fpsl.2016.12.001.
- Tian, S.P., J. Liu, C.F. Zhang, and X.H. Meng. 2010. Quality properties of harvested mango fruits and regulating technologies. New trends in postharvest management of fresh produce II. Fresh Produce 4:49–54.
- Tran, T.T.L., S. Aiamla-or, V. Srilaong, P. Jitareerat, C. Wongs-Aree, and A. Uthairatanakij. 2015. Application of Nitric Oxide to Extend the Shelf Life of Mango Fruit. Acta Hortic. 97–102. doi: https://doi.org/10.17660/ActaHortic.2015.1088.11.
- Tran, T.T.L., S. Aimla-or, V. Srilaong, P. Jitareerat, C. Wongs-Aree, and A. Uthairatanakij. 2013. Fumigation with ozone to extend the storage life of mango fruit cv. Nam Dok Mai No. 4. Agric. Sci. J. 44(2):663–672.
- Tzortzakis, N., T. Taybi, E. Antony, I. Singleton, A. Borland, and J. Barnes. 2013. Profiling shifts in protein complement in tomato fruit induced by atmospheric ozone-enrichment and/or wound-inoculation with Botrytis cinerea. Postharvest Biol. Tech. 78:67–75. doi: https://doi.org/10.1016/j.postharvbio.2012.12.005.
- Tzortzakis, N., T. Taybi, R. Roberts, I. Singleton, A. Borland, and J. Barnes. 2011. Low-level atmospheric ozone exposure induces protection against Botrytis cinerea with down-regulation of ethylene-, jasmonate- and pathogenesis-related genes in tomato fruit. Postharvest Biol. Technol. 61:152–159. doi: https://doi.org/10.1016/j.postharvbio.2011.02.013.
- Ullah, H., S. Ahmad, A.K. Thompson, W. Ahmad, and M.A. Nawaz. 2010. Storage of ripe mango (Mangifera indica L.) cv. Alphonso in controlled atmosphere with elevated CO2. Pak. J. Bot. 42(3):2077–2084.
- Ullah, H., S. Ahmad, M. Amjad, and M.A. Khan. 2012. Response of mango cultivars to modified atmosphere storage at an ambient temperature cv. alphanso and chounsa. Pak. J. Agri. Sci. 49(3):323–329. http://www.pakjas.com.pk
- Valente, M., F. Ribeyre, G. Self, L. Berthiot, and S. Assemat. 2011. Instrumental and sensory characterization of mango fruit texture. J. Food Qual. 34:413–424. doi: https://doi.org/10.1111/j.1745-4557.2011.00412.x.
- Vázquez-Celestino, D., H. Ramos-Sotelo, D.M. Rivera-Pastrana, M.E. Vázquez-Barrios, and E.M. Mercado-Silva. 2016. Effects of waxing, microperforated polyethylene bag, 1-methylcyclopropene and nitric oxide on firmness and shrivel and weight loss of ‘Manila’ mango fruit during ripening. Postharvest Biol. Tech. 111:398–405. doi: https://doi.org/10.1016/j.postharvbio.2015.09.030.
- Vega-Alvarez, M., N.Y. Salazar-Salas, G. López-Angulo, K.V. Pineda-Hidalgo, M.E. López-López, M.O. Vega-García, F. Delgado-Vargas, and J.A. López-Valenzuela. 2020. Metabolomic changes in mango fruit peel associated with chilling injury tolerance induced by quarantine hot water treatment. Postharvest Biol. Technol. 169:111299. doi: https://doi.org/10.1016/j.postharvbio.2020.111299.
- Wang, B., J. Wang, X. Feng, L. Lin, Y. Zhao, and W. Jiang. 2009. Effects of 1-MCP and exogenous ethylene on fruit ripening and antioxidants in stored mango. Plant Growth Regul. 57:185–192. doi: https://doi.org/10.1007/s10725-008-9335-y.
- Wang, H., Z. Yang, F. Song, F.W. Chen, and S. Zhao. 2016. Effects of heat treatment on changes of respiration rate and enzyme activity of Ivory mangoes during storage. J. Food Process. Preserv. 41. doi: https://doi.org/10.1111/jfpp.12737.
- Wang, J., B. Wang, W. Jiang, and Y. Zhao. 2007. Quality and shelf life of mango (Mangifera Indica L. cv. ‘Tainong’) coated by using chitosan and polyphenols. Food Sci. Tech. Int. 13(4):317–322. doi: https://doi.org/10.1177/1082013207082503.
- Wang, K.L.C., H. Li, and J.R. Ecker. 2002. Ethylene biosynthesis and signaling networks. Plant Cell. 14: S131–S151. www.plantcell.org/cgi/doi/ https://doi.org/10.1105/tpc.001768
- Wongmetha, O., and L. Ke. 2013. Changes in quality attributes and storage life of mango during storage. Adv. Mater. Res. 610:3518–3521. doi: https://doi.org/10.4028/www.scientific.net/AMR.610-613.3518.
- Wongmetha, O., L. Ke, and Y. Liang. 2013. The changes in physico-chemical compositions of ‘Irwin’ mango fruit after postharvest treatments under low temperature storage. Thai J. Agric. Sci. 46(1):11–19.
- Wright, A.H., J.M. Delong, J. Arul, and R.K. Prange. 2015. The trend toward lower oxygen levels during apple (Malus × domestica Borkh) storage. J. Hortic. Sci. Biotech. 90(1):1–13. doi: https://doi.org/10.1080/14620316.2015.11513146.
- Yashoda, H.M., T.N. Prabha, and R.N. Tharanathan. 2006. Mango ripening: Changes in cell wall constituents in relation to textural softening. J. Sci. Food Agric. 86:713–721. doi: https://doi.org/10.1002/jsfa.2404.
- Yimyong, S., T.U. Datsenka, A.K. Handa, and K. Seraypheap. 2011. Hot water treatment delays ripening-associated metabolic shift in ‘Okrong’ mango fruit during storage. J. Amer. Soc. Hort. Sci. 136(6):441–451. doi: https://doi.org/10.21273/JASHS.136.6.441.
- Zaharah, S.S., and Z. Singh. 2011a. Mode of action of nitric oxide in inhibiting ethylene biosynthesis and fruit softening during ripening and cool storage of ‘Kensington Pride’ mango. Postharvest Biol. Technol. 62(3):258–266. doi: https://doi.org/10.1016/j.postharvbio.2011.06.007.
- Zaharah, S.S., and Z. Singh. 2011b. Post-harvest fumigation with nitric oxide at the pre-climacteric and climacteric-rise stages influences ripening and quality in mango fruit. J. Horticu. Sci. Biotech. 86(6):645–653. doi: https://doi.org/10.1080/14620316.2011.11512817.
- Zaharah, S.S., and Z. Singh. 2013. Nitric Oxide fumigation delays mango fruit ripening. Acta Hortic. 543–550. doi: https://doi.org/10.17660/ActaHortic.2013.992.67.
- Zainuri, D.C., A.H. Joyce, L.C. Wearing, and L. Terry. 2001. Effects of phosphonate and salicylic acid treatments on anthracnose disease development and ripening of ‘Kensington Pride’ mango fruit. Aust. J. Exp. Agric. 41:805–813. doi: https://doi.org/10.1071/EA99104.
- Zeng, K., J. Cao, and W. Jiang. 2006. Enhancing disease resistance in harvested mango (Mangifera indica L. cv. ‘Matisu’) fruit by salicylic acid. J. Sci. Food Agric. 86:694–698. doi: https://doi.org/10.1002/jsfa.2397.
- Zerbini, P.E., M. Vanoli, A. Rizzolo, M. Grassi, R.M. De Azevedo Pimentel, L. Spinelli, and A. Torricelli. 2015. Optical properties, ethylene production and softening in mango fruit. Postharvest Biol. Technol. 101:58–65. doi: https://doi.org/10.1016/j.postharvbio.2014.11.008.
- Zhang, Z., Z. Gao, M. Li, M. Hu, H. Gao, D. Yang, and B. Yang. 2012. Hot water treatment maintains normal ripening and cell wall metabolism in mango (Mangifera indica L.) fruit. HortScience. 47(10):1466–1471. doi: https://doi.org/10.21273/HORTSCI.47.10.1466.
- Zheng, X., B. Hu, L. Song, J. Pan, and M. Liu. 2017. Changes in quality and defense resistance of kiwifruit in response to nitric oxide treatment during storage at room temperature. Sci. Hortic. 222:187–192. doi: https://doi.org/10.1016/j.scienta.2017.05.010.
- Zhu, X., Q. Wang, J. Cao, and W. Jiang. 2008. Effects of chitosan coating on postharvest quality of mango (Mangifera Indica L. cv. Tainong) fruits. J. Food Process. Preserv. 32:770. doi: https://doi.org/10.1111/j.1745-4549.2008.00213.x.