?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Azanza garckeana (snot apple) is an underutilized indigenous fruit of southern Africa. The study was aimed at determining the functional characteristics and potential use of extracted pectin from A. garckeana (snot apple) fruits from Zimbabwe. A. garckeana (snot apple) fruits were collected from a semi-arid communal area of Nhema, Zimbabwe. Pectin properties were determined using standard methods. Fruit jam was formulated with 53% (w/v) U. kirkiana fruit pulp, 45% (w/v) sugar, 1.5% (w/v) A. garckeana pectin, 0.5% (w/v) citric acid. The optimum extraction conditions were 90°C for 90 minutes at pH 3. Pectin yield was 9.21 ± 0.1% to 18.1 ± 0.1% and 10.1 ± 0.1 to 20.2 ± 0.2% for dried and fresh A. garckeana fruits respectively. Physicochemical properties of the pectin were (% w/w) degree of esterification (DE) 72.1–80.3, methoxyl content 6.1–6.8, anhydrouronic acid (AUA) content 60.3–67.6, moisture content 3.1–2.2, ash content 0.9–0.7 for dried and fresh fruits. Equivalent weight range was 816.7–848.2 mg/mol. Jam had total soluble solids (68.2 ± 0.1%), total titratable acid (2.1 ± 0.2 g/L), moisture content (32.6 ± 1.3%) and pH (3.4 ± 0.1). Jam’s overall acceptance score was 6. There was no significant difference in aroma, spreadability, taste, and texture of both jams at P < 0 .05. Pectin extracted from A. garckeana (snot apple) fruits has good functionality properties with potential for applications in food processing, especially in jam production.
Introduction
Azanza garckeana (snot apple) fruit tree is an underutilized indigenous fruit tree (IFT) that is well distributed in warm woodlands of Southern Africa (Maroyi, Citation2017). Thespesia garckeana (commonly known as A.garckeana) fruit tree belongs to the Malvaceae family and its domestication is important to support nutrition, health, and income generation to most communities in sub-Saharan Africa (World Agroforestry Centre, Citation2020). In Zimbabwe, the snot apple is known in a local vernacular as mutohwe in Shona and uxakuxaku in Ndebele and it ripens from February to September. The ripe fruit is 35 mm in diameter, round, and appears reddish when mature with short dense hairs on the outer surface (Schmidt et al., Citation2002). The ripe fruit is normally eaten raw and this is done by removing seeds and chewing of the fruit. The fruits produce a sweet glutinous slime and sometimes they are dried (Mojeremane and Tshwenyane, Citation2004).
In tropical Africa, the Azanza garckeana fruit tree is a popular multipurpose fruit tree that is characterized by edible fruits, plant parts with medicinal uses, and products sold to local markets (Glew et al., Citation2005). The ripe fruit is used as food additives in Sudan (Suliman et al., Citation2012). Jellies and syrups of the fruit are added to soups and/or used to make porridge in Zambia (Storrs, Citation1979). Azanza garckeana fruit contain (w/w) carbohydrate 28.4%, crude protein 12.0%, crude fiber 45.30%, fat 1.04 ± 0.01%, and moisture 6.50% (Nkafamiya et al., Citation2016). The mineral content (mg/100 g) of the fruit comprise of iron 12.0 ± 0.43, calcium 127.0 ± 0.04, potassium 1360 ± 1.3, magnesium 96.25 ± 0.67 and zinc 12.02 ± 0.9 (Nkafamiya et al., Citation2016). In most areas of Zimbabwe ripe wild fruits are often sold by street vendors on roadsides and in urban and rural markets in Sub-Saharan Africa (Chawafambira et al., Citation2020b, Citation2020a).
Pectin is a structural hetero-polysaccharide that is found in the plant primary cell walls and is used as a stabilizer and gelling agent in jam and jellies (Braidwood et al., Citation2014). Research on the underutilized but important A. garckeana fruit is limited to nutritional, phytochemicals, potential medicinal use, cultivation, agronomy, and productivity. The fruit has not been fully exploited including the existing potential for a wider application in food processing and other technological applications. The fruit has the potential to be utilized and used in the production of new food products (Van Wyk, Citation2011). Thus, this study was aimed at extracting and determining the functional characteristics of pectin extracts of A. garckeana (snot apple) fruits with potential for use in food processing.
Materials and Methods
Fruit Collection
Ripe A. garckeana (snot apple) fruits were collected from domesticated trees () in Nhema communal area in Shurugwi district (is a semi-dry communal area located 30°S 19°E in Agro farming region 3 and has poor sandy soils) as represented in . Permission to collect fruit samples in the study were granted by local ward councillor and participating families in the area. Fruit trees were chosen randomly using the randomised sampling method. Samples of 100 ripe fruits were collected, transported in clean polythene bags, and stored at room temperature (25°C) in a laboratory.
Sample Preparation
The collected fruits were sorted by selecting ripe fresh fruits and removing defective (rotten) fruits. The ripe and fresh fruits were cut open and the seeds were removed. The fruits were dried according to a standard procedure from the Association of Official Analytical Chemistry (AOAC) in an oven (UL 40, Memmert, Schwabach, Germany) at 110°C for 3 hours until a constant weight was achieved. The dried A. garckeana fruits were milled using a miller (DFT-150, Dickson, China) and sieved through a 500 μm sieve into a powder. The milled sample was packed in a polyethylene bag and stored at −15°C in a freezer (ACF15F, Acson, Malaysia).
Pectin Extraction
Extraction of pectin was carried out according to a method described by Tang et al. (Citation2011). Forty grams of A. garckeana fruit powder was measured using an analytical balance (B204-S, MK II, Mettler Toledo, Switzerland) and mixed with acid-water (40 g citric acid/200 mL distilled water) in a beaker until the pH had reached 3. The extraction process was done at 70, 90, and 105°C for 90 minutes in a shaking water bath (Lab Companion 37 L, Jeio Tech, Korea), and samples were cooled to 55°C then filtered using a muslin cloth. One volume of each sample extract was added to 95% ethanol (1:1 v/v) to allow pectin precipitation and pectin was filtered through a Whatman filter paper (No 1). Each pectin filtrate was further washed with excess 96% ethanol and distilled water to remove any impurities. The precipitates of each extraction were dried at 65°C in a hot oven (UL 40, Memmert, Schwabach, Germany) for 10 hours until a constant weight was reached and then cooled in a desiccator. Pectin obtained from each extraction was ground, sieved, and stored at 25°C. The experiments were performed in triplicate to ensure accuracy. The percentage yield of extracted pectin was calculated as follows:
where P = the amount of extracted pectin in grams (g), Q = the initial amount of A. garckeana fruit sample (40 g).
Functional and Physicochemical Characteristics of Pectin Extracts
Degree of Esterification
The degree of esterification of pectin was analysed according to a titrimetric method of the Food Chemical Codex (Citation1996). Dried pectin samples (0.2 g) were moistened with ethanol and dissolved in 20 ml distilled water. The sample was shaken in a water bath (Lab Companion 37L, Jeio Tech, Korea) at 45 °C until the pectin had completely dissolved. Three drops of phenolphthalein were added to the sample. Titration of the samples with 0.1 N sodium hydroxide was conducted and the initial titration volumes were noted. The volume of 0.1 N sodium hydroxide solution was used to determine the number of free carboxyl groups. Ten millilitres of 0.1 N sodium hydroxide was then added to neutralize poly galacturonic acid and the mixture was shaken vigorously. The mixture was allowed to stand at room temperature (26 °C) for 2 hours to de-esterify pectin. Ten (10) millilitres of 0.1 N hydrochloric acid was added to neutralize sodium hydroxide. Phenolphthalein drops were added and the sample was titrated with a known volume of 0.1 N sodium hydroxide until a pink colour appeared. The esterified carboxyl groups were determined using the final volume of 0.1 N sodium hydroxide solution. The DE was calculated using the formula:
Equivalent Weight Analysis
Equivalent weight analysis was determined according to a method by Ania et al. (Citation2012). The following formula was used:
Methoxyl Content Analysis
Methoxyl content analysis was determined using a method adopted by Ania et al. (Citation2012). The methoxyl content of the pectin extract samples was determined by mixing 25 ml of 0.25 M NaOH to the neutralized solution used in equivalent weight determination. The mixture was vigorously shaken and left to stand for 30 minutes at room temperature (26°C). To the mixture, 25 ml of 0.25 M HCl was added and titrated until a pink color appeared as an endpoint. The volume of the alkali used for the neutralization reaction was recorded and the methoxyl (MeO) content was calculated using the following formula:
Where: 31 is the molecular weight of methoxyl.
Anhydrouronic Acid (AUA) Test
The Anhydrouronic acid (AUA) test was determined using values obtained from the equivalent weight and the methoxyl content tests according to the method by Ania et al. (Citation2012). The anhydrouronic acid (AUA) content was calculated using the following formula:
where: z = volume cm3 of NaOH from equivalent weight analysis, y = volume cm3 of NaOH from methoxyl content test, w = weight of pectin extract sample.
Solubility Analysis
The solubility test was determined according to a method by Yang et al. (Citation2009). A 1 g of pectin extract was mixed with 10 ml distilled water and heated to 90°C. Five milliliters of the pectin-water mixture was then mixed with 70% rubbing alcohol in a test tube and observed until clots started forming in the mixture.
Gelling Test in Acid-sugar
The citric acid (5 g) was dissolved in 100 ml of distilled water and the pH of the solution was determined according to AOAC (Citation2000) method using a digital pH meter (BT-675, BOECO, Hamburg, Germany). Sixty grams of white sugar was then dissolved in the acidified water with a pH of 3 and stirred. A 1 g of pectin extract sample was added to the solution and heated at 105°C for 45 minutes. The heated mixture was allowed to cool and the time taken for the solution to start gel was recorded as the setting time.
Gelling Test in Calcium Ions
A weight of 0.1 g calcium carbonate was measured using an analytical balance (B204-S, MK II, Mettler Toledo, Switzerland) and mixed with acid- sugar solution with a pH of 3. The mixture was heated at 105°C for 45 minutes then cooled and the time is taken for the solution to start gelling (setting time) was noted.
Ash content
The ash content was determined according to a standard method by AOAC (Citation2000). A 2 g pectin extract sample was weighed and placed in a crucible and weighed. The crucible was placed in a desiccator and heated at 550°C for 60 minutes in a muffle furnace. The sample was cooled in a desiccator and the weight of the ash and crucible was determined. The ash content was determined using the formula:
Where W0 = weight of empty crucible (g), W1 = weight of crucible + sample (g), W2 = weight of crucible + ash (g)
Moisture Content
The moisture content was determined according to AOAC (Citation2000) standard method, using the following formula:
Where; W1 = weight of crucible (g); W2 = weight of crucible (g) + fresh sample (g); W3 = weight of crucible (g) + dried sample (g).
Pectin Extracts in the Jam
Pectin extracted at the optimum temperature of 90°C for 90 minutes was used in the production of jam. The production process of the jam was carried out using a standard process by FAO (Citation1995). Jam samples were prepared using U. kirkiana fruit pulp, citric acid, and pectin extracted from A. garckeana fruits. The formulation used to prepare the jam was adopted from Chawafambira et al. (Citation2020c) with few modifications in the quantity of pulp and sugar. In this study, the formulation had 53% pulp, 45% sugar, 1.5% A. garckeana pectin extract, and 0.5% citric acid. The pulp and sugar were mixed in a stainless steel pot and cooked at 110°C. Citric acid and pectin extracts (6 g) were added and the mixture was continuously stirred until the jam had 68 °Brix. A bench Brix refractometer (MA871, North Carolina, Milwaukee Instruments, USA) was used to check for oBrix. In a control experiment, commercial pectin (4 g) was used. A sensory evaluation process was conducted on the jam.
Physiochemical Analysis of Jam
pH Measurement
The pH of the pulp and jam was determined according to AOAC (Citation2000) standard method using a digital pH meter (BT-675, BOECO, Hamburg, Germany).
Total Soluble Solids
The total soluble solids content of the fruit pulp and jam was determined according to AOAC (Citation2000) standard method using a bench Brix refractometer (MA871, North Carolina, Milwaukee Instruments, USA) and distilled water was used to calibrate and rinse-off residual sample after each reading.
Total Titratable Acid
Jam acidity (expressed as total titratable acidity) was determined according to AOAC (Citation2000) standard method.
Sensory Evaluation
In the sensory evaluation process, 50 untrained panelists were selected from the Nhema communal area (n = 30) and Chinhoyi University of Technology students (n = 20). The panelists used in the study were aged between 15–35 years and consent was obtained from each panelist. The sensory evaluation process was conducted in individual temporary booths made of cardboard boxes in the Nhema area. A 9–point hedonic scale was designed using the following key: 1 = Dislike extremely, 2 = Dislike very much, 3 = Dislike moderately, 4 = Dislike slightly, 5 = Neither like or dislike, 6 = Like slightly, 7 = Like moderately, 8 = Like strongly, 9 = Like extremely and all instructions for the panelists was translated into Shona, a local language for easy understanding of the sensory process. Panelists were presented with a test sample weighing 20 g each and rinsing water. The panelists were asked to evaluate taste, aroma, texture, spreadability, and overall acceptance and rated their answers on the provided 9-point hedonic scale scorecard. Panelists were not allowed to discuss their responses during the sensory evaluation process.
Statistical Analysis
The results of functional, physicochemical characteristics of pectin and sensory properties of jam were expressed as mean ± standard deviation (SD). All experiments were conducted in triplicates analysis were carried out using SPSS package version 18.0 (Coakes and Ong, John Wiley & Sons, Queensland, Australia). The least significant difference (LSD) test was conducted to determine any significant differences at (P < 0.05). Customer acceptability and sensorial results were analyzed using one-way Analysis of Variance (ANOVA).
Results and Discussion
Pectin Extracts
The pectin yield from A. garckeana fruits both dried and fresh ranged from 9.21 ± 0.1% to 18.1 ± 0.1% and 10.1 ± 0.1 to 20.2 ± 0.2% respectively (). The pectin yield was significantly different at P < 0.05. The results of acid extraction showed an increase in pectin yield with an increase in extraction temperature up to 90°C and thereafter it began to decrease. In this study citric acid was used in acid extraction. Cho et al. (Citation2019) reported the positive effects of using food-grade organic acids such as citric, malic, and tartaric acids on the extraction of pectin in apple peel. This is also supported by Picot-Allain et al. (Citation2020) who reported no significant difference in the pectin yield obtained by extraction using citric acid as compared to treatment with 0.1 M hydrochloric acid. The pectin yield results from this study were higher as compared to ambarella (Spondias cytherea Sonn.) peel (10 to 13%) and mango (Anacardiaceae) peel (4.6 to 18.5%) extracted using deionized water (Koubala et al., Citation2008a, Citation2008b). Ndabikunze et al. (Citation2010) reported a pectin content of 0.28 ± 0.05% in U. kirkiana (Euphorbiaceae) fruits from Iringa forests in Tanzania. Conversely, Patra and Basak (Citation2020) reported a high pectin yield of 25.34 ± 0.77% dry wt, 14.86 ± 0.40% dry wt, and 11.94 ± 0.60% dry wt in citrus medica, phyllanthus emblica, and carissa carandas fruits respectively. More so, the increase in temperature and reaction of the acid solution and the fruit structure would increase the solubility of the extracted pectin hence giving a higher rate of pectin extraction (Ania et al., Citation2012). Also, Picot-Allain et al. (Citation2020) reported that heating of the fruit would cause a disruptive effect to the cell wall, thereby enabling the diffusion of the solvent and pectin extraction from the fruit matrix. Furthermore, the extraction at a higher temperature (105°C) indicated a decrease in pectin extraction because high temperatures cause the breakdown of α-1.4 linked units of galacturonic acid or methyl ester yielding low pectin of lower molecular weight which is unstable (Ania et al., Citation2012). This is supported by Rha et al. (Citation2011), who reported that extraction temperature conditions and the source of pectin determine the pectin yield. High extraction temperatures have been reported to promote energy loss through vaporization and this increases the cost of extracting the pectin (Rha et al., Citation2011).
The pH affects the extraction of pectin because the presence of hydrogen ions at a high concentration in the solvent will stimulate the hydrolysis of protopectin by combining cellulose and pectin molecules (Liew et al., Citation2014; Redgwell et al., Citation1997). Liew et al. (Citation2014) reported that the increase in hydrogen ion concentration of the solution will promote the ionization of the carboxylate groups, that is, there is the conversion of highly hydrated carboxylate group into hydrated carboxylic acid groups (BeMiller, Citation1986). Furthermore, the ionization process will reduce the repulsion of polysaccharide molecules which support the gelling action of pectin and precipitates the pectin at lower pH (BeMiller, Citation1986). In the study, a pH of 3 was used in the extraction process because low pH was found to produce high pectin yield regardless of the plant material (Udonne et al., Citation2016).
Barclay et al. (Citation2014) noted that the maturity index affects the amount of pectin in the fruit because as the fruit becomes overripe, the pectin is broken down by pectinase and pectin esterase into simple sugars that are become water-soluble. Low temperature (< 60°C) reduces the viscosity of pectin and promotes poor diffusion of the pectin between the extraction phases and leads to low pectin yield (Xue et al., Citation2011). Our results were in agreement with those obtained by Drusch (Citation2007) and Ania et al. (Citation2012). The process of oven drying of fruits might have affected the pectin structure. This is supported by Liew et al. (Citation2014) who reported the destructive and swelling effects of oven drying on the pectin structure of yellow passion fruits as mound-shaped pellets were formed on smooth surfaces of pectin. Also, a possible explanation by Xue et al. (Citation2011) reported that acid interactions might have destroyed the glycosidic bond and ester bond of pectin and results in low yields.
Degree of Esterification
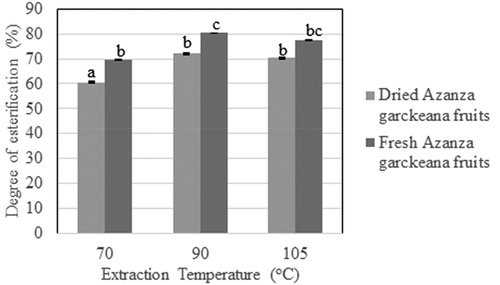
There was a significant difference in the degree of esterification between the pectin extracted from dried and fresh A. garckeana fruits at P < 0.05. The degree of esterification (also known as the degree of methylation) is used as an expression of the molar ratio of methyl-ester to galacturonic acid (Picot-Allain et al., Citation2020). The DE indicated the gelling property of pectin, hence its analysis in this study was important to understand the application of pectin. The degree of esterification from Azanza garckeana fruits both dried and fresh ranged from 60.5 ± 0.1% to 70.3 ± 0.1% and 69.4 ± 0.2 to 77.4 ± 0.2% respectively (). Pectin is classified as low methoxyl pectin with ≤ a 50% degree of esterification and high methoxyl pectin with > 50% degree of esterification (Grant et al., Citation2007). This suggests that our study indicated that snot apple (A. garckeana) pectin is classified as high methoxyl pectin because the degree of esterification was 72.1 ± 0.3% and 80.3 ± 0.1 at optimum extraction temperature of 90°C. This is supported by Mota et al. (Citation2020) who reported a high methoxyl index in fruit peel of Opuntia robusta. Canteri-Schemin et al. (Citation2005) noted that a 50% DE in pectin indicates high-methoxyl (HM) pectin which is essential when making high sugar products. Fishman et al. (Citation1984) reported the importance of DE in determining the gel-setting time. The DE noted in this study can explain rapid-set pectin that is useful in the manufacture of jams (Rolin, Citation2002). According to Sundar Raj et al. (Citation2012), DE depends on fruit species and stages of maturity.
Functional and Physicochemical Properties
The results of functional and physicochemical properties of the extracted pectin from Azanza garckeana fruits under optimum conditions of 90°C for 90 minutes at pH 3 are given in . The values of methoxyl contents obtained were 6.1 ± 0. 2 and 6.8 ± 0.2% for dried and fresh pectin extracts of Azanza garckeana fruits respectively. The methoxyl content is important in determining the setting time and the gelling ability of the pectin (Ania et al., Citation2012; Constenla and Lozano, Citation2003). The results from this study were lower than those for the peel of mango (Anacardiaceae) (7.33%), banana (Musaceae) (7.03%), pomelo peel (Rutaceae) (8.57%), Lime (Rutaceae) (9.92%), (Madhav and Pushpalatha, Citation2002), and passion (8.81% – 9.61%) but were much higher than dragon fruit pectin (2.98% to 4.34%) (Ismail et al., Citation2012). More so, the methoxyl content of A. garckeana fruits might have been reduced due to the increase in sugars during ripening of the fruits (Sirisakulwat et al., Citation2008). The spreading quality and sugar-binding capacity of pectin are increased with increase methoxyl content (Madhav and Pushpalatha, Citation2002). The methoxyl indicated that the pectin was of low ester content (< 7.5%) and this suggests a good quality pectin (Braddock, Citation2004).
Table 1. Functional and physiochemical properties extracted pectin at optimum conditions of 90°C, 90 minutes and pH 3
The AUA was determined to show the purity of the extracted pectin from A. garckeana fruits. The AUA value must be > 65% (Food Chemical Codex, Citation1996). The AUA results from this study (60.3–67.6%) were high as compared to apple pomace pectin (Rosaceae) (59.52 to 70.50%), (Kumar and Chauhan, Citation2010), commercial apple (Rosaceae) pectin (61.72%), and dragon (Cactaceae) fruit pectin which was 45.25 to 52.45% (Ismail et al., Citation2012) respectively. The AUA results were relatively high (> 65%). This suggests that our extracted pectin contained was relatively pure and did not contain some protein groups (Ismail et al., Citation2012). The moisture content of the pectin indicated that the pectin had good storage and utilization properties. The pectin extracts can be considered to be of good keeping quality.
The equivalent weight was low and this could be attributed to a higher partial degradation of pectin. Ramli and Asmawati (Citation2011), reported that the degradation of the pectin is dependent on the amount of free acid used in the extraction process.
Solubility and Gelation Property
Qualitative analysis of the solubility and gelation properties of the pectin extracts showed a positive test result for solubility in water at 90°C and gelation in acidified sugar with calcium ions at pH 3 (). The poor solubility and gelation properties in alcohol can be explained by the effect of dipole interaction, van der Waals and hydrophobic interactions which are induced by the alcohol in water rather than hydrogen bonds (McMurry, Citation2012).
Table 2. Qualitative analysis for pectin extracts
Table 3. Chemical properties of the jam
Chemical Properties of the Jam
The total soluble solids (TSS) were 68.2 ± 0.1 and 69.0 ± 0.2 in jam with A. garckeana pectin and control jam respectively. The chemical properties of the jam are shown in . The TSS values observed in this study are in agreement with a study by Ndabikunze et al. (Citation2010) who reported a TSS of 68.53 in U. kirkiana jam produced using commercial pectin. FAO (Citation1995) reported that a good jam must be produced with a final TSS range of 65–68%. The high TSS could be attributed to the presence of pectinase and the heat treatment used in processing, which causes the breakdown of the insoluble pectin from complex polysaccharides into simpler sugars (Kumar, Citation2015).
The pH of the jams was noted to be in the range of 3.0–3.5 which is the recommended limit by the Food and Agriculture Organization. Citric acid was used to set the pH which affects the viscosity (Nwosu et al., Citation2014) and improves gel formation in the jam (Featherstone, Citation2016). At low pH, hydrogen ions are high in solution, which tends to suppress the ionization of the galacturonate carboxyl group, and the repelling effect of the negatively charged carboxyl group to each other. Citric acid addition will lower the pH, reduce the negative charge of the pectin molecules, and then facilitates the hydrogen bonding of pectin molecules (Featherstone, Citation2016) resulting in the precipitation of pectin molecules. The high methoxyl index of pectin extracts from A. garckeana pectin was able to cause nonionic mechanisms in the presence of sugars and citric acid. This is supported by Picot-Allain et al. (Citation2020) who reported that the addition of sucrose (table sugar) as an ingredient in jam making at low pH favors gel formation of high methoxyl pectin. Furthermore, the high methoxyl pectin gels become stable due to the hydrophobic interactions and intermolecular hydrogen bonding of methyl esters in two pectin chains (Picot-Allain et al., Citation2020). The low pH in the jam inhibits the growth and survival of many food pathogens and microbes thereby ensuring its microbiological safety (International Commission on Microbiological Specifications for Foods, Citation2002).
Ndabikunze et al. (Citation2010) noted a total titratable acidity (TTA) content of 0.05 ± 0.02% in U. kirkiana pulp. The TTA observed in jams could be attributed to the presence of natural acids in the fruits. Vertuani et al. (Citation2002) reported the presence of citric, ascorbic, tartaric, succinic, and malic acids in fruit pulps. The acidity of the fruit pulp plays an important technological role in gel formation at low pH in jam production.
Organoleptic Characteristics of the Jam
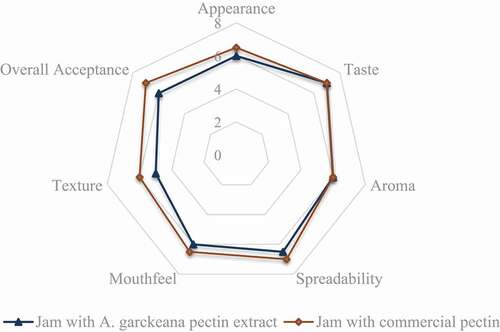
The overall acceptance of the jam produced with A. garckeana pectin extract was 5.5. This was significantly different from the control jam sample at P < 0.05 (). There was no significant difference in spreadability, aroma, taste, appearance, and mouthfeel of both jams at P < 0.05. This suggests a jam can be produced using A. garckeana pectin with similar sensorial properties to jam with commercial pectin although its acceptability was low.
Conclusion
This study revealed that an underutilized fruit, A. garckeana (snot apple) can be used as a source of pectin with technological functional properties. A novelty jam was produced by adding pectin extracts from A. garckeana (snot apple) fruits as one of the ingredients. The optimum conditions for pectin extraction from A. garckeana fruits was 90°C for 90 minutes at pH 3. Dried and fresh A. garckeana (snot apple) fruits produced a high pectin yield of 21.1 ± 0.2% w/w and 23.1 ± 0.1% w/w. The pectin for A. garckeana (snot apple) fruits can be classified as high methoxyl pectin with a DE of > 70%. A. garckeana (snot apple) fruits possess pectin with relative good functional and physiological properties such as moisture (2.2–3.1% w/w), ash (0.7–1.36% w/w), methoxyl (6.1–6.8%), and AUA (52.3–58.3%) and pH (4–4.1). Qualitatively, the pectin is soluble in warm water and gelatinizes in acidified solution in the presence of calcium ions. The prepared jam had a TSS (68.2%), TTA (2.1), moisture content (32.6%), and pH (3.4). The low pH of the jam makes it microbiological safe. The functional properties of the jam were significant in enhancing the sensorial qualities and overall acceptance of the jam. There was no significant difference in the preference of the jam prepared with commercial and extracted pectin. This study has provided more insights on the need to utilize pectin extracted from A. garckeana (snot apple) fruits in food processing. Research on the use of other indigenous fruits as sources of pectin is therefore recommended to increase access to pectin in most processing industries in Africa.
References
- Ania, A.O., M.M. Barau, O.A. Mamman, Z. Amina, H. Hauwa, M.S.U. Hauwa, and B.A. Yagana. 2012. Extraction and characterization of pectin from peels of lemon (citrus lemon), grape fruit (citrus paradise) and sweet orange (Citrus sinensis). Br. J. Pharmacol. Toxicol. 3(6):259–262.
- Association of Official Analytical Chemistry. 2000. Official method of analysis (AOAC) International. 17th edition ed. AOAC International, Maryland. USA.
- Barclay, A., P. Sandall, and C. Shwide-Slavin. 2014. The ultimate guide to sugars and sweeteners: Discover the taste, use, nutrition, science, and lore of everything from agave nectar to Xylitol. The Experiment.
- BeMiller, J.N. 1986. An introduction to pectins: Structure and properties. Chemistry and function of pectin. Int. J. Sci. Eng. Res. 310:2–12. doi: https://doi.org/10.1021/bk-1986-0310.ch001.
- Braddock, R.J. 2004. Importance of byproducts to citrus juice processing. Fruit Process 14:310–313.
- Braidwood, L., C. Breuer, and K. Sugimoto. 2014. My body is a cage: Mechanisms and modulation of plant cell growth. New Phytol. 201(2):388–402. doi: https://doi.org/10.1111/nph.12473.
- Canteri-Schemin, M.H., H.C.R. Fertonani, N. Waszczynskyj, and G. Wosiacki. 2005. Extraction of pectin from apple pomace. Brazilian Arch. Biol. Techno. 48(2):259–266. doi: https://doi.org/10.1590/S1516-89132005000200013.
- Chawafambira, A., M.M. Sedibe, A. Mpofu, and M. Achilonu. 2020b. multivariate analyses of functional and chemical properties of Uapaca Kirkiana fruits from Zimbabwe. Int. J. Fruit Sci. 20(sup3):S1174–S1191. doi: https://doi.org/10.1080/15538362.2020.1781022.
- Chawafambira, A., M.M. Sedibe, A. Mpofu, M. Achilonu, and F. Yildiz. 2020a. Uapaca kirkiana, an indigenous fruit tree in sub-Saharan Africa: A comprehensive review. Cogent Food Agric. 6(1):1766735. doi: https://doi.org/10.1080/23311932.2020.1766735.
- Chawafambira, A., M.M. Sedibe, A. Mpofu, and M.C. Achilonu. 2020c. The potential of Uapaca kirkiana fruit jam for the delivery of Lactobacillus rhamnosus yoba as a probiotic food. Afr. J. Food Agric. Nutr. Dev. 20(4):16161–16177. doi: https://doi.org/10.18697/ajfand.92.19355.
- Cho, E., H. Jung, B. Lee, H. Kim, J. Rhee, and S. Yoo. 2019. Green process development for apple-peel pectin production by organic acid extraction. Carbohydr Polym. 204:97–103. doi: https://doi.org/10.1016/j.carbpol.2018.09.086.
- Codex, F.C. 1996. IV monographs. National Academy Press, Washington DC, USA, 283.
- Constenla, D., and J.E. Lozano. 2003. Kinetic model of pectin demethylation. Latin Am.Appl. Res. 33:91–96.
- Drusch, S. 2007. Sugar beet pectin: A novel emulsifying wall component for microencapsulation of lipophilic food ingredients by spray-drying. Food Hydrocoll 21(7):1223–1228. doi: https://doi.org/10.1016/j.foodhyd.2006.08.007.
- FAO. 1995. Fruit and vegetable processing, FAO agricultural services bulletin number 119. Food and Agriculture Organization of the United Nations, Rome, Italy.
- Featherstone, S. 2016. A complete course in canning and related processes. - Processing procedures for canned food products. 14th Edition ed. Woodhead Publishing. Cambridge, England.
- Fishman, M.L., P.E. Pferffe, R.A. Barford, and K.W. Donar. 1984. Studies of pectin solution properties by high performance exclusion chromatography. J. Agric. Food Chem. 32(2):372–378. doi: https://doi.org/10.1021/jf00122a048.
- Glew, R.S., D.J. Vanderjagt, L.T. Chuang, Y.S. Huang, M. Millson, and R.H. Glew. 2005. Nutrient content of four edible wild plants from west Africa. Plant Foods Hum. Nutr. 60(4):187–193. doi: https://doi.org/10.1007/s11130-005-8616-0.
- Grant, G.T., E.R. Morris, D.A. Rees, P.J. Smith, and D. Thom. 2007. Biological interactions between polysaccharides and divalent cations: The egg‐box model. FEBS Lett. 32(1):195–198. doi: https://doi.org/10.1016/0014-5793(73)80770-7.
- International Commission on Microbiological Specifications for Foods. 2002. Microbiological testing in food safety management. - microorganisms in food 7. USA, Springer Science, New York.
- Ismail, N.S.M., N. Ramli, N.M. Hani, and Z. Meon. 2012. Extraction and characterization of pectin from dragon fruit (Hylocereus polyrhizus) using various extraction conditions. Sains Malaysiana 41:41–45.
- Koubala, B.B., G. Kansci, L.I. Mbome, M.J. Crepeau, J.F. Thibault, and M.C. Ralet. 2008a. Effect of extraction conditions on somephysicochemical characteristics of pectins from“Amelioree” and “Mango” peels. Food Hydrocoll 22(7):1345–1351. doi: https://doi.org/10.1016/j.foodhyd.2007.07.005.
- Koubala, B.B., L.I. Mbome, G. Kansci, F.T. Mbiapo, M.J. Crepeau, J.F. Thibault, and M.C. Ralet. 2008b. Physicochemical properties of pectins from ambarella peels (Spondias cytherea) obtained using different extraction conditions. Food Chem. 106(3):1202–1207. doi: https://doi.org/10.1016/j.foodchem.2007.07.065.
- Kumar, A., and G.S. Chauhan. 2010. Extraction and characterization of pectin from apple pomace and its evaluation as lipase (steapsin) inhibitor. Carbohydr. Polym. 82(2):454–459. doi: https://doi.org/10.1016/j.carbpol.2010.05.001.
- Kumar, S. 2015. Role of enzymes in fruit juice processing and its quality enhancement. Adv. Appl. Sci. Res. 6(6):114–124.
- Liew, S.Q., N.L. Chin, and A.Y. Yusof. 2014. Extraction and characterization of pectin from passion fruit peels. Agric. Agric. Sci. Procedia 2(231):236. doi: https://doi.org/10.1016/j.aaspro.2014.11.033.
- Madhav, A., and P.B. Pushpalatha. 2002. Characterization of pectin extracted from different fruit wastes. J. Trop.Agric. 40:53–55.
- Maroyi, A. 2017. Azanza garckeana fruit tree: Phytochemistry, pharmacology, nutritional and primary healthcare applications as herbal medicine: A review. Res. J. Med. Plants 11(4):115–123. doi: https://doi.org/10.3923/rjmp.2017.115.123.
- McMurry, J. 2012. Organic Chemistry. 8TH Edition ed. Cengage, Canada.
- Mojeremane, W., and S.O. Tshwenyane. 2004. Azanza garckeana: A valuable edible indigenous fruit tree of Botswana. Pakistan J. Nutr. 3(5):264–267. doi: https://doi.org/10.3923/pjn.2004.264.267.
- Mota, J., C. Muro, J. Illescas, O.A. Hernández, A. Tecante, and E. Rivera. 2020. Extraction and Characterization of Pectin from the Fruit Peel of Opuntia robusta. Chem. Select 5(37):11446–11452. doi: https://doi.org/10.1002/slct.202002181.
- Ndabikunze, B.K., B.N. Masambu, and B.M. Tiisekwa. 2010. Vitamin C and mineral contents, acceptability and shelf life of juice prepared from four indigenous fruits of the Miombo woodlands of Tanzania. J. Food Agric. Environ. 8(2):91–96.
- Nkafamiya, I.I., B.P. Ardo, S.A. Osemeahon, and A. Akinterinwa. 2016. Evaluation of nutritional, non-nutritional, elemental content and amino acid profile of Azanza garckeana (Goron Tula). Curr. J. Appl. Sci.Technol. 12(6):1–10. doi: https://doi.org/10.9734/BJAST/2016/19811.
- Nwosu, J.N., L.O. Udeozor, C.C. Ogueke, N. Onuegbu, G.C. Omeire, and I.S. Egbueri. 2014. Extraction and utilization of pectin from purple star-apple (Chrysophyllum cainito) and African star-apple (Chrysophyllum delevoyi) in Jam production. Austin J. Of Nutrition Food Sci. 1(1):1003–1009.
- Patra, P.A., and U.C. Basak. 2020. Physicochemical characterization of pectin extracted from six wild edible fruits in Odisha, India. Curr. Res. Nutr. Food Sci. 8:2. doi: https://doi.org/10.12944/CRNFSJ.8.2.05.
- Picot-Allain, M.C.N., B. Ramasawmy, and M.N. Emmambux. 2020. Extraction, characterisation, and application of pectin from tropical and sub-tropical fruits: A review. Food Rev. Int. 1–31. doi: https://doi.org/10.1080/87559129.2020.1733008.
- Ramli, N., and Asmawati. 2011. Effect of ammonium oxalate and acetic acid at several extraction time and pH on some physicochemical properties of pectin from cocoa husk (Theobroma cacao). Afr. J. Food Sci. 15(5):790–798. doi: https://doi.org/10.5897/AJFS11.107.
- Redgwell, R.J., E. MacRae, I. Hallett, M. Fischer, J. Perry, and R. Harker. 1997. In vivo and in vitro swelling of cell walls during fruit ripening planta. Planta. 203(2):162–173. doi: https://doi.org/10.1007/s004250050178.
- Rha, H.J., I.Y. Bae, S. Lee, S.H. Yoo, P.S. Chang, and H.G. Lee. 2011. Enhancement of anti-radical activity of pectin from apple pomace by hydroxamation. Food Hydrocoll 25(3):545–548. doi: https://doi.org/10.1016/j.foodhyd.2010.08.010.
- Rolin, C. 2002. Commercial pectin preparations. Pectins and their manipulation, Blackwell Publishing, CRC Press, USA, 222–241.
- Schmidt, E., M. Lotter and W. McCleland. 2002. Trees and Shrubs of Mpumalanga and Kruger National Park. Jacana Publishers, Johannesburg, South Africa. p. 702.
- Sirisakulwat, S., A. Nagel, P. Sruamsiri, R. Carle, and S. Neidhart. 2008. Yield and quality of pectins extractable from the peels of Thai mango cultivars depending on fruit ripeness. J. Agric. Food Chem. 56(22):10727–10738. doi: https://doi.org/10.1021/jf802173c.
- Storrs, A.E.G. 1979. know your trees: Some of the most common trees found in Zambia. The Forest Department, Ndola, Zambia, 380.
- Suliman, A.M.E., I.Y. Difa, and Z.A. Salih. 2012. The nutritive value of jakjak (Azanza garckeana L.) fruit and its utilization in juice production. Asian J. Biol. Sci. 5(4):209–215. doi: https://doi.org/10.3923/ajbs.2012.209.215.
- Sundar Raj, A.A., S. Rubila, R. Jayabalan, and T.V. Ranganathan. 2012. A review on pectin: Chemistry due to general properties of pectin and pharmaceutical uses. Sci. Rep. 1(2):1–4. doi: https://doi.org/10.4172/scientificreports.
- Tang, P.Y., C.J. Wong, and K.K. Woo. 2011. Methods of preparation, characterisation and applications. Asian J. Biol. Sci. 4(2):189–195. doi: https://doi.org/10.3923/ajbs.2011.189.195.
- Udonne, J.D., O.O. Ajani, and O.P. Akinyemi. 2016. A comparative study of extraction of pectin from wet and dried peels using water based and microwave method. International Journal of Scientific and Engineering Research 7(3):416–432.
- Van Wyk, B.E. 2011. The potential of South African plants in the development of new medicinal products. S. Afr. J. Bot. 77(4):812–829. doi: https://doi.org/10.1016/j.sajb.2011.08.011.
- Vertuani, S., E. Braccioli, V. Buzzoni, and S. Manfredini. 2002. Antioxidant capacity of Adansonia digitata fruit pulp and leaves, Acta phytotherapeutica. 2(5):2.
- World Agroforesty Centre. 2020. Uapaca kirkiana - Its uses, ecology, identity, propagation, pests. World Agroforesty Centre. Retrieved, 2020, April 24, from http://www.worldagroforestry.org/
- Xue, Z.H., X. Zhang, Z.J. Zhang, J.H. Liu, Y.F. Wang, D.X. Chen, and L.S. Long. 2011. Optimization of pectin extraction from citrus peel by response surface methodology. Food Sci. 18:128–132.
- Yang, D., J.Z. Zhang, S. Fu, Y. Xue, and J. Hu. 2009. Evolution process of polymethacrylate hydrogels investigated by rheological and dynamic light scattering techniques. Colloids Surf A Physicochem Eng Asp 353(2–3):197–203. doi: https://doi.org/10.1016/j.colsurfa.2009.11.013.