?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Zoochoric plants usually produce fruits with a mechanical barrier in the exocarp, or chemical inhibitors in the fruit pulp (mesocarp) or seed coat (endocarp) to achieve required dormancy. Tamarindus indica L. is one such zoochoric tree species, but the role of possible bioactive chemicals in the ecology of its fruit and seed dispersal has not been explored before. We investigated the germination inhibitory effects of T. indica fruit pulp by comparing germination of intact T. indica fruits with depulped T. indica seeds. This experiment revealed that attached pulp (including exocarp) delayed or decreased germination via an unverified mechanism. We then analyzed the pulp (including exocarp) to find out if there was a dominant bioactive chemical, by creating extracts using both alcohol and aqueous fractionation, and then analyzed them using GLC-MS analysis. We discovered that tartaric acid was the main bioactive chemical present in the methanol fraction (but not aqueous), so decided to test its effect on T. indica seed germination. We exposed viable depulped T. indica seeds to varying concentrations of pure tartaric acid in distilled water and found germination inhibition was observed at a concentration of 0.4 mg/ml. Therefore, since tartaric acid is the major chemical component in T. indica fruit pulp, it is possible that it could play a crucial role in the zoochoric dispersal of seeds by inhibiting germination while they are still attached to the tree or have just fallen close to the parent tree so that they have an opportunity to reach distant sites favorable for germination.
Introduction
Many plants produce fruits that are dependent on zoochoric agents (e.g. birds or mammals) for dispersal of their seeds, and can have unique mechanisms to delay the germination of seeds until they can reach distant sites that are favorable for germination and growth (Mayer and Poljakoff-Mayber, Citation1982; Sato, Citation2012). Such plants usually have mechanical or chemical inhibitors in the fruit, either in the exocarp, pulp or seed coat, to achieve the needed dormancy (Jordano, Citation2000). Once the fruit is consumed by the zoochoric agent, these inhibitors are removed either through digestion by saliva or passage through their alimentary canal and thus improve germination potential when finally dispersed to distant locations (Kiepiel and Johnson, Citation2019; Kissmann and Habermann, Citation2013; Pegman et al., Citation2017; Yagihashi et al., Citation1998).
Tamarindus indica L. is a leguminous tree (Fabaceae) that is solely dependent on animals for long distance seed dispersal and is a native and widely distributed species in Asia, with immense economic as well as traditional importance, and produces edible pod-like fruits. It is used for food, medicinal, cultural, social, environmental amelioration and income generation purposes (Havinga et al., Citation2010). The fruit pulp is thick, paste-like, strongly sour and highly acidic in nature and is used as food preservatives and as a traditional medicine in India (Ebifa-Othieno et al., Citation2017). However, little is known about the chemical nature of fruit pulp and its role in seed germination (Fergnani et al., Citation2020; Han and Xu, Citation2010; Mohammed et al., Citation2019). Hence, in our study, we analyzed the potential germination inhibitors present in T. indica fruit pulp, its chemical characterization, and ecological significance, using a protocol outlined below.
Materials and Methods
Experimental Rationale and Protocol
Our hypothesis is that there is an unspecified bioactive chemical in T. indica fruit pulp that inhibits germination. Therefore, we first performed a deinhibition germination test to determine if attached pulp inhibits germination. We then analyzed the pulp (including exocarp) to find out if a dominant bioactive chemical is present, by creating extracts using both alcohol and aqueous fractionation, since bioactive compounds can exist in either or both, and then analyzed them with gas spectral and liquid chromatography. The main bioactive candidate was then tested in pure uncontaminated form against germination of viable depulped T. indica seeds.
Collection of Fruits
Fruits of T. indica were collected in Ranaghat, Nadia, West Bengal, India, in November and December 2018. Some intact fruits were kept for the deinhibition experiment and others were de-seeded by knife and the pulp was collected and stored in a refrigerator at 4°C for analysis. Seeds were also collected, tested for viability, and saved for use in the germination experiment.
Preparation of Pulp Extract for Determination of Bioactive Compounds
Approximately 250 g of T. indica pulp was soaked in 500 ml each of methanol (Methanol CAS 67–56-1 × 106009 – Merck Millipore) as well as in water (double distilled) separately. The mixtures were vortexed at a speed of 3000 rpm using a Mechanical Stirrer (Model No. DC Stirrer NZ-1000s AC220V, EYELA) for two hours and filtered through sintered disc funnels. The extracts were collected and concentrated in a rotary vacuum evaporator (EYELA, Model No. N1-NW) and are henceforth referred to as TrMF (Methanol Fraction of T. indica) and TrWF (Water Fraction of T. indica). Both extracts were further purified by consecutive runs through column chromatography, followed by thin-layer chromatography, until a single spot was observed under UV light (365 nm).
Germination Deinhibition Experiment to Detect the Effect of T. indica Fruit Pulp on Germination of T. indica Seeds
A deinhibition experiment was performed using T. indica seeds that had pulp and exocarp attached versus manually depulped seeds (Pegman et al., Citation2017). Intact fruits and depulped seeds were kept on wet cotton on Petri dishes for 14 days in greenhouse conditions (Abubakar and Muhammad, Citation2013). Germination percentage was calculated for each seed type, post incubation. The experiment was replicated six times (N = 480). The percentage of seeds that germinated (EquationEquation 1(1)
(1) ) was determined based on the standard criteria of appearance of plant parts (Yagihashi et al., Citation1998).
GLC-MS Analysis of Purified TrMF and TrWF
Purified TrMF and TrWF were subjected to GLC-MS (gas chromatography mass spectrometry) Analysis (Agilent Technologies, MS Q-TOF, Model: G6550A; HiP Sampler, Model: G4226A; Binary Pump, Model: G4220B; Column Comp., Model: G1316C) for detecting bioactive compounds. The GLC-MS analysis was done at the Sophisticated Analytical Instrument Facility, IIT Bombay, Powai, Mumbai 400076, India. Hypersil gold C18 column (100 mm × 2.1 mm, 3 µm) was used and 5 μl of sample (dissolved in chloroform) was injected into column in the split mode for analysis at an injector temperature of 250°C. The identification of compounds was performed by comparing the mass spectra with the spectral data of the NBS75K library provided by the GLC/MS control and data processing software.
Germination Inhibitory Experiment Using Pure Tartaric Acid at Different Concentrations on Viable T. indica Seeds
Germination inhibitory activity of pure commercial tartaric acid (Sigma Aldrich, CAS-87-69-4) was performed at different concentrations on viable T. indica seeds without pulp. Viability of seeds was first assessed using the floatation test, with only seeds that sank being used (Gribko and Jones, Citation1995). Seeds were then surface sterilized with 0.1% mercuric chloride solution, washed three times with distilled water and placed on filter paper in Petri dishes (20 seeds in each Petri dish). One hundred mg of tartaric acid were dissolved in 100 ml of distilled water (i.e. 1 mg/ml). Further dilutions to 0.1 mg/ml, 0.2 mg/ml, 0.4 mg/ml, 0.6 mg/ml and 0.8 mg/ml were made and applied to seed replicates. A ‘control’ was maintained by treating the seeds with equal volumes of distilled water instead of tartaric acid solutions. There were seven sets of experiments including control (N = 420). The experiments were run for 14 days incubation in controlled greenhouse conditions. The percentage of seeds that germinated was determined based on EquationEquation 1(1)
(1) (Yagihashi et al., Citation1998).
Statistical Analysis
Results for germination deinhibition experiment were tested for odds ratio (CI 95%) using intact seeds as control. One-way ANOVA was used to determine the influence of tartaric acid on germination percentage of T. indica seeds. Post hoc comparisons using the Tukey HSD test were performed to detect whether the mean values of germination between different tartaric acid concentrations were significantly different from each other and the control. All statistical analyses were carried out using SPSS Statistics 21 software.
Results
Germination Deinhibition Experiment to Detect the Effect of T. indica Fruit Pulp on Germination of T. indica Seeds
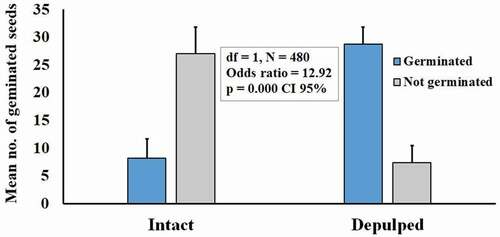
Germination deinhibition using T. indica seeds with pulp and exocarp attached versus manually depulped seeds showed a significant difference, with an odds ratio of 12.92 (df = 1, p = 0.000) based on the count of germinated seeds ( and EquationEquation 1)(1)
(1) . This means that the odds of germination of manually depulped seeds are 12.92 times greater than the odds of seeds with pulp. This means that the higher rate of germination of manually depulped seeds was not a matter of chance and that the fruit pulp significantly inhibited germination. A mean value of 28 ± 3 seeds germinated when manually depulped, compared with 8 ± 3 intact seeds.
GLC-MS Analysis of TrMF and TrWF
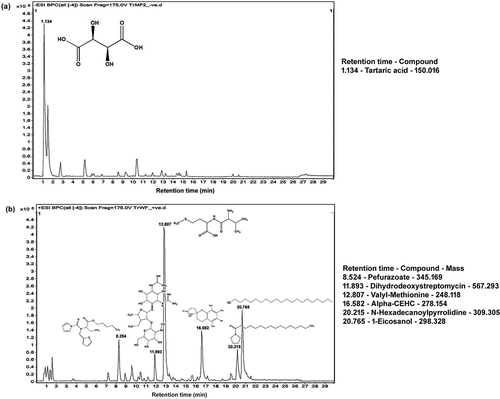
GLC-MS spectra of purified methanol extract of T. indica fruit pulp (TrMF) showed one sharp peak at 1.134 minutes (retention time, RT) that corresponded to the chemical compound tartaric acid (C4H6O6) with mass 150.016 g/mol as confirmed by comparing the mass spectra with the spectral data of the NBS75K library provided by the GLC/MS control and data processing software (). GLC-MS spectra of aqueous extract of T. indica fruit pulp (TrWF) showed six sharp peaks at retention time of 8.254, 11.893, 12.807, 16.582, 20.215 and 20.765 minutes that corresponded to the compounds pefurazoate (345.169 g/mol), dihydrodeoxystreptomycin (567.293 g/mol), valyl-methionine (248.118 g/mol), alpha–CEHC (278.154 g/mol), N-hexadecanoyl pyrrolidine (309.305 g/mol) and eicosanol (298.328 g/mol) respectively. Tartaric acid was absent in the TrWF.
Germination Experiment Using Pure Tartaric Acid at Different Concentrations on Viable T. indica Seeds
Dose dependent germination inhibition was observed using pure tartaric acid applied to manually depulped viable T. indica seeds. A complete inhibition of germination was observed at a concentration of 0.4 mg/ml (). In the control replicate, c. 36% germination was observed, whereas at 0.1 mg/ml and 0.2 mg/ml concentrations, c. 23% and 10% germination were detected, respectively. The germination percentage showed strong negative correlation with the concentration of tartaric acid with R2 = 0.937 ().
Figure 3. Germination percentage data depicting dose-dependent germination inhibition using tartaric acid (commercial) at different concentrations on viable Tamarindus indica L. seeds. Each value in the graph shows mean from three independent experiments (N = 420). The effect was significantly dose- dependent with R2 = 0.937
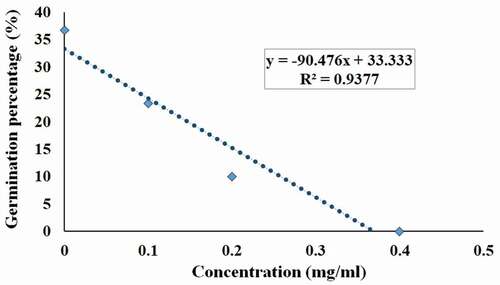
One-way ANOVA was performed to compare the inhibitory effect of the different tartaric acid concentrations on the tested samples. There was a significant effect at p < 0.05 (F (6,14) = 67.66, p = 0.000, N = 420). Post hoc comparisons using the Tukey HSD test indicated that the mean values of germination between different tartaric acid concentrations were significantly different from control.
Discussion
Zoochoric fruits, such as those in T. indica L., can contain mechanical or chemical inhibitors in the exocarp, pulp or seed coat to achieve the required dormancy for seeds to have enough time for dispersal to distant locations where conditions for germination are often more favorable. Result of germination deinhibition experiment using T. indica seeds with pulp and exocarp attached versus manually depulped seeds suggested that the attached pulp (including the exocarp) decreases germination via an unverified mechanism. GLC-MS analysis of methanol fraction (TrMF) and water fraction (TrWF) of T. indica fruit pulp indicated the presence of tartaric acid as the main bioactive compound in the methanol fraction, whereas amino acids and metabolites, such as valyl-methionine, alpha–CEHC, N-hexadecanoyl pyrrolidine, eicosanol, were present in the water fraction along with the antimicrobial compounds pefurazoate and dihydrodeoxystreptomycin (Patil and Lade, Citation2018). The germination experiment revealed 100% germination inhibition at a concentration of 0.4 mg/ml pure tartaric acid. One-way ANOVA validated the hypothesis that tartaric acid may play a key role in the inhibitory nature of the pulp of T. indica fruits since there was a significant effect on germination compared with the control. Post hoc comparisons using the Tukey HSD test further supported this claim because the mean values of germination between all tartaric acid concentrations were significantly different from the control. There had been previous reports of organic acids from the leaves of T. indica having allelopathic effects (Syed et al., Citation2014), but the possible role of tartaric acid in inhibiting seed germination and prolonging seed dormancy has never been explored before. Therefore, we can draw a hypothesis that the inhibition may be due to the tartaric acid that was shown to be present in the pulp of T. indica.
Plants produce fruits with chemicals that can inhibit germination, as well as containing nutrients that attract animals who spread the seeds to distant sites from the parent plant, thus contributing to plant regeneration and colonization of new sites (Willson and Traveset, Citation2000). Furthermore, the fruit pulp can decrease and even preclude germination by altering the seeds’ micro environment (e.g. osmotic pressure and light regime; Traveset et al., Citation2007). In T. indica fruit, seeds are surrounded by a coating of thick paste like pulp that offers a return to frugivores, but might also have other functions such as the protection of the seeds and the prevention of germination while still on the plant, or when dropped below the plant, so that they are able to reach distant favourble sites before germination occurs (Robertson et al., Citation2006). Fruit pulp also possesses antibacterial activity, which protects the seeds from microbial damage and decay (Abukakar et al., Citation2008). Our study specifically suggests that chemical components, such as tartaric acid, may enhance endozoochory in our focal plant species by slowing seed germination, potentially enhancing seed dispersal and increasing community composition in distant ecosystems.
Conclusion
Zoochoric fruits often have mechanical or chemical inhibitors in the fruit either in the exocarp or in the pulp to achieve the required dormancy. This provides seeds enough time for dispersal to distant places where conditions for germination are often more favorable. We hypothesized that germination inhibitors are stored in the T. indica fruit pulp to acquire the needed dormancy. Germination deinhibition experiment indicated that possible inhibitory chemicals are stored in the pulp of the fruits. The GLC-MS analysis revealed the presence of tartaric acid as the predominant bioactive compound in the methanol fraction of T.indica fruit pulp (including exocarp), whereas the aqueous fraction contained amino acids, metabolites and antimicrobial agents. Dose-dependent germination inhibition activity of pure tartaric acid on viable T. indica seeds suggested tartaric acid play a key role in inhibitory nature of the fruit pulp. Tartaric acid was previously known to be a main chemical component of T. indica fruit pulp but its role in the ecology of fruit and seed dispersal was not explored before. Tartaric acid is thus proposed to have a role in the zoochoric dispersal of seeds by inhibiting germination through an unknown mechanism while seeds are still attached to the tree or have just fallen close to the parent tree.
Author’s contribution
Design of the study: SMB (Suparna Mandal Biswas) and EB (Ekta Bhattacharya); Collected test data: EB; Data analysis and interpretation: SMB and EB; Preparation of draft manuscript and critical revision - SMB and EB; Supervision and Project administration: SMB; Funding acquisition: SMB.
Acknowledgments
We thank Director, Prof. Sanghamitra Bandhopadhyay for providing laboratory facilities and financial support for performing this research work. We are indebted to Prof. Susmita Mukhopadhyay, Prof-in-Charge, Biological Sciences Division and Prof. Sabyasachi Bhattacharya, Head, Agricultural and Ecological Research Unit, Indian Statistical Institute, for their encouragement, valuable advice and laboratory facilities.
Additional information
Funding
Literature cited
- Abubakar, Z.A., and A. Muhammad. 2013. Breaking seed dormancy in Tamarind (Tamarindus indica) a case study of gombe local government area. J. Appl. Sci. Environ. Manage. 17:83–87.
- Abukakar, M.G., A.N. Ukwuani, and R.A. Shehu. 2008. Phytochemical screening and antibacterial activity of Tamarindus indica pulp extract. Asian J. Biochem. 3:134–138. doi: https://doi.org/10.3923/ajb.2008.134.138.
- Ebifa-Othieno, E., A. Mugisha, P. Nyeko, and J.D. Kabasa. 2017. Knowledge, attitudes and practices in tamarind (Tamarindus indica L.) use and conservation in Eastern Uganda. J. Ethnobiol. Ethnomed. 13:5. doi: https://doi.org/10.1186/s13002-016-0133-8.
- Fergnani, D.M., M.M. Kowalewski, and V.A. Fernandez. 2020. Germination of native and exotic seeds dispersed by wild black-and-gold howler monkeys (Alouatta caraya): Assessing deinhibition and scarification effects. Primates. 61:519–527. doi: https://doi.org/10.1007/s10329-020-00791-9.
- Gribko, L.S., and W.E. Jones. 1995. Test of the float method of assessing northern red oak acorn condition. Tree Planters’ Notes. 46:143–147.
- Han, B., and L. Xu. 2010. Study on the germination inhibitors in the fruits of Panax quinquefolium Linne. Med. Plant. 1:61–63.
- Havinga, R.M., A. Hartl, J. Putscher, S. Prehsler, C. Buchmann, and C.R. Vogl. 2010. Tamarindus indica L. (Fabaceae): Patterns of use in traditional African medicine. J. Ethnopharmacol. 127:573–588. doi: https://doi.org/10.1016/j.jep.2009.11.028.
- Jordano, P. 2000. Fruits and frugivory, p. 125–166. In: M. Fenner ed.. Seeds: The ecology of regeneration in plant communities. 2nd. CABI Publ., Wallingford, UK.
- Kiepiel, I., and S.D. Johnson. 2019. Spit it out: Monkeys disperse the unorthodox and toxic seeds of Clivia miniata (Amaryllidaceae). Biotropica. 51:619–625. doi: https://doi.org/10.1111/btp.12698.
- Kissmann, C., and G. Habermann. 2013. Seed germination performances of Styrax species help understand their distribution in Cerrado areas in Brazil. Bragantia 72(3):199–207. doi: https://doi.org/10.1590/brag.2013.030.
- Mayer, A.M., and A. Poljakoff-Mayber. 1982. The germination of seeds. 3rd ed. Pergamon Press, Oxford and New York.
- Mohammed, S., V. Turečková, D. Tarkowská, M. Strnad, K. Mummenhoff, and G. Leubner-Metzger. 2019. Pericarp-mediated chemical dormancy controls the fruit germination of the invasive hoary cress (Lepidium draba), but not of hairy whitetop (Lepidium appelianum). Weed Sci. 67:560–571. doi: https://doi.org/10.1017/wsc.2019.33.
- Patil, A.S., and B.D. Lade. 2018. Wound stress induced secondary metabolites in Passiflora foetida: Exploration of antimicrobial compounds. Saudi J. Med. Pharm. Sci. 4:613–627. doi: https://doi.org/10.21276/sjmps.2018.4.5.21.
- Pegman, A.P.M., G.L. Perry, and M.N. Clout. 2017. Size-based fruit selection by a keystone avian frugivore and effects on seed viability. N. Z. J. Bot. 55(2):118–133. doi: https://doi.org/10.1080/0028825X.2016.1247882.
- Robertson, A.W., A. Trass, J.J. Ladley, and D. Kelly. 2006. Assessing the benefits of frugivory for seed germination: The importance of the deinhibition effect. Funct. Ecol. 20: 58–66. http://www.jstor.org/stable/3598961
- Sato, H. 2012. Frugivory and seed dispersal by brown lemurs in a malagasy tropical dry forest. Biotropica. 44:479–488. doi: https://doi.org/10.1111/j.1744-7429.2011.00838.x.
- Syed, S., Z.I. Ahmed, M.I. Al-Haq, A. Mohammad, and Y. Fujii. 2014. The possible role of organic acids as allelochemicals in Tamarindus indica L. leaves. Acta Agric. Scand. B, Soil Plant Sci. 64:511–517. doi: https://doi.org/10.1080/09064710.2014.927525.
- Traveset, A., A.W. Robertson, and J. Rodríguez-Pérez. 2007. A review on the role of endozoochory in seed germination. Seed dispersal: Theory and its application in a changing world. Chapter 4:78–103.
- Willson, M.F., and A. Traveset. 2000. The ecology of seed dispersal, p. 85–110. In: M. Fenner (ed.). Seeds: The ecology of regeneration in plant communities. CAB International, UK.
- Yagihashi, T., M. Hayashida, and T. Miyamoto. 1998. Effects of bird ingestion on seed germination of Sorbus commixta. Oecologia 114(2):209–212. doi: https://doi.org/10.1007/s004420050438.