ABSTRACT
In recent years, the economic impact of the grape and wine industry within Texas has significantly increased. The majority of grapes grown in Texas are produced within the Texas High Plains American Viticultural Area (AVA). Vineyards within this AVA are subject to late spring frosts that may potentially reduce crop yields, and lower fruit quality. Objectives of this experiment were to compare the growth and fruitfulness of shoots grown from primary and secondary buds of V. vinifera ‘Grenache’ vines grafted onto three rootstocks (110 R, 1103P, and Freedom). Over two growing seasons, field-grown vines were exposed to the following treatments: primary bud growth and forced secondary bud growth (simulated late spring frost). Leaf gas exchange, pruning cane weight, vine yield, and fruit maturity data were collected each year. Bud treatment or rootstock did not influence leaf gas exchange. Cane weight and yield data were greater for shoots grown from primary bud shoots, and rootstock 1103P. In addition, berries grown from primary buds and 1103P rootstocks were more mature compared to fruits from secondary buds or vines grown on other rootstocks. Results offer insight into the potential interaction of ‘Grenache’ scions with grafted rootstocks during a late spring frost within the Texas High Plains AVA.
Introduction
Although the commercial viticulture industry within Texas is relatively young, within the United States Texas ranks fifth among all states in wine production (Townsend et al., Citation2016). In the mid-1980s, several American Viticultural Areas (AVAs) were established within Texas (Hellman et al., Citation2011). An AVA is a strictly geographically focused “delimited grape growing region distinguishable by geographical features, the boundaries of which have been recognized and defined” (Code of Federal Regulations, Citation2020). An AVA is one appellation allowed on American wine labels that provides consumers information on the growing location of grapes used in the wine (Hellman et al., Citation2011). Although several vineyards are located outside an AVA, there are currently eight AVAs within the state of Texas () (Takow et al., Citation2013; Townsend and Hellman, Citation2014).
Figure 1. Location of the eight American Viticultural Areas (AVA) in Texas: 1) Mesilla Valley, 2) Texas Davis Mountains, 3) Escondido Valley, 4) Fredericksburg in the Texas Hill Country, 5) Bell Mountain, 6) Texas High Plains, 7) Texas Hill Country, and 8) Texoma (Takow et al., Citation2013).
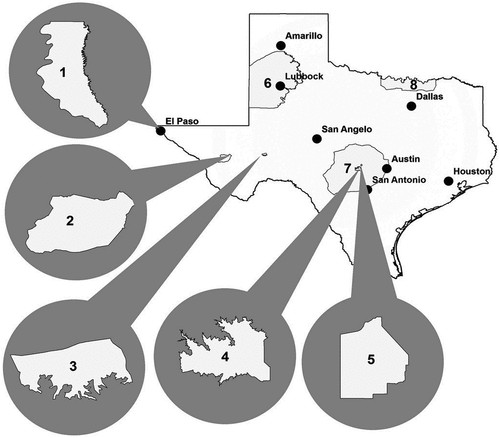
The vast majority of grape bearing hectares in Texas (60%), and grape production (73%) are located within the Texas High Plains AVA (United States Department of Agriculture, Citation2019). Because of the hot growing season climate, Hellman et al. (Citation2011) and Kamas (Citation2017) indicate the Texas High Plains AVA may be best suited for warm and hot climate grape cultivars. In addition, climate conditions within the Texas High Plains AVA (high wind speeds, thunderstorms, hail, extreme diurnal winter and spring temperatures) can be challenging for growing grapes (Lipe and Perry, Citation1988; Townsend and Hellman, Citation2014). Furthermore, because late spring frosts are common to Texas High Plains AVA vineyards, producers have implemented numerous mitigation strategies (cultivar and rootstock selection, late or double pruning, cover crop management, overhead winter irrigation, site selection, vine training, wind turbines, and heaters) to reduce post-budburst crop loss due to late spring frosts (Kamas, Citation2017; Lipe et al., Citation1992; Townsend and Hellman, Citation2014). However, despite implementing numerous precautions, late spring frosts continue to reduce yield in many Texas High Plains AVA vineyards (Lipe et al., Citation1992; Townsend and Hellman, Citation2014), and vineyards throughout the world (Evans et al., Citation2019; Friend et al., Citation2011; Frioni et al., Citation2017).
Rootstocks are frequently used in grape production to address soil biotic pests (phylloxora, nematodes, cotton root rot), and abiotic challenges (high pH, salinity, water logged soils, drought) (Fort et al., Citation2017; Fort and Walker, Citation2011; Moyer et al., Citation2018; Ollat et al., Citation2016). In addition, use of rootstocks may assist with above-ground vine challenges, such as timing of fruit maturity, fruit composition, yield, scion vigor, budburst, cold tolerance, disease tolerance or resistance, and vine physiology (Cousins et al., Citation2007; Moyer at al., Citation2018; Ollat et al., Citation2016; Padgett-Johnson et al., Citation2003).
Although it is thought 80% of vineyards throughout the world use grafted vines (Ollat et al., Citation2016; Tandonnet et al., Citation2010), there are likely between 70 and 80 rootstocks commonly used worldwide (Ollat et al., Citation2016). However, it is estimated that fewer than 10 rootstocks are grafted to the vast majority of vines (Ollat et al., Citation2016; Ruehl and Schmid, Citation2014). Today, most growers use several rootstocks to address interactions between scions, biotic, and abiotic vineyard stress factors (Fort et al., Citation2017; Moyer et al., Citation2018; Sharma and Upadhyay, Citation2004). Despite the large number of rootstocks available, today’s growers still face several biotic and abiotic stresses. Consequently, research has been ongoing and continues to seek better adapted, more tolerant, and resistant rootstocks (Bordenave et al., Citation2014; Heinitz et al., Citation2015; Ollat et al., Citation2016). One of the key challenges new growers face is to select rootstocks that are resistant or tolerant of abiotic and biotic stresses, and adapted to the specific vineyard site (Kamas, Citation2017; Ruehl and Schmid, Citation2014; Tandonnet et al., Citation2010). Although 1103P may lack resistance to some nematode species (Smith et al., Citation2016), within the Texas High Plains AVA commonly used rootstocks include 1103P, 110 R, SO4, and 420A. In addition, many Texas High Plains AVA growers prefer self-rooted (SR) vines (P. Helwi, personal communication).
Risk and incidence of damage due to late spring frost depends on plant susceptibility factors, minimum air temperature achieved, and length of time at, or below, a critical temperature (Friend et al., Citation2011). Generally, −2.0°C is considered the critical air temperature for injury of non-dormant grapevines (Barlow, Citation2010; Evans et al., Citation2019). However, vine tissue temperature can differ from air temperature (Evans et al., Citation2019; Leuning and Cremer, Citation1988). Therefore, additional variables such as grapevine cultivar, soil and surface moisture levels, presence of a cover crop, soil cultivation, soil temperature, presence and quantity of ice nucleating bacteria, and stage of vine phenological development may also affect the critical temperature of vine frost injury (Evans et al., Citation2019; Johnson and Howell, Citation1981; Sun et al., Citation2017). Friend et al. (Citation2011) report that damaged vines replace shoots killed in a late spring frost event at the cost of the depletion of vine carbohydrate reserves that negatively affects future yield and vine productivity. Therefore, late spring frost damage can reduce yield during the current growing season, and possibly reduce yield in the subsequent growing season (Stafne and Puckette, Citation2011; Trought et al., Citation1999).
Of the three compound buds found in each grapevine leaf axil, primary buds exhibit the least amount of hardiness, while tertiary buds (the smallest of the three compound buds) are the most hardy. Although primary buds are the least hardy, primary buds are the most fruitful of the three compound buds (Barlow, Citation2010; Dry, Citation2000). Therefore, when compared to vines unaffected by late spring frosts, vines damaged by late spring frosts tend to have lower yields (fruits borne primarily from secondary buds) (Barlow, Citation2010; Jones et al., Citation2010; Sánchez and Dokoozlian, Citation2005). In addition, shoots from secondary buds generally have lower cane weights and fewer clusters, but may have similar cluster mass, berries per cluster, berry weight, and fruit composition (Barlow, Citation2010; Evans et al., Citation2019; Jones et al., Citation2010).
Because frost damaged primary shoots are generally replaced by shoots produced from quiescent, secondary buds (Friend et al., Citation2011), grape growers desire to know the disparity of grapevine fruitfulness and fruit maturity between primary and secondary bud shoot growth. In addition, Texas High Plains AVA grape producers require knowledge of the influence rootstocks may have on scion fruit maturity and fruitfulness following a late spring frost event. However, because late spring frost events are challenging to predict and generally damage most shoots produced from primary buds, the extent of fruitfulness and fruit maturity differences between shoots grown from primary, and shoots grown from secondary buds, have not been adequately described for V. vinifera grape cultivars grown within the Texas High Plains AVA. Therefore, the goal of this study was to expose grafted, field-grown, Texas High Plains AVA V. vinifera ‘Grenache’ vines to simulated frost injury, and compare impact a simulated late spring frost event may have on gas exchange, growth, yield, and fruit maturity of simulated and non-simulated frost damaged vines.
Materials and Methods
Experimental Site
Experiments were conducted at the Texas A&M AgriLife Research and Extension Center, Lubbock, TX (lat. 33°41ʹ33”N, long. −101°49ʹ17”W). The vineyard is located within the US. Department of Agriculture hardiness zone 7b (United States Department of Agriculture, Citation2012) and is approximately 981 m above sea level. Research was conducted during the 2016 and 2017 growing seasons. Soil characteristics for the vineyard site are a deep, well-drained Olton series of fine sandy loam, and have been described previously (Lipe and Perry, Citation1988).
Experimental Site Weather Conditions
Weather data (precipitation, air temperature) were collected using an onsite weather station (Campbell Scientific, Logan, UT). Each year, growing degree days (GDD) were calculated from 1 April to 31 October (Moyer et al., Citation2018). To calculate daily GDD units, base temperature (10°C) was subtracted from the average daily temperature. If GDD calculations resulted in a negative number, the negative number was reset to zero (Moyer et al., Citation2018).
Plant Material
Experimental vines were established V. vinifera ‘Grenache’ FPS 06 vines, grafted to 110 R, 1103P, and Freedom rootstocks. Though ‘Grenache’ tends to produce poor color in many Texas vineyards, ‘Grenache’ is thought to be adapted to hot climates found within the High Plains AVA (Hellman et al., Citation2011; Kamas, Citation2017). In addition, ‘Grenache’ is generally not used as a varietal in Texas wines, but is commonly grown within the High Plains AVA and is frequently used to produce blends with other Rhone cultivars (Hellman et al., Citation2011; Kamas, Citation2017). 110 R and 1103P are rootstocks commonly used within the Texas High Plains AVA. Freedom is well adapted to the sandy, well drained soils common to the region (Kamas, Citation2017; Montague et al., Citation2020), and rootstock characteristics have been described previously (Ibacache et al., Citation2016; Kamas, Citation2017). Vines were planted in 2004 at a 1.8 × 3 m vine by row spacing, with a north-south row orientation. Vines were bilateral cordon trained (cordons established 1 m above soil), and spur-pruned to four or five spurs for each cordon, and two buds for each spur. Canopies were managed in a sprawl configuration, with single foliage catch wires at ≈ 15 cm and ≈ 35 cm above each cordon (Plank et al., Citation2019).
Bud Treatments
Each year, initial dormant pruning was completed 1 to 2 weeks prior to budburst. To simulate late spring frost damage in new shoots (secondary shoot treatment, SST), following initial dormant bud pruning, shoot growth was allowed to grow until new shoots reached 15 cm in length. At this time, the initial 15 cm of growth from primary buds was removed by pruning, and secondary buds were induced to break (Schrader et al., Citation2019). Shoots for control plants (primary shoot treatment, PST) were not pruned after initial dormant pruning. To simulate late spring frost damage for consecutive growing seasons, individual vines received the same treatment for two consecutive growing seasons. Initial dormant pruning dates for each growing season were noted ().
Table 1. Annual growing degree day (GDD) accumulation, precipitation, minimum temperataure, maximum temperature, mean minimum temperature, and mean maximum temperautre from an on-site weather station located at the Texas AgriLife Experiment Station (Lubbock, TX) during the 2016 and 2017 growing seasons. In addition, secondary shoot pruning date and harvest date for ‘Vitis vinifera ‘Grenache’ grafted to grafted to 1103P, 110 R, and Freedom rootstocks each growing season
Experimental Design
Vines were organized in a randomized complete block design with three blocks within one vineyard row. Within each block, there were four adjacent vines of each rootstock. Within adjacent rootstock vines, PST, and secondary shoot treatment SST vines were randomly assigned to two vines. Therefore, there were 36 total vines, and six vines for each rootstock shoot treatment combination. Data were collected from each vine within each block. Prior to, and during experiment years, vines were irrigated through drip irrigation lines, and managed according to viticultural practices common for the Texas High Plains AVA (Kamas, Citation2017; Townsend and Hellman, Citation2014).
Gas Exchange Measurements
During three cloudless days in 2016 (6 May, 13 June, and 25 July), and three cloudless days in 2017 (6 June, 20 July, and 7 August), midday (1 hour on either side of solar noon) leaf stomatal conductance (gs), transpiration (E), leaf to air vapor pressure deficit (LVPD), and CO2 assimilation (A) were estimated using two LI-6400 XT infrared gas analyzers (IRGA) (Li-Cor Biosciences Inc., Lincoln, NE). Each IRGA was equipped with a 6400–02B red/blue external LED light source, and CO2 mixer. To simulate ambient growing conditions, during each daily measurement period light intensity within each LI-6400 XT chamber was maintained at ambient light conditions, and chamber reference CO2 was sustained at 400 ppm. In addition, prior to and several times during daily measurement periods, each chamber was clamped to a nearby non-sample leaf, and leaf temperature, and LVPD were estimated. Conditions within each chamber were then set to closely represent these ambient conditions (Soar et al., Citation2009). Using each IRGA, gas exchange data were measured on two arbitrarily selected leaves from a randomly selected block × rootstock × bud treatment combination. Leaves selected for gas exchange estimates were full sun, recently matured leaves (7th to 9th node from the tip of the shoot) (Padgett-Johnson et al., Citation2003). Measurements then continued on random vines within the block. Once measurements were completed on all vines within the first block, another block was randomly selected, and measurements were completed as described until gas exchange had been measured on each vine in the experiment (144 total gas exchange measurements each measurement day).
Berry Maturity and Shoot Vigor Measurements
Fruit maturity was determined by berry juice assays of total soluble solids (TSS). To estimate TSS (°Brix) content, 50 berries from each vine were sampled from top, middle, and bottom of random clusters. Berries were sealed in zipper locked plastic bags, placed in a cooler with ice, and brought to an onsite lab. Berries were juiced with a benchtop juicer, juice was poured in test tubes, and test tubes were centrifuged for 3 minutes. Berry juice TSS content was then measured with a temperature compensating refractometer (3150 Smart Refractometer; ATAGO, Bellevue, WA). Berries were considered to be at harvest maturity when TSS of PST fruit were 22 to 24 °Brix (Boulton et al., Citation1999; Moyer et al., Citation2018). Following harvest, individual berry weight (50 berries from each vine), number of clusters per vine, cluster weight, and individual vine yield were measured and recorded. During spring of 2017 and 2018 dormant cane weights were measured for each vine (Moyer et al., Citation2018). Yield to cane weight ratio (Ravaz Index) was calculated for each individual vine.
Statistical Analysis
Daily gas exchange, cane weight, yield, and fruit data treatment (rootstock, SST, and PST) means were exposed to analysis of variance using the General Linear Models procedure appropriate for a randomized complete block design (SAS version 9.4, SAS Institute, Cary, NC). For each growing season, gas exchange daily means indicated similar trends (no interaction found for data from separate years). Therefore, daily gas exchange data from within each growing season were pooled. However, gas exchange data from 6 May 2016 (prior to instigating SST) were not pooled with other gas exchange dates. In addition, pre-SST mid-day leaf gas exchange data for the 2017 growing season were not available for analysis. Cane weight, yield, Ravaz, index, and fruit data means from 2016 to 2017 followed similar trends for each growing season (no interaction found for data from separate years). Therefore, these variables were also pooled for statistical analysis. If mean differences were detected, least square means were separated by Tukey-Kramer’s procedure (α = 0.05). Pooled least square means of pruning weight, berry yield, Ravaz Index, cluster weight, number of clusters from each vine, and TSS were plotted by bud treatment and rootstock (no treatment × rootstock interactions). However, for berry weight data, there was a treatment × rootstock interaction.
Results
Meteorological Parameters
Growing degree data from each experimental growing season (1 April–31 October) indicate the 2016 growing season was slightly warmer when compared to the 2017 growing season (). However, the greatest air temperature recorded during the experiment (44.4°C) was recorded 17 June 2017. In addition, during the 2017 growing season approximately 36% more precipitation fell when compared to the 2016 growing season ().
Leaf Gas Exchange
For each rootstock, initial (pre-SST) gas-exchange measurements from 2016 indicate no differences between SST and PST for leaf A, gs, LVPD, or E measurements (data not shown). In addition, pooled (2016 and 2017) leaf gas exchange data indicated leaf gas exchange did not differ between PST and SST vines, or rootstocks (Table S1 of Supplementary Materials).
Vine Growth, Yield, and Berry Maturity
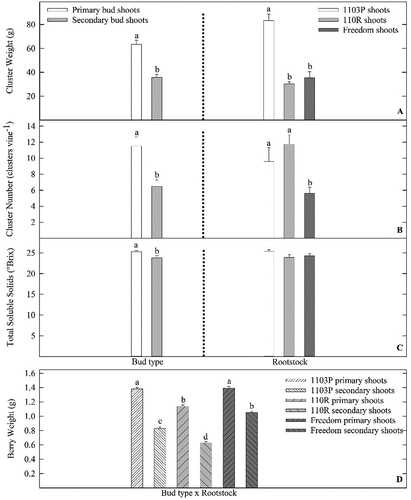
Cane weights were greatest for vines grown from PST buds and vines grown on 1103P rootstocks (). Yield was nearly 66% greater for PST vines compared to SST vines. Compared to 1103P, yield for 110 R and Freedom vines was 45% and 75% less, respectively (). Ravaz Index also was greater for PST vines when compared to SST vines (). Furthermore, Ravaz Index values for 1103P and 110 R vines were greater when compared to Freedom vines. Similar trends were found for fruit characteristics. Cluster weight was 40% greater for PST vines when compared to SST vines, and cluster weight for 1103P vines was 65% greater when compared to 110 R and Freedom vines (). Number of clusters produced by each vine was greatest for PST, 1103P, and 110 R vines. Vine bud growth treatment (PST or SST) and rootstock had a significant interaction on vine berry mass (). Individual berry mass was greatest for PST vines grown on 1103P and Freedom rootstocks, while intermediate berry mass was found on 110 R PST, and Freedom SST vines. SST vines grafted to 110 R rootstocks produced fruit with the least berry weight (). Fruit TSS for vines exposed to the SST treatment had 10% lower °Brix when compared to PST vines. TSS did not differ among rootstocks ().
Figure 2. Influence of primary shoot treatment (PST) or secondary shoot treatment (SST) bud shoot growth and rootstock on cane weight (A), vine yield (B), and Ravaz Index (C) of Vitis vinifera ‘Grenache’ vines grown on 1103P, 110 R, and Freedom rootstocks at the Texas AgriLife vineyard in Lubbock, TX. Data pooled from 2016 and 2017 growing seasons. Letters above each bar represent differences between bud type, or rootstock (LS Means, Tukey–Kramer test, P ≤ 0.05.). Only main effects (no bud type x rootstock interaction) presented. Errors bars represent SE of the mean.
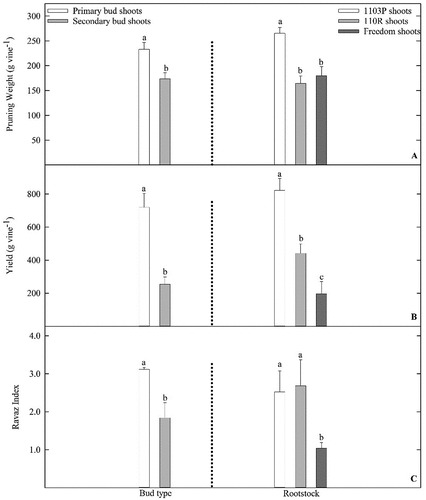
Figure 3. Influence of primary shoot treatment (PST) or secondary shoot treatment (SST) bud shoot growth and rootstock on cluster weight (A), number of cluster from each vine (B), and berry total soluble solids (°Brix) (C) of Vitis vinifera ‘Grenache’ vines grown on 1103P, 110 R, and Freedom rootstocks at the Texas AgriLife vineyard in Lubbock, TX. In addition, influence of bud type x rootstock interaction on individual berry weight (D). Data pooled from 2016 and 2017 growing seasons. Letters above each bar represent differences between main effects (A, B, C), or bud type x rootstock interaction (D) (LS Means, Tukey–Kramer test, P ≤ 0.05.). Errors bars represent SE of the mean.
Discussion
Meteorological Parameters
Mean, yearly precipitation for the Texas High Plains AVA ranges from 41.4 to 63.7 cm (Hellman et al., Citation2011), while the mean annual precipitation for the experimental vineyard is 48.5 cm (National Oceanic and Atmospheric Administration, Citation2020). Therefore, precipitation each year of the experiment was less than mean annual precipitation (). In addition, mean cumulative GDD (10°C) for the Texas High Plains AVA ranges from 2,028 to 2,653 (Hellman et al., Citation2011). During the 2016 growing season, cumulative GDD was 2,779, while in 2017 cumulative recorded GDD was 2,644 (). Although weather during each growing season of the experiment was near average, data indicate variability between growing seasons, which is typical for weather within the Texas High Plains AVA (Kamas, Citation2017; Widrlechner et al., Citation2012).
Leaf Gas Exchange
Earlier studies indicate vine leaf gas exchange may respond to rootstock (Düring, Citation1994; Iacono et al., Citation1998), or scion cultivar (Bota et al., Citation2016; Tomás et al., Citation2012), and foliage gas exchange may have a scion by rootstock interaction (Padgett-Johnson et al., Citation2003; Tramontini et al., Citation2013). Düring (Citation1994) reported field grown ‘Riesling’ vines grafted onto 5BB and SO4 rootstocks had greater A, and gs when compared to SR ‘Riesling’ vines. However, Williams and Smith (Citation1991) found few gas exchange differences on field grown ‘Cabernet Sauvignon’ vines grafted to A x R1, St. George, and 5 C rootstocks. Various authors report sensitivity of grape foliage, yield, or fruit composition to late spring frosts and primary bud damage (Evans et al., Citation2019; Frioni et al., Citation2017; Moyer et al., Citation2018). Montague et al. (Citation2020) found leaf gas exchange differences between primary and secondary buds grown on ‘Grenache’ and ‘Cabernet Sauvignon’ scions grafted to the same rootstock (110 R). However, the current study appears to be the first research indicating vine leaf gas exchange does not differ between leaves from primary and secondary shoots grown on the same cultivar, but differing rootstocks (Table S1 of Supplementary Materials). Because rootstocks are adapted to numerous edaphic soil factors and soil moisture conditions (Ollat et al., Citation2016; Tandonnet et al., Citation2010), genetic factors (density of roots, influence of rootstock on scion water status, gas exchange, yield, nutrient translocation, etc.) associated with each rootstock are expressed differently with each scion and vineyard soil characteristic (Düring, Citation1994; Kodur et al., Citation2010; Williams and Smith, Citation1991). Therefore, in this study, it appears rootstock differences did not influence leaf gas exchange of PST or SST shoots, and ‘Grenache’ leaf gas exchange is likely more dependent upon a combination of leaf age (Poni et al., Citation1994; Shultz et al., Citation1996), leaf shoot position (Poni et al., Citation1994), leaf area (Poni and Giachino, Citation2000), leaf exposure to sunlight (Chiarawipa et al., Citation2012), and source to sink relationships (Petrie et al., Citation2000). In addition, leaf gas exchange means from this study appear to be similar when compared to leaf gas exchange data from previous studies of irrigated vines (Bota et al., Citation2016; Santesteban et al., Citation2009; Tomas et al., Citation2012). Although vine water status and irrigation volume were not directly measured, because experimental SST vines were irrigated with the same volume as PST vines, experimental vines likely would not have been exposed to water stress.
Vine Growth, Yield, and Berry Maturity
Relatively few studies directly compare cane weight data from primary and secondary bud shoots (Creasy and Creasy, Citation2018). The mean length of shoots (a representation of cane weight) grown from primary buds of five-year old, interspecific hybrid (‘Marquette’) vines were 28% greater when compared to the mean length of shoots grown from secondary buds (Frioni et al., Citation2017). In addition, Jones et al. (Citation2010) indicated cane weights from 20 year old, spur pruned ‘Pinot Noir’ vines grown from secondary buds had 47% less weight when compared to vines grown from primary buds. These results are comparable to our data in which mean cane weights from shoots grown from PST buds was nearly 27% greater when compared to mean cane weights of shoots grown from SST buds ().
In addition to lower cane weights, mean vine yield was less for shoots grown from SST buds, compared to shoots of PST buds (). Once again, the literature indicates a decrease in yield would be expected when primary buds are damaged during late spring frosts, and vineyard yield is dependent upon secondary bud fertility (Barlow, Citation2010; Friend et al., Citation2011; Frioni et al., Citation2017). Low fruitfulness of secondary buds is likely related to the lack of dormant inflorescence primordia found in secondary buds of V. vinifera cultivars (Sánchez and Dokoozlian, Citation2005). Reduced numbers of inflorescence primordia would lead to fewer flower clusters, fewer flowers, lower cluster weights, and lower yield for SST vines (Friend et al., Citation2011; Frioni et al., Citation2017; Jones et al., Citation2010).
Furthermore, across PST and SST vines, a rootstock effect was found in terms of cane weight and yield (). Whether shoots from scions grafted to rootstocks exhibit greater, or lesser yield compensation is likely rootstock dependent (Kodur et al., Citation2010; Williams and Smith, Citation1991). In the current experiment, although no effects of rootstock on gas exchange rates were found (Table S1 of Supplementary Materials), when compared to shoots grown on 110 R and Freedom rootstocks, it is apparent photosynthates were preferentially allocated to shoots and fruit grown on 1103P rootstocks (Petrie et al., Citation2000) (). This indicates carbon assimilation rate is likely not a critical variable explaining the strong rootstock effect on cane weights, yield, and Ravaz Index (Tadonnet et al., Citation2010). These data indicate vine yield appears to be highly dependent upon rootstock (), and growers should be aware of possible rootstock × scion yield differences.
Ravaz Index is often used as an expression of vine balance (Reynolds and Vanden Heuvel, Citation2009). For V. vinifera cultivars, crop loads in the range of 5 to 10 are often the goal (Bravdo et al., Citation1984, Citation1985), but vine balance is known to differ with cultivar, training system, climate, and soil type (Reynolds and Vanden Heuvel, Citation2009; Scheiner et al., Citation2020). For many grape cultivars, it is thought crop loads above 12 (over cropping) delay fruit maturation, and compromise wine composition (reduced color, titratable acidity, and proline concentrations) (Bravdo et al., Citation1984, Citation1985). In the current study, yield and cane weights of PST, and 1103P shoots were greater when compared to yield and cane weights of SST, 110 R, and Freedom shoots, respectively (). In addition, the ratio of yield to cane weight differed between PST and SST vines, and Ravaz Index was less for Freedom vines when compared to 1103P and 110 R vines (). For numerous grape cultivars, previous work indicates an increase in vine balance ratio is mainly related to an increase in vine yield, and a decrease in vine cane weight (Reynolds and Vanden Heuvel, Citation2009; Scheiner et al., Citation2020). However, for SST vines compared to PST vines, Ravaz Index decreased because yield was reduced in relation to lower cane weights. The same may be said for Ravaz Index of Freedom shoots compared to 1103P and 110 R shoots (). In general, when compared to SST vines, PST vines had greater vigor and were more fruitful. Similar results are true for 1103P vines when compared to 110 R, and Freedom vines (). These data are an indication that within the Texas High Plains AVA, the propensity of primary buds, and ‘Grenache’ vines grafted to 1103P rootstocks, is to produce greater shoot growth, and have greater bud fertility when compared to secondary buds, and vines grown with Freedom or 110 R rootstocks. In different locations and with differing plant material, others (Dry, Citation2000; Friend et al., Citation2011; Jones et al., Citation2010) found similar results. These data indicate understanding the role of vine carbon allocation (source to sink relationship), rootstock, and scion genotype is critical for proper vine management and vineyard production (Kodur et al., Citation2010; Petrie et al., Citation2000; Williams and Smith, Citation1991).
For all vines, overall yield was closely related to cluster mass (PST and 1103P vines produced clusters with greater mass), number of clusters produced from each vine (PST, 1103P, and 110 R vines produced the greatest number of clusters), and berry mass (largest berries were produced by PST, 1103P, and Freedom vines) (). It is interesting to note when comparing yield characteristics across rootstock treatments, 1103P vines had greater yield and greater cluster weights. However, 1103P and 110 R vines produced a similar number of clusters. It is known rootstocks influence scion water status, nutrient uptake, and dry matter partitioning (Kodur et al., Citation2010; Williams and Smith, Citation1991). In addition, rootstock effect on scion budburst, anthesis, fruit set, fruit ripening, and yield is documented (Menora et al., Citation2015). Greater yield, cluster weights, and cluster numbers found on vines grafted to 1103P rootstocks (, 3) could likely be attributed to a combination of the influence of rootstock on scion flowering, fruit set, vigor, or competition for carbon assimilates (Ferroni and Scalabrelli, Citation1995; Menora et al., Citation2015).
Furthermore, each rootstock had greater berry weights for PST fruit when compared to SST fruit (). When compared to shoots grown from primary buds, smaller clusters from shoots grown from secondary buds are recognized (Dry, Citation2000). However, others have found cluster and berry mass of fruit grown from secondary buds and primary buds do not differ (Barlow, Citation2010; Evans et al., Citation2019; Friend et al., Citation2011). Our data indicate vine balance and yield (cluster mass, clusters produced from each vine, and berry mass) response to SST and PST tends to be genotype specific (Kodur et al., Citation2010; Williams and Smith, Citation1991), and relates well to previous research (Jones et al., Citation2010; Sánchez and Dokoozlian, Citation2005; Stafne and Puckett, Citation2011). Lack of secondary bud fruitfulness is likely related to absence of dormant inflorescence primordia found within secondary buds of V. vinifera (Sánchez and Dokoozlian, Citation2005). When extrapolated by row and vine spacing, mean yield for each vine treatment or cultivar are much lower than expected yield for Texas High Plains AVA commercial vineyards (6.7 to 8.9 ton/ha) (Kamas, Citation2017). However, historical yield data from the experimental vineyard is traditionally low. Lack of yield for the experimental vineyard, and for the present study, is likely due to low bud density, and close proximity of the experimental vineyard to trees and hedgerows, and challenges associated with managing vineyards near high bird populations (Pagay et al., Citation2013).
As with gas exchange variables, berry fruit maturity (TSS) did not differ between rootstocks. However, TSS differed for berries from PST and SST shoots (). While vegetative growth is a strong carbohydrate pre-veraison sink, during post-veraison berries become the main carbohydrate sink (Kamas, Citation2017; Petrie et al., Citation2000). In addition, Kodur et al. (Citation2010) indicate 1103P rootstocks tend to have greater vigor when compared to Freedom and 110 R rootstocks. Kasimatis and Kissler (Citation1974) report TSS for berries from primary and secondary buds did not differ for many grapevine cultivars including ‘Grenache.’ Therefore, not finding TSS differences between 1103P, 110 R, and Freedom rootstocks is likely related to genetic differences and allocation of carbohydrates pre (shoots are strong sinks for 1103P) and post-veraison (fruit are strong sinks for 1103P, 110 R, and Freedom).
Current and previous research indicate maturation of fruit from secondary buds is likely to be delayed compared to maturation of fruit from primary buds (Barlow, Citation2010). In the present study, leaves from each rootstock, PST, and SST vines had similar leaf gas exchange parameters (Table S1 of Supplementary Materials). For SST vines, the need to replace shoots, leaves, inflorescences, and fruit lost from simulated late spring frosts likely redirected carbon partitioning, and further stressed limited vine carbohydrate reserves (Stafne and Puckett, Citation2011; Trought et al., Citation1999). In addition, amount of sunlight received by primary buds has been shown to have a significant effect on inflorescence primordia size (Sánchez and Dokoozlian, Citation2005). Due to lower cane weights (), SST vines likely had fewer shoots, less leaf area, and a more open canopy when compared to PST vines (Frioni et al., Citation2017; Jones et al., Citation2010). However, quantity of sunlight does not appear to have an influence on size of inflorescence primordia for secondary buds (Dry, Citation2000; Sánchez and Dokoozlian, Citation2005). Therefore, when compared to berries from primary buds, berries from secondary buds are likely a weaker sink for carbohydrates produced from leaf A.
Throughout the world, vineyards are subjected to yield losses, and management challenges related to late spring frosts. Our research reveals that for ‘Grenache’ vines grown within the Texas High Plains AVA, shoots from secondary buds produced 25% less growth, 40% less berry weight, 44% fewer clusters from each vine, and 64% less total vine yield when compared to shoots from primary buds. In a similar manner, rootstock selection can influence vine shoot growth, berry weight, cluster weight, and yield. These data are likely the first to compare primary and secondary bud shoot gas exchange, growth, and fruit characteristics of a single grape cultivar grafted to three different rootstocks. Although under actual late spring frost condition vines may have differing results, under these simulated late spring frost conditions we conclude ‘Grenache’ scions grafted to 1103P rootstocks produced vines with greater shoot growth, berry weight, cluster weight, clusters from each vine, and yield compared to ‘Grenache’ vines grown on 110 R or Freedom rootstocks. As previously detailed, yield data for this study were less than commonly found within Texas High Plains AVA commercial vineyards. However, despite low experiment yields, we believe trends for SST, PST, and rootstocks are valid, and indicate how ‘Grenache’ vines and examined rootstocks interact in relation to vine gas exchange, growth, and fruit production. Furthermore, in response to late spring frosts, it is likely similar interactions will occur for other grapevine scions and rootstocks. Grape producers now have initial information on response of ‘Grenache’ scions grafted to several rootstocks in response to a simulated late spring frost. Further research investigating response of additional cultivars and rootstocks to late spring frosts will likely improve grower insight when selecting vines to plant within areas subjected to late spring frosts.
Supplemental Material
Download MS Word (19.8 KB)Acknowledgments
Research presented is a portion of Emily Graff’s thesis project and was partially supported by funds provided by Texas AgriLife Research and Extension, and State of Texas Viticulture and Enology Research, Education, and Engagement Funding.
Disclosure statement
Mention of a trademark, proprietary product, or vendor does not constitute a guarantee of warranty of the product by Texas Tech, or Texas A&M University, and does not imply its approval to the exclusion of other products, or vendors that also may be suitable.
Supplementary Material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Barlow, S. 2010. Improved frost management in the Goulburn & Yarra Valleys. Final report to Grape and Wine Research and Development Corporation, Project Number RT 06/04-1. University of Melbourne: Melbourne, Victoria, Australia.
- Bordenave, L., J.P. Tandonnet, S. Decroocq, E. Marguerit, S. Cookson, D. Esmenjaud, and N. Ollat. 2014. Wild Vitis as a germplasm resource for rootstocks. Exploitation of autochthonous and more used vines varieties – Oenoviti International Network I. Geisenheim, Germany. https://www.researchgate.net/profile/Nathalie_Ollat/publication/329357525_Wild_vitis_as_a_germplasm_resource_for_rootstocks/links/5c03a9a345851523d1593ad3/Wild-vitis-as-a-germplasm-resource-for-rootstocks.pdf (accessed 10 November, 2020).
- Bota, J., M. Tomás, J. Flexas, H. Medrano, and J.M. Escalona. 2016. Differences among grapevine varieties in their stomatal behavior and water use efficiency under progressive water stress. Agricultural Water Management. 164:91–99. doi: 10.1016/j.agwat.2015.07.016.
- Boulton, R.B., V.L. Singleton, L.F. Bisson, and R.E. Kunkee. 1999. Principles and practices of winemaking. Springer Science+Business Media, Inc, New York, NY.
- Bravdo, B., Y. Hepner, C. Loinger, S. Cohen, and H. Tabacman. 1984. Effect of crop level on growth, yield and wine quality of a high yielding Carignane vineyard. Am. J. Enol. Vitic. 35:247–252.
- Bravdo, B., Y. Hepner, C. Loinger, S. Cohen, and H. Tabacman. 1985. Effect of crop level and crop load on growth, yield, must, and wine composition, and quality of Cabernet Sauvignon. Am. J. Enol. Vitic. 36:125–131.
- Chiarawipa, R., Y. Wang, X.Z. Zhang, Z.H. Han, and M. Rueangkhanab. 2012. Modeling light acclimation of photosynthetic response in different ages of vine leaves. Acta Hortic. 956(956):255–260. doi: 10.17660/ActaHortic.2012.956.28.
- Code of Federal Regulations. 2020. https://ecfr.federalregister.gov/current/title-27/chapterI/subchapter-A/part-4/subpart-C/section-4.25 accessed 9 November, 2020.
- Cousins, P., D. Johnston, S. Switras-Meyer, L. Boyden, J. Vidmar, and C. Meyer. 2007. USDA ARS research in grape rootstock breeding and genetics. Acta Hortic. 733(733):51–58. doi: 10.17660/ActaHortic.2007.733.5.
- Creasy, G.L., and L.L. Creasy. 2018. Grapes. 2nd ed. CABI. Oxfordshire, UK.
- Dry, P.R. 2000. Canopy management for fruitfulness. Australian Journal of Grape and Wine Research 6(2):109–115. doi: 10.1111/j.1755-0238.2000.tb00168.x.
- Düring, H. 1994. Photosynthesis of ungrafted and grafted grapevines: Effects of rootstock genotype and plant age. Am. J. Enol. Vitic. 45:297–299.
- Evans, K.J., P.K. Bricher, and S.D. Foster. 2019. Impact of frost injury incidence at nodes of Pinot Noir on fruitfulness and growth-stage lag. Australian Journal of Grape and Wine Research 25(2):201–211. doi: 10.1111/ajgw.12381.
- Ferroni, G., and G. Scalabrelli. 1995. Effect of rootstock on vegetative activity and yield in grapevine. Acta Hortic. 388(388):37–42. doi: 10.17660/ActaHortic.1995.388.5.
- Fort, K., J. Fraga, D. Grossi, and M.A. Walker. 2017. Early measures of drought tolerance in four grape rootstocks. Journal of the American Society for Horticultural Science 142(1):36–46. doi: 10.21273/JASHS03919-16.
- Fort, K., and A. Walker. 2011. Breeding salt tolerant rootstocks. Foundation Plant Services Grape Program Newsletter. October. http://iv.ucdavis.edu/files/134523.pdf (accessed 9 November, 2020).
- Friend, A.P., M.C.T. Trought, C. Stushnoff, and G.H. Wells. 2011. Effect of delaying budburst on shoot development and yield of Vitis vinifera L. Chardonnay ‘Mendoza’ after a Spring Freeze Event. Australian Journal of Grape and Wine Research. 17:378–382.
- Frioni, T., A. Green, J.E. Emling, S. Zhuang, A. Palliotti, P. Sivilotti, R. Falchi, and P. Sabbatini. 2017. Impact of spring freeze on yield, vine performance and fruit quality of Vitis interspecific hybrid Marquette. Sci. Hortic. 219:302–309. doi: 10.1016/j.scienta.2017.03.026.
- Heinitz, C.C., K. Fort, and M.A. Walker. 2015. Developing drought and salt resistant grape rootstocks. Acta Hortic. 1082(1082):305–312. doi: 10.17660/ActaHortic.2015.1082.42.
- Hellman, E.W., E.A. Takow, M.D. Tchakerian, and R.N. Coulson. 2011. Geographic information system characterization of four appellations in West Texas, USA. Geoscience Canada 38:6–20.
- Iacono, F., A. Buddella, and E. Peterlunger. 1998. Water stress and rootstock influence on leaf gas exchange of grafted and ungrafted grapevines. Sci. Hortic. 75(1–2):27–39. doi: 10.1016/S0304-4238(98)00113-7.
- Ibachace, A., F. Albornoz, and A. Zurita-Silva. 2016. Yield responses in Flame seedless, Thompson seedless and Red Globe table grape cultivars are differentially modified by rootstocks under semi arid conditions. Scientia Horticulturae. 25–32.
- Johnson, D.E., and G.S. Howell. 1981. The effect of cane morphology and cultivar on the phenological development and critical temperatures of primary buds of grape vines. Journal of the American Society for Horticultural Science 106:545–549.
- Jones, J.E., S.J. Wilson, G. Lee, and A.M. Smith. 2010. Effect of frost damage and pruning on current crop and return crop of Pinot Noir. New Zealand Journal of Crop and Horticultural Science 3(3):209–216. doi: 10.1080/01140671.2010.498402.
- Kamas, J. 2017. Growing grapes in Texas: From the commercial vineyard to the backyard vine. 2nd ed. Texas A&M University Press, College Station.
- Kasimatis, A.N., and J.J. Kissler. 1974. Responses of grapevines to shoot break-out following injury by spring frost. Am. J. Enol. Vitic. 25:17–20.
- Kodur, S., J.M. Tisdall, C. Tang, and R.R. Walker. 2010. Accumulation of potassium in grapevine rootstocks (Vitis) as affected by dry matter partitioning, root traits and transpiration. Australian Journal of Grape and Wine Research 16:272–282.
- Leuning, R., and K.W. Cremer. 1988. Leaf temperatures during a radiation frost. I. Observations. Agricultural and Forest Meteorology. 42: 1221–133.
- Lipe, W.N., L. Baumhardt, C.W. Wendt, and D.J. Rayburn. 1992. Differential thermal analysis of deacclimating Chardonnay and Cabernet Sauvignon grape buds as affected by evaporative cooling. Am. J. Enol. Vitic. 43:355–361.
- Lipe, W.N., and R.L. Perry. 1988. Effects of rootstocks on wine grape scion vigor, yield, and juice quality. HortScience 23:317–321.
- Menora, N.D., V. Joshi, V. Kumar, D. Mijaya, M.K. Debnath, S. Pattanashetty, A.S. Padmavathamma, M.T. Variath, S. Biradar, and S. Khadakabhavi. 2015. Influence of rootstock on bud break, period of anthesis, fruit set, fruit ripening, heat unit requirement and berry yield of commercial grape varieties. International Journal of Plant Breeding and Genetics 9(3):126–135. doi: 10.3923/ijpbg.2015.126.135.
- Montague, T., E. Graff, and S. Kar. 2020. Secondary bud gas exchange, growth, and fruitfulness of Vitis vinifera L. cultivars, ‘Grenache’ and ‘Cabernet Sauvignon’ grown on the Texas High Plains. Viticulture Data Journal. 2:e60430. doi: 10.3897/vdj.2.e60430.
- Moyer, N.M., J. King, and G. Moulton. 2018. Evaluation of rootstocks on harvest metrics of ‘Pinot noir clone 02A’ wine grapes in maritime Western Washington. HortTechnology 28(6):830–835. doi: 10.21273/HORTTECH04170-18.
- National Oceanic and Atmospheric Administration. 2020. https://www.ncdc.noaa.gov/cdo-web/. (accessed 9 November, 2020).
- Ollat, N., L. Bordenave, J.P. Tandonnet, J.M. Boursiquot, and E. Marguerit. 2016. Grapevine rootstocks: Origins and perspectives. Acta Hortic. 1136(1136):11–22. doi: 10.17660/ActaHortic.2016.1136.2.
- Padgett-Johnson, M., L.E. Williams, and M.A. Walker. 2003. Vine water relations, gas exchange, and vegetative growth of seventeen Vitis species grown under irrigated and nonirrigated conditions in California. Journal of the American Society for Horticultural Science 128(2):269–276. doi: 10.21273/JASHS.128.2.0269.
- Pagay, V., A.G. Reynolds, and K.H. Fisher. 2013. The influence of bird netting on yield and fruit, juice, and wine composition of Vitis vinifera L. Journal International Des Sciences de La Vigne Et Du 47:35–45.
- Petrie, P., M. Trought, and G. Howell. 2000. Influence of leaf ageing, leaf area and crop load on photosynthesis, stomatal conductance and senescence of grapevine (Vitis vinifera L. cv. Pinot noir) leaves. Vitis 39:31‑36.
- Plank, C., E. Hellman, and T. Montague. 2019. Light and temperature independently influence methoxypyrazine content of Vitis vinifera (cv. Cabernet Sauvignon) berries. HortScience 54(2):282–288. doi: 10.21273/HORTSCI13634-18.
- Poni, S., and E. Giachino. 2000. Growth, photosynthesis and cropping of potted grapevines (Vitis vinifera L. cv. Cabernet Sauvignon) in relation to shoot trimming. Australian Journal of Wine and Grape Research 6(3):216–226. doi: 10.1111/j.1755-0238.2000.tb00182.x.
- Poni, S., C. Intrieri, and O. Silvestroni. 1994. Interactions of leaf age, fruiting, and exogenous cytokinins in Sangiovese grapevines under non-irrigated conditions. I. Gas Exchange. American Journal of Enology and Viticulture 45:71–77.
- Reynolds, A.G., and J.E. Vanden Heuvel. 2009. Influence of grapevine training systems on vine growth and fruit composition: A review. Am. J. Enol. Vitic. 60:251–268.
- Ruehl, E.H., and J. Schmid. 2014. Rootstock breeding between site adaptation and abiotic stress tolerance. Acta Hortic. 1045(1045):117–122. doi: 10.17660/ActaHortic.2014.1045.15.
- Schultz, H.R., W. Kiefer, and W. Gruppe. 1996. Photosynthetic duration, carboxylation efficiency and stomatal limitation of sun and shade leaves of different ages in field-grown grapevine (Vitis vinifera L.). Vitis. 35:169–176.
- Sánchez, L.A., and N.K. Dokoozlian. 2005. Bud microclimate and fruitfulness in Vitis vinifera L. Am. J. Enol. Vitic. 56:319–329.
- Santesteban, L., G.C. Miranda, and J.B. Royo. 2009. Effect of water deficit and rewatering on leaf gas exchange and transpiration decline of excised leaves for four grapevine (Vitis vinifera L.) cultivars. Sci. Hortic. 121(4):434–439. doi: 10.1016/j.scienta.2009.03.008.
- Scheiner, J., J. Anciso, and F. Westover. 2020. Impact of training system on Blanc Du Bois vegetative growth, yield components, and fruit composition. Viticulture Data Journal. 2:1–11. doi: 10.3897/vdj.2.e53118.
- Schrader, J.A., D.R. Cochran, P.A. Domoto, and G.R. Nonnecke. 2019. Phenology and winter hardiness of cold-climate grape varieties and advanced selections in Iowa climate. HortTechnology 29(6):906–922. doi: 10.21273/HORTTECH04475-19.
- Sharma, J., and A.K. Upadhyay. 2004. Effect of moisture stress on performance of own rooted and grafted vines of Tas-A-Ganesh (Vitis vinifera L.). Acta Hortic. 662(662):253–257. doi: 10.17660/ActaHortic.2004.662.36.
- Smith, B.P., N.B. Morales, M.R. Thomas, H.M. Smith, and P.R. Clingeleffer. 2016. Grapevine rootstocks resistant to the root-knot nematode Meloidogyne javanica. Australian Journal of Grape and Wine Research 23(1):125–131. doi: 10.1111/ajgw.12242.
- Soar, C.J., M.J. Collins, and V.O. Sadras. 2009. Irrigated Shiraz vines (Vitis vinifera) upregulate gas exchange and maintain berry growth in response to short spells of high maximum temperature in the field. Functional Plant Biology 36(9):801–814. doi: 10.1071/FP09101.
- Stafne, E.T., and J.A. Puckett. 2011. Aerial root formation on winegrape varieties after Spring 2007 freeze events. American Journal of Viticulture and Enology 62(1):118–121. doi: 10.5344/ajev.2010.10062.
- Sun, L.L., Y.P. Du, Q.U. Duan, and H. Zhai. 2017. Root temperature regulated frost damage in leaves of the winegrape Vitis vinifera L. Australian Journal of Grape and Wine Research 23:1–9.
- Takow, E.A., E.W. Hellman, A.G. Birt, M.D. Tchakerian, and R.N. Coulson. 2013. A web-based geographic information system application for description of American Viticultural Areas in Texas. HortTechnology 23(2):165–172. doi: 10.21273/HORTTECH.23.2.165.
- Tandonnet, J.-P., S.J. Cookson, P. Vivin, and N. Ollat. 2010. Scion genotype controls biomass allocation and root development in grafted vines. Australian Journal of Grape and Wine Research 16(2):290–300. doi: 10.1111/j.1755-0238.2009.00090.x.
- Tomás, M., H. Medrano, A. Pou, J.M. Escalona, S. Martorell, M. Ribas-Carbó, and J. Flexas. 2012. Water-use efficiency in grapevine varieties grown under controlled conditions: Effects of water stress at the leaf and whole-plant level. Australian Journal of Grape and Wine Research 18(2):164–172. doi: 10.1111/j.1755-0238.2012.00184.x.
- Townsend, C.G., D.R. Butler, and R.W. Dixon. 2016. The challenge of growing wine grapes in the Hill Country: An evaluation of changing grower perceptions of natural hazards in Texas vineyards. Papers in Applied Geography 2(1):105–112. doi: 10.1080/23754931.2015.1114511.
- Townsend, C.G., and E.W. Hellman. 2014. Viticulture in Texas: The challenge of natural hazards. Journal of Wine Research 25(4):211–228. doi: 10.1080/09571264.2014.967338.
- Tramontini, S., M. Vitali, L. Centioni, A. Schubert, and C. Lovisolo. 2013. Rootstock control of scion response to water stress in grapevine. Environ. Exp. Bot. 93:20–26. doi: 10.1016/j.envexpbot.2013.04.001.
- Trought, M.C.T., G.S. Howell, and N. Cherry. 1999. 7 Jun. 2020. Practical considerations for reducing frost damage in vineyards. Report to New Zealand Winegrowers 1999 (Lincoln University: Canterbury, New Zealand). https://researcharchive.lincoln.ac.nz/bitstream/handle/10182/4236/frost_damage_in_vineyards.pdf?sequence=1&isAllowed=y%20/%20handle/10182/4236/frost_damage_in_vineyards.pdf?sequence=1&isAllowed=y>. (accessed 9 November, 2020).
- United States Department of Agriculture. 2012. https://planthardiness.ars.usda.gov/PHZMWeb/ accessed 9 November, 2020.
- United States Department of Agriculture. 2019. https://www.nass.usda.gov/Statistics_byState/Texas/Publications/Current_News_Release/2019_Rls/tx-wine-grape-varieties-2019.pdf. (accessed 27 January, 2021).
- Widrlechner, M.P., C. Daly, M. Keller, and K. Kaplan. 2012. Horticultural applications of a newly revised USDA Plant Hardiness Zone Map. HortTechnology 22(1):6–19. doi: 10.21273/HORTTECH.22.1.6.
- Williams, L.E., and R.J. Smith. 1991. The effect of rootstock on the partitioning of dry weight, nitrogen and potassium, and root distribution of Cabernet Sauvignon grapevines. Am. J. Enol. Vitic. 42:118–122.
