ABSTRACT
Raspberries (Rubus idaeus L.) are important vegetatively propagated fruit plants. They are used as food, as medicinal plants, and in pharmaceutical and cosmetic industry. For the protection of intellectual property rights, it is very important to have efficient methods that enable fast and accurate identification of cultivars. Raspberry cultivars can be differentiated in two ways: on the phenotypic level with morphological descriptors and on the molecular level with molecular markers. Among the frequently used are SSRs, which are highly informative, easy to score and have a good genome coverage. Considering 19 previously published SSR molecular markers, we selected the most specific loci for each selected genotype and developed specific fingerprint for each of the genotypes included in the study: four red raspberry cultivars (‘Polka,’ ‘Glen Ample,’ ‘Meeker,’ ‘Rose de Côte d’Or’) and two genotypes named Sicoly and Dieffenbach. The aim of our investigation was to demonstrate the differentiation between studied raspberry genotypes by specific amplified fragment patterns, which were unique for each genotype and were based on simple sequence repeats (SSRs). Furthermore, the developed fingerprints were successfully tested on randomly chosen, unknown genotypes.
Introduction
Among berry fruits, the raspberry is considered to be a highly valuable horticultural crop due to its unique flavors and other sensory qualitative characteristics, as well as medicinal properties. The global production of raspberries is estimated to exceed 870,000 tonnes annually. The world market of raspberries has increased by 66% in the last decade (FAOSTAT, Citation2020). However, the number of successfully grown cultivars in commercial raspberry fruit production is still limited. In the future, new highly productive and high-quality raspberry cultivars are needed to satisfy the increasing demands of the existing markets.
Cultivar registration is an important issue in plant genetic resource evaluation and characterization, and is closely linked with crop’s utilization. Verification of cultivar authenticity (genuineness) is important in raspberry cultivation to guarantee productivity (plant growth, plant health, fruit quality, etc.). It is also important for the protection of plant breeder’s rights in order to reduce the extent of mislabeled plants on the market or illegal propagation. The unregulated propagation and distribution of patented cultivars is considered to be a serious problem (Kunihisa, Citation2011).
The current plant cultivar protection system is largely based on morphological description of plant varieties. Many of these traits are at least partly influenced by the environment. The incorporation of new methodologies, using DNA-based approaches, into plant material certification schemes can accelerate and optimize the cultivar identification process, by allowing fingerprinting of each genotype at any stage of development, and independently of environmental factors (Noli et al., Citation2008) that may influence the phenotype (Jamali et al., Citation2019). Since raspberries are vegetatively propagated, the molecular characterization of genotypes can be a reliable reference for certification and control of its propagation and distribution.
Different kinds of DNA markers have been successfully used for identification of cultivars. Differentiation of cultivated raspberries using random amplified polymorphic DNA (RAPD), amplified fragment length polymorphisms (AFLPs), and simple sequence repeats (SSRs) have been reported by several authors (e.g., Ercisli et al., Citation2008; Fernández-Fernández et al., Citation2011; Miyashita et al., Citation2015; Pinczinger et al., Citation2020; Simlat et al., Citation2018; Umar et al., Citation2010).
Polymorphic SSR markers appear to be more desired for genotyping vegetatively propagated plants than other DNA fingerprinting methods because they are highly informative (show high polymorphism), reproducible, technically simple (they are simpler than several other techniques), robust, and suitable for automated allele detection and sizing (Nybom and Lācis, Citation2021; Rafalski and Tingey, Citation1993). SSR markers for the Rubus species were first developed in early 2000s (Amsellem et al., Citation2001) and, in subsequent years, a number of studies were conducted involving several plant species (Castillo et al., Citation2010; Graham et al., Citation2004, Citation2002; Kalia et al., Citation2011; Pinczinger et al., Citation2020).
Pinczinger et al. (Citation2020) used sixteen SSR markers, divided into six multiplexes, successfully identified the raspberry cultivars, and found that of the 33 samples considered, only 24 samples appeared to be true-to-type, whereas nine samples were found to be wrongly labeled. This proves that the extent of mislabeled plants on the market is high. Also, Girichev et al. (Citation2015) have established DNA fingerprints for 79 genotypes of raspberry and three blackberry cultivars of different origins, including both primocane and floricane fruiting types, using 16 SSR markers. The study found a very narrow genetic base of Rubus resources in Germany. The data provided by Girichev et al. (Citation2015) and Pinczinger et al. (Citation2020) provides a good basis for cultivar identification, and emphasizes the necessity of DNA fingerprints for growers and breeders.
Improvements in molecular marker technology, such as multiplex PCR for DNA amplification, consisting of simultaneous amplifications of more than one SSR in a single reaction, combined with the utilization of fluorescence-based automated DNA detection and fragment sizing, allow faster, more accurate, and cost-effective acquisition of data. This offers a potential improvement of the efficiency and affordability of cultivar testing (Akin et al., Citation2016; Fernández-Fernández et al., Citation2011; Hayden et al., Citation2008; Honjo et al., Citation2011; Pinczinger et al., Citation2020).
In the future, new raspberry cultivars will have to satisfy the increasing demands of the existing markets, and producers will have to reduce the pollution of the environment (i.e., the use of pesticides will have to be avoided or drastically reduced). Cultivars will have to be resistant to all critical pests and diseases, well-adapted to cultivar’s environments (changes of humidity and temperature) and, at the same time, should possess high values of “health promotion – disease prevention” compounds. For each raspberry breeding programme, available genetic resources are essential. Germplasm collections may include traditional cultivars, genotypes resulting from previous breeding programmes (various hybrids, mutants, transformed genotypes), and primitive and wild genotypes. Until now, about 200 raspberry cultivars have been registered and described in plant collections, but only around 20 have been largely commercialized. There is a great need for physiological and morpho-agronomic testing of the existing germplasm and its diversity in order to exploit it in breeding programmes aimed at producing new raspberry cultivars with higher quantity of bioactive compounds, natural resistance to fungi, viruses and other parasites, and responding to lowering rates of chemical treatments. Rationalization of germplasm collections appears to be a necessity as soon as there are more than one hundred accessions. To rationalize a collection, a curator must have reliable data about the existing phenotypic and genotypic variation, and genetic relationships among individual accessions. Consequently, practical breeders will know where they can find a genotype, cultivar or cultivar group with a peculiar trait and data about its variation. If they have several options, they will probably consider the accessions with the highest number of other crucial traits.
The main objective of the presented study was to develop a protocol for an efficient and fast testing of the authenticity of raspberry cultivars based on specific multiplex of SSR markers. We wanted to reveal the fingerprinting patterns for a certain number of randomly chosen genotypes, which could be used as an example in testing of raspberry cultivars. Even though red raspberry cultivars have low genetic diversity (Girichev et al., Citation2015; Pinczinger et al., Citation2020), we wanted to find the minimal number of markers for successful identification of an individual genotype. With a reduced number of used markers, the identification method is quicker and cheaper, and thus more suitable for practical application. The second objective of our study was to test the applicability of the developed protocol.
Materials and Methods
Plant Material and DNA Isolation
The study includes six genotypes: four commercial, clonally propagated red raspberry cultivars (‘Polka’ (Poland), ‘Glen Ample’ (UK), ‘Meeker’ (USA), ‘Rose de Côte d’Or’ (France)) and two genotypes named Sicoly (France) and Dieffenbach (Switzerland).
DNA was extracted from fresh, young and healthy leaves using the CTAB method (Doyle and Doyle, Citation1987) with some modifications as described by Šiško et al. (Citation2009). Two separate extractions per plant were performed. The DNA concentration was estimated using a DNA fluorometer DQ 300 (Hoefer, Inc., Holliston, Massachusetts).
SSR Markers and PCR Protocol
Nineteen microsatellite loci used in previous studies were tested: four of the Rim series (Rim15, Rim17, Rim19, Rim36), seven of the Rub series (Rub25a, Rub108a, Rub157b, Rub223a, Rub228, Rub262, Rub277), seven of the Rhm series (Rhm1, Rhm3, Rhm11, Rhm18, Rhm21, Rhm23, Rhm43) and one of the Rig series (Rig1) (Castillo et al., Citation2010; Graham et al., Citation2004, Citation2002). Among them, 12 loci were found suitable to obtain specific fragment patterns for six raspberry genotypes (). All primers were ordered from Sigma-Aldrich. Lyophilized primers were diluted to a working concentration of 500 pmol/μl.
Table 1. The list of 12 selected SSR primers used in the study, their sequences, repeat motifs, allele sizes, optimized annealing temperatures (Ta), and numbers of cycles
The annealing temperature (Ta) and the number of cycles for each primer pair were optimized. For primers Rim19, Rim36, Rhm1, Rhm3, Rhm11, Rhm18, Rhm21, Rhm23, Rhm43, Rig1, the best amplification was obtained at 55°C, while, 59°C was found to be the optimal temperature for primers Rim15, Rim17, Rub25a, Rub108a, Rub157b, Rub223a, Rub228, Rub262, Rub277 (). Regarding optimization of the number of cycles, the best amplification for most primer sets (Rim19, Rim36, Rhm1, Rhm3, Rhm11, Rhm18, Rhm21, Rhm23, Rhm43, Rig1 Rim15, Rub25a, Rub108a, Rub157b, Rub223a, Rub262) was achieved in 30 cycles, while three primer sets (Rim17, Rub228, Rub277) required a lower number of cycles (25 cycles).
Ten μl of PCR mixture contained 20 ng DNA, 0.25 U Taq DNA polymerase (Fermentas), 1 x PCR buffer (Fermentas), 2 mM MgCl2 (Fermentas), 0.5 μl of each primer and 0.2 mM of each dNTP’s (Sigma). PCR was performed separately for each primer pair. The forward primer of each primer pair was fluorescently labeled with dyes Cy 5 or Cy 5.5, while the reverse primer was unlabeled. PCR condition consisted of 25–30 cycles with hot start for 5 minutes at 94°C, denaturation at 94°C for 30 seconds, annealing at 55°C or 59°C for 45 seconds, and an extension step at 72°C for 75 seconds, and a final extension step at 72°C for 8 min. Capillary electrophoresis of PCR products was performed on Beckman Coulter CEQ8000 according to manufacturer’s instructions. Fragment size analysis was done with the in-build software. A fluorescent-labeled size marker (Beckman Coulter DNA Size Standard Kit 400 bp) was used as a molecular weight reference.
The procedure of protocol development for cultivar identification consisted of several steps. In the first step, we optimized the conditions of all 19 selected primer sets in order to obtain the best amplification with each primer set. Then we conducted PCR reactions for all 19 primers with each of six selected raspberry genotypes. To reduce labor and costs for PCR products detection, the multiplex of three primer sets was combined according to the obtained allele sizes of each primer set (). To better distinguish primers in the multiplex, primers were labeled with two different fluorescent dyes: Cy 5 and Cy 5.5.
All unambiguous fragments were scored for the presence (1) or absence (0) of each band. The binary data matrix was used to calculate Dice’s similarity coefficients (Dice, Citation1945), and a neighbor-joining tree was constructed using the DARWIN computer package (Perrier and Jacquemoud-Collet, Citation2005). For each microsatellite locus, the number of alleles per locus (n), allele frequencies, observed (H0) and expected heterozygosity (HE), and polymorphic information content (PIC) were calculated using the Cervus 3.0.7 computer programme (Marshall et al., Citation1998, 2014 version).
Results and Discussion
Fragment sizes for 12 analyzed loci for all tested genotypes are listed in . From the completed data (allele sizes) obtained with SSR primers, we selected the most specific microsatellite loci for each of the six studied genotypes. For identification of the cultivar ‘Meeker,’ we used seven selected microsatellite loci, while for the remaining five genotypes (‘Glen Ample,’ ‘Polka,’ ‘Rose de Côte d’Or’, Sicoly, Dieffenbach), groups of six loci were used.
Table 2. Fragment sizes (bp) of the six genotypes, at 12 microsatellite loci. Specific sizes are in bold
We validated our results for cross comparison with those obtained by Pinczinger et al. (Citation2020) on a set of three genotypes (‘Glen Ample,’ ‘Polka,’ ‘Meeker’). In accordance with Girichev et al. (Citation2015) and Fernández-Fernández et al. (Citation2011), we compared our results only on cultivar ‘Glen Ample.’ The differences in some fragment sizes of all three genotypes, which were true-to-type, suggested that the method was successful. The occurrence of differences in some fragment sizes, which were observed from 1 to 3 bp, could be related to the marker itself or to some technical issues (e.g., different sequencers used, different fluorescence dyes used). These small differences are not problematic as long as the researcher is aware of them.
A total of 49 alleles were detected at 12 microsatellite loci, while the number of alleles detected per locus ranged from 2 (Rub277 and Rhm21) to 7 (Rim19 and Rub262), with an average of 4.083 alleles per locus (). The observed heterozygosity ranged between 0.167 (loci Rim36, Rub277 and Rhm21) and 1.00 (locus Rhm43), with an average of 0.514. The expected heterozygosity ranged between 0.167 (loci Rim36, Rub277 and Rhm21) and 0.894 (loci Rim19 and Rub262), with an average of 0.617. The largest difference between the observed and expected heterozygosity was observed on loci Rim17 and Rub262 (0.394). There were no differences on three loci (Rim36, Rub277, Rhm21). The highest PIC value (polymorphic information content, PIC, is a measure of the quality of informativeness of molecular markers) was obtained on loci Rim19 and Rub262 (0.796) and the lowest on loci Rim36, Rub272 and Rhm21 (0.141). Averages of observed and expected heterozygosity are in line with Girichev et al. (Citation2015); however, average PIC value was higher in our research (0.526).
Table 3. SSR loci analyzed and parameters of genetic variability calculated for different microsatellite loci of the six Rubus genotypes: number of alleles (n), observed (Ho) and expected (He) heterozygosity, and polymorphic information content (PIC)
Even though red raspberry cultivars have a small genetic diversity (Girichev et al., Citation2015; Pinczinger et al., Citation2020), we wanted to find the minimal number of markers for successful identification of an individual genotype. With a reduced number of used markers, the identification method is quicker and cheaper, and thus more suitable for practical application.
With the selected microsatellite loci, a unique fingerprint for each cultivar was obtained. Each fingerprint was cultivar-specific and could be used for its identification. The first group of six loci was found to be useful for identification of the cultivar ‘Glen Ample’: two homozygous (Rim19 and Rub262) and three heterozygous (Rub25a, Rub277 and Rhm11) (). Another group of six loci was found suitable for identification of the cultivar ‘Rose de Côte d’Or’: two homozygous (Rub108a and Rhm3) and four heterozygous (Rub25a, Rim19, Rim17 and Rub 162) (). The cultivar ‘Meeker’ could be identified by seven loci: one homozygous (Rhm11) and six heterozygous (Rub108a, Rim19, Rub262, Rhm3, Rim15 and Rhm43) (). The third group of six loci was chosen for identification of the genotype named Dieffenbach: Rhm3 and Rim17 (homozygous) and Rim19, Rhm11, Rim15 and Rhm43 (heterozygous) (). The fourth group of six loci was used for identification of the genotype named Sicoly: one homozygous (Rub262) and five heterozygous (Rim19, Rim17, Rhm21, Rhm11 and Rim15) (). The last group of six loci was used for identification of the cultivar ‘Polka’: two homozygous loci (Rim17 and Rim15) and four heterozygous (Rim19, Rub262, Rhm11 and Rim36) were selected ().
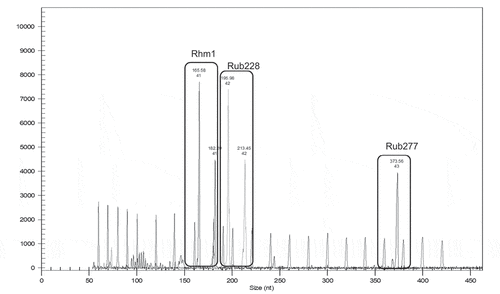
Figure 2. Electrotraces for identification of the cultivar ‘Glen Ample’ based on combination of six microsatellite loci
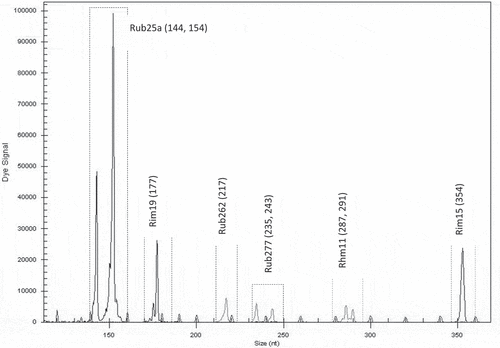
Figure 3. Electrotraces for identification of the cultivar ‘Rose de Côte d’Or’ based on combination of six microsatellite loci
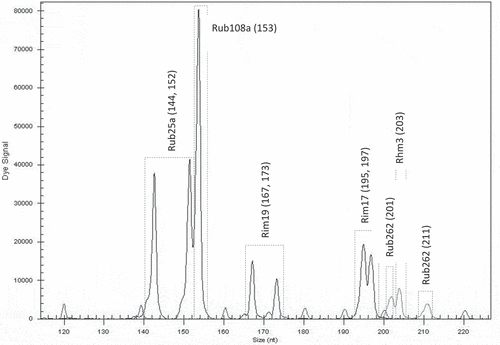
Figure 4. Electrotraces for identification of the cultivar ‘Meeker’ based on combination of seven microsatellite loci
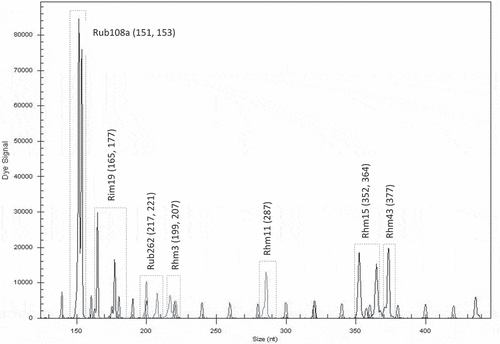
Figure 5. Electrotraces for identification of the raspberry genotype Dieffenbach based on combination of six microsatellite loci
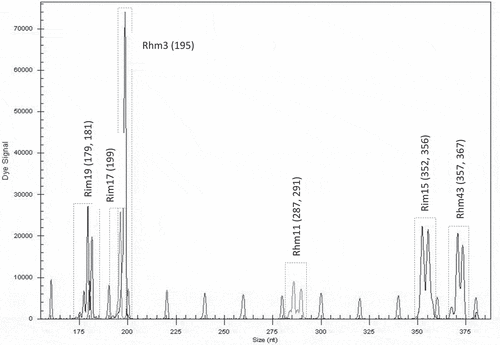
Figure 6. Electrotraces for identification of the raspberry genotype Sicoly based on combination of six microsatellite loci
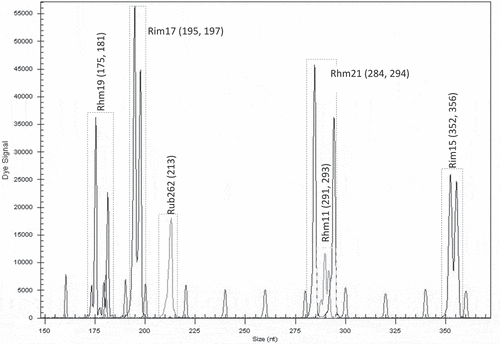
Figure 7. Electrotraces for identification of the cultivar ‘Polka’ based on combination of six microsatellite loci
In the study, we used two different fluorescence labels (Cy 5, Cy 5.5), which is enough for differentiation within three markers; for electrotraces with six or seven markers, additional fluorescent labels should be used.
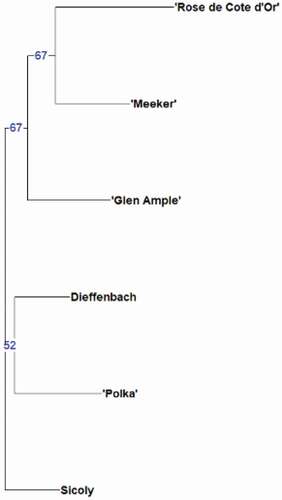
The dendrogram obtained from neighbor-joining cluster analysis () based on Dice genetic similarity coefficient matrix showed two clusters and one outside genotype, which was Sicoly, a selection from France. The cluster analysis shows greater similarity between ‘Polka’ and Dieffenbach, and a genetic distance to the other three cultivars (‘Glen Ample,’ ‘Rose de Côte d’Or’, ‘Meeker’).
Figure 8. Neighbor-joining dendrogram showing similarities among six raspberry genotypes based on 12 SSR markers
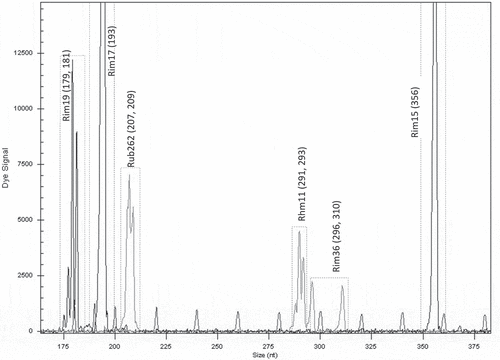
The applicability of the developed protocol for cultivar identification was tested on the cultivar ‘Polka.’ From the Slovenian Plant Gene Bank (Raspberry Collection), five genotypes (including ‘Polka’) were sampled and marked with numbers. Each genotype was represented by one plant. DNA was extracted from all of them and PCR was performed using specific primer sets from the fingerprint (electrotrace) developed for the identification of ‘Polka.’ The obtained electrotraces of five genotypes were then compared with the electrotrace of ‘Polka.’ The differentiation of ‘Polka’ from other unknown samples was based on the identity of electrotraces under consideration.
In raspberry cultivation practice and practical genetic breeding, the morphological descriptors will probably remain crucial. If there are few cultivars, it is generally not difficult to identify them. The list of traits published by UPOV (International Union for the Protection of New Cultivars of Plants (Citation2006) : – Guidelines for the Conduct of Tests for Distinctness, Uniformity and Stability: Blackberry, Rubus subg. Rubus) are generally sufficient and highly reliable for determination of cultivars. However, problems appear when there are several very similar cultivars. We also have to consider that some of the traits only partially reflect the heritable genetic variability, due to the modifying impact of various environmental and ontogenic factors, as well as the interaction between genetic structure and environment. These difficulties can be solved by using a combination of morphological and molecular markers. The molecular marker techniques allow the detection of specific sequence differences among individuals and in this way overcome the limitations due to morphological and ontogenic variation. Molecular markers are even more important when we have to identify a cultivar from a small part of a plant (e.g., a seed or a leaf part).
Cultivar identification with various approaches based on molecular markers has been used in many vegetatively propagated plant species like strawberry (Congiu et al., Citation2000; Hirata et al., Citation2020; Honjo et al., Citation2011; Kunihisa, Citation2011; Kunihisa et al., Citation2003), hazelnut (Akin et al., Citation2016), black raspberry (Dossett et al., Citation2012), bermudagrass (Wang et al., Citation2010), and also raspberries (Pinczinger et al., Citation2020).
Raspberry cultivars are propagated vegetatively; therefore, all individuals belonging to a particular cultivar share the same (identical) genome. This simplifies the task of molecular identification because any difference between two given individuals unambiguously indicates that they do not belong to the same cultivar.
The developed protocol enabled us to differentiate and identify all included genotypes. Five genotypes could be identified by six and one by seven microsatellite loci. Our approach was found to be relatively simple, fast, reliable, and cost-efficient. A similar approach for identification of cultivars and verification of their authenticity can also be used for other vegetatively propagated species.
Acknowledgments
We thank Dr Vesna Novak, Anja Ivanuš and Andreja Šober for technical assistance in the laboratory. This work would not have been possible without the financial support of the Research Project ‘New Agricultural Practices for Quality Production of Red Fruits Enriched in Healthy Compounds’ funded under FP7-SME, grant agreement ID: 262030.
References
- Akin, M., A. Nyberg, J. Postman, S. Mehlenbacher, and N.V. Bassil. 2016. A multiplexed microsatellite fingerprinting set for hazelnut cultivar identification. Eur. J. Hortic. Sci. 81(6):327–338. doi: https://doi.org/10.17660/ejhs.2016/81.6.6.
- Amsellem, L., C. Dutech, and N. Billotte. 2001. Isolation and characterization of polymorphic microsatellite loci in Rubus alceifolius Poir. (Rosaceae), an invasive weed in La Réunion island. Mol. Ecol. Notes 1(1–2):33–35. doi: https://doi.org/10.1046/j.1471-8278.2000.00013.x.
- Castillo, N.R.F., B.M. Reed, J. Graham, F. Fernández-Fernández, and N.V. Bassil. 2010. Microsatellite markers for raspberry and blackberry. J. Amer. Soc. Hort. Sci 135(3):1–8. doi: https://doi.org/10.21273/jashs.135.3.271.
- Congiu, L., M. Chicca, R. Cella, R. Rossi, and G. Bernacchia. 2000. The use of random amplified polymorphic dna (RAPD) markers to identify strawberry varieties: A forensic application. Mol. Ecol. 9:229–232. doi: https://doi.org/10.1046/j.1365-294x.2000.00811.x.
- Dice, L.R. 1945. Measures of the amount of ecologic association between species. Ecology. 26:297–302. doi: https://doi.org/10.2307/1932409.
- Dossett, M., N.V. Bassil, and C.E. Finn. 2012. SSR fingerprinting of black raspberry cultivars shows discrepancies in identification. Acta Hort. 946. doi: https://doi.org/10.17660/actahortic.2012.946.4.
- Doyle, J.J., and J.L. Doyle. 1987. A rapid isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 19(1):11–15.
- Ercisli, S., I. Badjakov, V. Kondakova, A. Atanassov, and E. Todorovska. 2008. AFLP-based genetic relationships in wild and cultivated red raspberry genotypes (Rubus idaeus L.). Biotechnol. Biotec. Eq. 22(4):907–910. doi: https://doi.org/10.1080/13102818.2008.10817576.
- FAOSTAT. 2020. Food and agricultural commodities production. 16 Sept 2020. http://faostat.fao.org/site/339/default.aspx.
- Fernández-Fernández, F., L. Antanaviciute, C.L. Govan, and D.J. Sargent. 2011. Development of a multiplexed microsatellite set for fingerprinting red raspberry (Rubus idaeus) germplasm and its transferability to other Rubus species. J. Berry Res. 1(4):177–187. doi: https://doi.org/10.3233/jbr-2011-019.
- Girichev, V., M.V. Hanke, A. Peil, and H. Flachowsky. 2015. SSR fingerprinting of a German Rubus collection and pedigree based evaluation on trueness-to-type. Genet. Resour. Crop Evol. 64:189–203. doi: https://doi.org/10.1007/s10722-015-0345-0.
- Graham, J., K. Smith, K. MacKenzie, L. Jorgenson, C. Hackett, and W. Powell. 2004. The construction of a genetic linkage map of red raspberry (Rubus idaeus subsp. idaeus) based on AFLPs, genomic-SSR and EST-SSR markers. Theor. App. Genet. 109:740–749. doi: https://doi.org/10.1007/s00122-004-1687-8.
- Graham, J., K. Smith, M. Woodhead, and J. Russel. 2002. Development and use of simple sequence repeat SSR markers in Rubus species. Mol. Ecol. 2:250–252. doi: https://doi.org/10.1046/j.1471-8286.2002.00203.x.
- Hayden, M.J., T.M. Nguyen, A. Waterman, and K.J. Chalmers. 2008. Multiplex-Ready PCR: A new method for multiplexed SSR and SNP genotyping. BMC Genomics. 9:80. doi: https://doi.org/10.1186/1471-2164-9-80.
- Hirata, C., T. Waki, K. Shimomura, T. Wada, S. Tanaka, H. Ikegami, Y. Uchimura, K. Hirashima, Y. Nakazawa, K. Okada, et al. 2020. DNA markers based on retrotransposon insertion polymorphisms can detect short DNA fragments for strawberry cultivar identification. Breed. Sci. 70:231–240. doi: https://doi.org/10.1270/jsbbs.19116.
- Honjo, M., T. Nunome, S. Kataoka, T. Yano, H. Yamazaki, M. Hamano, S. Yui, and M. Morishita. 2011. Strawberry cultivar identification based on hypervariable SSR markers. Breed. Sci. 61(4):420 425. doi: https://doi.org/10.1270/jsbbs.61.420.
- Jamali, S.H., J. Cockram, and L.T. Hickey. 2019. Insights into deployment of DNA markers in plant variety protection and registration. Theor. App. Genet. 132:1911–1929. doi: https://doi.org/10.1007/s00122-019-03348-7.
- Kalia, R.K., M.K. Rai, S. Kalia, R. Singh, and A.K. Dhawn. 2011. Microsatellite markers: An overview of the recent progress in plants. Euphytica. 177:309–334. doi: https://doi.org/10.1007/s10681-010-0286-9.
- Kunihisa, M. 2011. Studies using DNA markers in Fragaria × ananassa: Genetic analysis, genome structure, and cultivar identification. J. Japan Soc. Hort. Sci. 80(3):231–243. doi: https://doi.org/10.2503/jjshs1.80.231.
- Kunihisa, M., N. Fukino, and S. Matsumoto. 2003. Development of cleavage amplified polymorphic sequence (CAPS) markers for identification of strawberry cultivars. Euphytica. 134:209–215. doi: https://doi.org/10.1023/b:euph.0000003884.19248.33.
- Marshall, T.C., J. Slate, L.E.B. Kruuk, and J.M. Pemberton. 1998. Statistical confidence for likelihood-based paternity inference in natural populations. Mol. Ecol. Notes. 7:639–655. doi: https://doi.org/10.1046/j.1365-294x.1998.00374.x.
- Miyashita, T., H. Kunitake, N. Yotsukura, and Y. Hoshino. 2015. Assessment of genetic relationships among cultivated and wild Rubus accessions using AFLP markers. Sci. Hortic. 193:165–173. doi: https://doi.org/10.1016/j.scienta.2015.07.004.
- Noli, E., M.S. Teriaca, M.C. Sanguineti, and S. Conti. 2008. Utilization of SSR and AFLP markers for the assessment of distinctness in durum wheat. Mol. Breed. 22:301–313. doi: https://doi.org/10.1007/s11032-008-9176-4.
- Nybom, H., and G. Lācis. 2021. Large-scale genotyping and phenotyping of plant genetic resources of vegetatively propagated crops. Plants 10(2):1–30. doi: https://doi.org/10.3390/plants10020415.
- Perrier, X., and J.P. Jacquemoud-Collet. 2005. DARwin-5.0. Dissimilarity analysis and representation for Windows. User’s manual. CIRAD, Montpellier
- Pinczinger, D., M. von Reth, M.V. Hanke, and H. Flachowsky. 2020. SSR fingerprinting of raspberry cultivars traded in Germany clearly showed that certainty about the genotype authenticity is a prerequisite for any horticultural experiment. Eur. J. Hortic. Sci. 85(2):79–85. doi: https://doi.org/10.17660/ejhs.2020/85.2.1.
- Rafalski, J.A., and S.V. Tingey. 1993. Genetic diagnostics in plant-breeding– RAPDs, microsatellites and machines. Trends Genet. 9:275–280. doi: https://doi.org/10.1016/0168-9525(93)90013-8.
- Simlat, M., A. Ptak, A. Kula, and A. Orzel. 2018. Assessment of genetic variability among raspberry accessions using molecular markers. Acta Sci. Pol. Hortorum Cultus. 17(5):61–72. doi: https://doi.org/10.24326/asphc.2018.5.6.
- Šiško, M., B. Javornik, A. Šiftar, and A. Ivančič. 2009. Genetic relationships among Slovenian pears assessed by molecular markers. J. Amer. Soc. Hort. Sci. 134:97–108. doi: https://doi.org/10.21273/jashs.134.1.97.
- Umar, G., H.K. Vasanthaiah, D. Kambiranda, S.M. Basha, B.R. Phills, and W. Hunter. 2010. Assessment of genetic diversity among selected raspberry cultivars. Proc. Fla. State Hort. Soc. 123:26–28.
- UPOV (International Union for the Protection of New Varieties of Plants). 2006. Guidelines for the conduct of tests for distinctness, uniformity and stability: Raspberry - Rubus subg. Rubus. UPOV, Geneva.
- Wang, Z., Y. Wu, D.L. Martin, H. Gao, T. Samuels, and C. Tan. 2010. Identification of vegetatively propagated turf bermudagrass cultivars using simple sequence repeat markers. Crop Sci. 50:2103–2111. doi: https://doi.org/10.2135/cropsci2010.02.0116.