ABSTRACT
São Francisco River Valley is the main mango Brazilian growing region, where almost 85% of Brazilian exports are produced. In this region, the fructification pruning is the first and one of the most influential cultural treatments of the productive cycle. The objective of this study was to evaluate the effect of mechanical pruning on growth, development, physiology, and yield of mango cv. Tommy Atkins grown in the tropical semiarid. An experiment was carried out in Casa Nova, Bahia, Brazil, with mangoes ‘Tommy Atkins.’ The experimental design was in randomized blocks, with two treatments (manual and mechanical pruning), evaluated in subdivided plots over time (evaluation dates), with five replications and four plants per plot. There was a significant interaction between the types of pruning and the dates of evaluation for the number of flushes emitted per branch, branch diameter, light intensity, nitrogen leaf content, leaf proline, branch proline, and branch soluble carbohydrates. Mechanical pruning increases the length of sprout and decreases the percentage of plants with symptoms of floral malformation. Mechanical pruning presented a lower production compared with manual pruning in the first evaluation cycle after continuous manual pruning, suggesting an initial impact, but with the potential for adaptation of the plants in the next productive cycles.
Introduction
The mango is a fruit of great economic importance for Brazil, which is the largest mango producer in South America and the seventh in the world ranking, with an average annual production of 1,319,296 tons, in a cultivated area of 65,646 hectares (IBGE, Citation2018). São Francisco River Valley, a Brazilian semiarid region, produces nearly 85% of the mangoes exported by Brazil (MAPA, 2019).
The high yield and quality of the mango produced in the São Francisco River Valley is a result of the association between favorable climatic conditions for cultivation and well-established management techniques, which include, among others, pruning, adequate availability of nutrients, use of growth regulators and strict control of irrigation for floral induction (Cavalcante et al., Citation2018; Lobo et al., Citation2019b; Oldoni et al., Citation2018). From all agronomic treatments usually performed for growing mangoes in Brazilian semiarid, pruning comprises the first stage of the production cycle (Albuquerque et al., Citation2002).
The fructification pruning, i.e., the pruning of the outer branches of the canopy performed after fruit harvest, aims to guide the plant canopy and maintain uniformity in the development of new branches, in order to induce a uniform flowering and avoid alternating harvests (Mouco and Albuquerque, Citation2004).
Some studies in the scientific literature demonstrate that pruning improves the light penetration in the mango canopy, optimizes the production and use of photoassimilates (Solanki et al., Citation2016), allows harvesting fruits out of season, affects the yield and quality of fruits (Asrey et al., Citation2013; Sarkhosh et al., Citation2018), and also influences the occurrence of the pests and diseases (Singh et al., Citation2010).
Usually, mango pruning is performed manually, however, the use of mechanical pruning in crops such as apple trees (Mika et al., Citation2016), olive trees (Albarracín et al., Citation2018) and vines (Poni et al., Citation2016), indicate a possible alternative for mechanization of pruning also in mango.
According to Mayande (Citation2019) the advances on experienced technologies for the automation of harvesting, sorting, processing, and packaging mangoes, should shortly be extended to pruning. Therefore, understanding the effects of the mechanical pruning is essential given the rising expansion of the area cultivated with mangoes and the lack of labor to carry out most hand operations, especially in larger orchards.
In this sense, the objective of this study was to evaluate the effect of mechanical pruning on the growth, development, physiology, and production of the mango cv. Tommy Atkins grown in the tropical semiarid.
Materials and Methods
Plant Material and Growing Conditions
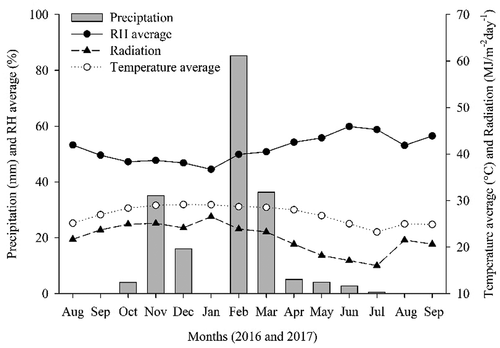
Fifteen years old mango (Mangifera indica L.) plants, cultivar Tommy Atkins, with uniform size and vigor were used in this study. The experiment was accomplished from August 2016 to September 2017 in experimental orchards located in Casa Nova (09°12’S and 40°55ʹW; at an altitude of 402 m above sea level), Bahia, Brazil. The climate of this region is classified as BSh (Köeppen), which corresponds to a semi-arid region. The climatic data for the study period were monitored and recorded by a weather station ().
Figure 1. Average air temperature, radiation, relative humidity (RH) and precipitation during the experiment
The plants, spaced with 10.0 m between the rows and 6.0 m between the plants, were daily irrigated (micro sprinkler) with one emitter per plant, for a flush of nearly 60 L h−1 each. All management practices such as pruning, control of weeds, pests and diseases, plant growth regulators for gibberellin inhibition (Cultar®) and dormancy break (calcium nitrate and potassium nitrate) were performed following the instructions of Genú and Pinto (Citation2002). The nutrient management was performed through a fertirrigation system, according to plant demand (Barbosa et al., Citation2016; Genú and Pinto, Citation2002). Tip pruning was performed to synchronize vegetative flush events in the canopy.
Floral management includes practices common to mango grown in the semiarid region, based on the recommendations of Albuquerque et al. (Citation2002).
Paclobutrazol (PBZ) was applied at 123 days after pruning (DAP), the dose used was 22.9 ml of Cultar 250 SC® (Syngenta Crop Protection) per plant, the equivalent to 5.72 g of a.i. diluted in water and applied via soil in the canopy projection.
The branch maturation started at 57 days after PBZ, totalizing five foliar sprays of potassium fertilizers and biostimulants, at weekly intervals: 1st and 2nd – BULK (Alltech®) 1% (12% KCL; 9.87% CO; 20% amino acids); 3rd – MKP (Dripsol®) 500 g (52% P2O5; 34% K2O); 4th – Ethrel® (25 ml) + Theion (500 g) + K2SO4 (2.5%); 5th – Ethrel® (25 ml) + Theion (500 g) + K2SO4 (2.5%).
Floral induction started at 78 days after PBZ, with five sprays of calcium nitrate and biostimulants: 1st and 3rd – 3% of Ca (NO3)2; 2nd and 3rd – 3% of Ca (NO3)2 + S; 4th and 5th – 2.0% of Ca (NO3)2 + Naturamin® (80% free amino acids; 12.8% N).
Treatments and Experimental Design
The experiment followed a randomized blocks design with two treatments, evaluated in scheme of split-plot in time, referring to the evaluation dates, five replications per treatment and four plants per replication. The treatments consisted of manual pruning (T1) and mechanical pruning (T2).
Manual pruning was performed according to the recommendations of Albuquerque et al. (Citation2002), in which the branches that bloomed and those that did not bloom in the previous harvest were removed. The mechanical pruning was performed with a side and top pruning machine coupled to a tractor, which cut 45° vertically on both sides of the plant and cut straight at the top, performing a trapezoidal pruning. Both prunings were carried out on 08/12/2016.
Evaluations and Statistical Analysis
To determine the effects of treatments, 6 branches were identified and monitored on the east side and 6 on the west side of the middle third of the crown. The diameter of the pruned branches (DB), the length of sprout (LS) and the diameter of sprout (DS) were evaluated, with the aid of a digital caliper and a graduated ruler (mm); number of flushes emitted per branch (NFB) and number of sprouts (NS), at 30, 60 and 90 days after pruning. Readings were taken in the middle of the canopy, avoiding necrotic areas by the attack of pests and diseases, and the edges of the leaf midrib, in the recently fully mature leaf pair from the apex of sprout.
Luminous intensity (LI) inside the canopy was measured at 44, 60, 90 and 120 days after pruning. These evaluations were always performed at the 10:00 a.m. and with a maximum difference of 10 minutes between the first and the last reading, using an Instrutherm® digital lux meter calibrated to 50000 Lux/Fc.
The levels of leaf nitrogen were determined during floral induction, in a total of 9 evaluation dates (02/19/2017, 02/26/2017, 03/05/2017, 03/12/2017, 04/02/2017, 04/16/2017, 04/29/2017 and 05/06/2017), following the Kjeldahl method, according to the methodology described by Tedesco et al. (Citation1995).
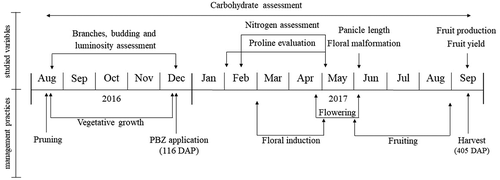
The free proline content was determined according to the methodology described by Bates et al. (Citation1973), being performed during pre-induction and floral induction, totalizing 8 evaluation dates. The same sample was used to determine total soluble carbohydrates in leaves and branches, following the methodology proposed by Dubois et al. (Citation1956), however, determined on 17 dates that comprised the entire production cycle ().
Figure 2. Descriptive scheme of the variables analyzed and the main events of the productive cycle of ‘Tommy Atkins’ Mango in the tropical semiarid
The incidence of plants with floral malformation was determined by observing and counting plants with corresponding symptoms, thus obtaining the percentage of plants with malformation (Dias et al., Citation2003). The number of panicles with malformation per plant was also counted.
At the end of the production cycle, at the time of harvest, the yield was counted in kg plant−1 and the fruit yield was estimated in t ha-1, multiplying fruit yield per plant by the density of plants per hectare.
In there is a descriptive scheme of the variables analyzed and the main events of the productive cycle of ‘Tommy Atkins’ mango.
The data obtained was subjected to the analysis of variance (ANOVA). All statistical analyses were performed using the R statistical software, version 3.5.0 (R CORE TEAM, Citation2018), and averages were compared by the Tukey test at p < .05.
Results and Discussion
According to the result of the ANOVA () it is possible to state that the type of pruning affected the length of sprouts (LS), plants with floral malformation (FM) and yield per plant, while the evaluation dates significantly affected all the growth variables evaluated, proline in the leaves (PL), proline in the branches (PB), and leaf carbohydrates.
Table 1. F-value for the variables length of sprout (LS), number of sprouts (NS), diameter of sprouts (DS), number of flushes per branch (NFB), diameter of the branch (DB), light intensity inside the canopy (LI), leaf nitrogen content (N), proline in leaves (PL), proline in branches (PB), total soluble carbohydrates in leaves (CL) and total soluble carbohydrates in branches (CB) in ‘Tommy Atkins’ Mango as a function of manual and mechanical pruning
There was a significant interaction between the types of pruning and the evaluation dates for the number of flushes emitted per branch (NFB), branch diameter, light intensity (LI), leaf nitrogen (N), and carbohydrates in the branches (CB) demonstrating the dependence between the factors for these variables ().
As the evaluation dates progressed from the pruning, an increase in the length and diameter of the sprouts can be observed, both presenting an increase in relation to the first evaluation (30 days) of 65.62% for the length and 71.04% for the diameter (), and the highest values at 90 days after pruning (DAP).
Figure 3. Effect of the evaluation date on the length of sprout (A), number of sprout (B) and diameter of sprout (C) in ‘Tommy Atkins’ Mango subjected to manual and mechanical pruning
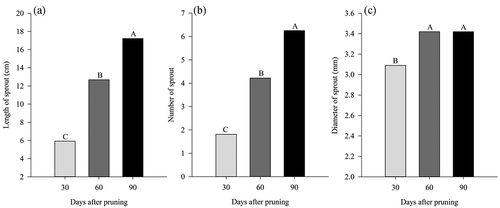
It is also possible to note that the increase in both variables was more intense up to 60 DAP, with a gain of 114.19% to LS and 133.15% to DS was observed concerning the previous evaluation (30 DAP). Likewise, the number of sprouts varied according to the days, however, it reached its maximum value at 60 DAP, remaining constant at 90 DAP ().
The increase in the length and diameter of the sprouts can be justified by the break-in apical dominance caused by the removal of the apexes of the branches through pruning, resulting in the reduction of the auxin content in the branches (Solanki et al., Citation2016) inducing the emission and development of side shoots. Taiz et al. (Citation2017) explain that the phenomenon of apical dominance is caused by the auxin produced at the apex of the stem, which inhibits the growth of axillary buds, also the stem apex exerts a strong carbohydrate drain action.
Solanki et al. (Citation2016) evaluating different pruning intensities in mango cv. Kesar obtained sprouts with 29.38, 28.23 and 28.29 cm for pruning with 25, 50 and 75 cm (remaining branches) respectively, values higher than those encountered in the present work, which at 90 DAP the sprouts length average was 17.22 cm.
Regarding the length of sprouts (LS), plants mechanically pruned obtained results 24.28% higher than those pruned manually (), while the diameter and number of sprouts did not present statistical differences, with averages of 4.1 mm and 3.31 sprouts, respectively.
Figure 4. Length of sprout in ‘Tommy Atkins’ Mango subjected to manual and mechanical pruning
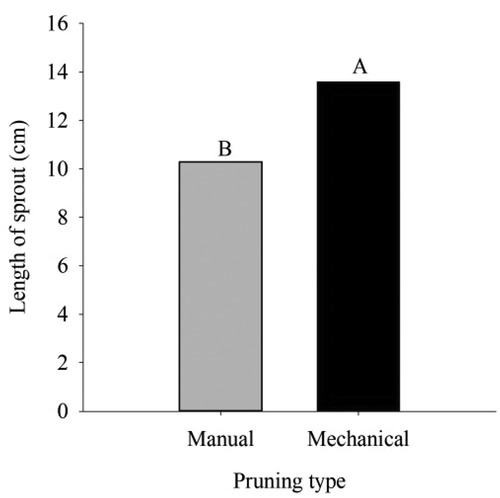
Nafees et al. (Citation2010) evaluated PBZ doses in different mango cultivars and obtained values of LS at 70 days after PBZ application, ranging from 13.63 cm for plants without application up to 8.81 cm in plants in which was applied the highest dose of PBZ (12 g a.i. per plant). These results are similar to those encountered in the present study, in which T1 (manual) and T2 (mechanical) presented length of sprouts of 10.29 cm and 13.59 cm, respectively, at 90 days after pruning.
The highest values achieved in this work are due to the fact that the evaluations were carried out before the application of PBZ, the sprouts being in full growth, once PBZ is a plant growth regulator that inhibits the synthesis of gibberellin, and it is used for reducing branch elongation and managing vegetative growth (Wongsrisakulkaew et al., Citation2017). In comparison to Palmer cultivar, Cavalcante et al. (Citation2020) registered an average sprout length of nearly 22 cm at 30 DAP for manually pruned plants, so a higher value that those quoted in . It important to infer that such plants were in their first growing season, while those of the present study ten, i.e., they were manually pruned ten times.
The number of flushes per branch presented a similar data distribution for both types of pruning as a function of time elapsing, in both cases, there was a significant increase from 30 DAP to 60 DAP, but without significant increases at 90 DAP. In the first evaluation (30 DAP) the plants pruned in both ways did not present differences in the number of flushes, but at 60 and 90 DAP mechanical pruning achieved a superiority of 19.10% and 21.60%, respectively, concerning manual pruning ().
Figure 5. Number of flushes per branch (A) and diameter of the branch (B) in ‘Tommy Atkins’ Mango subjected to manual and mechanical pruning at 30, 60 and 90 days after pruning
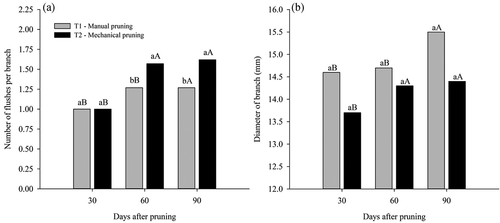
At 90 DAP the plants subjected to both prunings had more than one vegetative flush, before applying the PBZ. For Cavalcante et al. (Citation2018), it is essential to manage the application of PBZ in the mango after the emission of more than one vegetative flush, this recommendation is necessary in order that after the emission of the vegetative flushes, they can accumulate reserves and only then the floral induction can be initiated.
Additionally, there was an increase in the branch diameter in plants pruned manually at 90 DAP. The mechanically pruned plants, on the other hand, reached an earlier increase at 60 DAP, but stabilizing and not differing at 90 DAP (). Farias et al. (Citation2019) evaluating the pruning intensity, long, medium and short in guava, detected the precocity at the beginning of the phenophases in the branches medium and long pruned concerning those subjected to short pruning. This result corroborates the tendency that plants accelerate their metabolic processes to replace the vegetative growth of these species after intense pruning.
Medina-Urrutia and Nuñez-Elisea (Citation1997) assessed the effect of mechanical pruning on mango cv. Tommy Atkins at different vertical angles (0º, 15º and 30º) and concluded that the increase in NFB and DB in plants submitted to mechanical pruning can be attributed to the lower aerial/root ratio caused by the intense extraction of plant material and apical buds, providing greater production and action of hormones, such as the cytokinin that acts on the growth sites, which provided a higher rate of regeneration in plants where the removal of plant material was greater, that is, at an angle of 30°. Result similar to that of our study, in which the 45º angle was adopted and a higher NFB was obtained in relation to the plants pruned manually.
The plant position in the canopy from where the plant material is removed influences the incidence of light inside the canopy of the plants over time. It is observed that between 44 and 62 DAP there was no variation in the light intensity in the canopy of mechanically pruned plants, but there was a significant decrease of 91.34% at 90 DAP, however, stabilizing at the end of the period (122 DAP) ().
Figure 6. Light intensity inside the canopy in ‘Tommy Atkins’ Mango subjected to manual and mechanical pruning
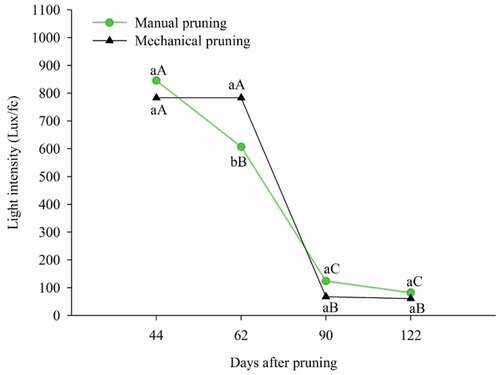
While in plants manually pruned, the decrease in light intensity occurred just at 62 DAP, differing from 44 DAP in 238.03 lux/fc, and again at 90 DAP, where there was a further decline, remaining stable in the last period of evaluation (122 DAP) ().
The decline in light intensity in the canopy of plants subjected to both types of pruning is attributed to the fact that, during this period, the plants were in full vegetative growth, which promoted the closure of the canopy through the emission of new sprouts and overlapping of the branches, in addition to their increase in diameter, as presented in and 5.
It appears that the closing of the canopy in manually pruned plants occurred faster than in those mechanically pruned, due to the increase in pruning intensity that implies a greater removal of plant material (Solanki et al., Citation2016), thereby, the plants mechanically pruned had lower active photosynthetic area, delaying the sprout of new leaves.
Regarding leaf nitrogen levels, although there was an oscillation over the days for T2, these were not statistically different (). However, T1 average values were affected by the evaluation dates, with the highest value obtained on 4/29/2017, corresponding to the evaluation after the 4th floral induction with nitrate.
Figure 7. Leaf nitrogen content on different evaluation dates during floral induction in ‘Tommy Atkins’ Mango subjected to manual and mechanical pruning
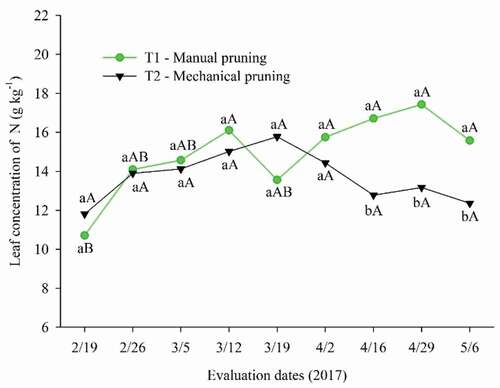
When comparing the types of pruning in each evaluation, it is observed that the pruning only differed statistically from the 7th evaluation (4/16/2017), right after the 3rd induction with nitrate, a period in which the average N content in the T1 plants was higher in 3.80 g kg−1 to that of T2 plants.
In terms of means the N values recorded in plants of both types of pruning in the evaluated period are compatible with those reported by Fernandes and Nascimento (Citation2004) for the pre-flowering period (12.2 g kg−1), being even higher, once, according to the authors, in this phase there are the highest levels of nitrogen, phosphorus, and potassium in the leaves, followed by a decrease due to flowering, fruit formation and maturation. This fact can be observed in the present study, since the peak in leaf N content for T1 occurred after the 4th floral induction with nitrate, coinciding with the beginning of flowering, demonstrating its main role in inducing the growth of flower buds.
Probably the increase in leaf nitrogen concentration and oscillations throughout the evaluation dates that correspond to floral induction occurred in the form of nitrate, a result of the frequent foliar applications of calcium nitrate to induce floral differentiation (Silva et al., Citation2014).
For Quaggio et al. (Citation1996), adequate nitrogen levels must be between 12 and 14 g kg−1, therefore, the result for mechanical pruning is considered adequate between 04/16/2017 and 05/06/2017 (12.36 g kg−1) corresponding to the beginning of flowering, while the average value recorded for manual pruning is considered excessive on the same dates.
However, the plants subjected to T1 demonstrate a pattern of reduction in values from 17.43 g kg−1 to 15.58 g kg−1, from the penultimate to the last evaluation date, which may be associated with an increase in the activity of the nitrate reductase enzyme, rising the absorption and translocation of N in the form of nitrate (Helaly et al., Citation2017), demonstrating that the plants subjected to T1 were later in the use of N.
The excess of nitrogen causes excessive vegetative growth, and the plant has difficulty in floral differentiation (Pinto et al., Citation2009). For this reason, it is desirable that the N levels are reduced at the beginning of floral induction, as can be noted in the present study, in which, the leaf nitrogen levels were at their minimum levels of 10.71 g kg−1 in plants pruned manually and 11.82 g kg−1 in those mechanically pruned.
As for proline levels, there is a strong relationship between the levels in leaves and branches, where the increases and decreases follow the same tendency ().
Figure 8. Proline levels in leaves and branches in ‘Tommy Atkins’ Mango during pre-induction and floral induction
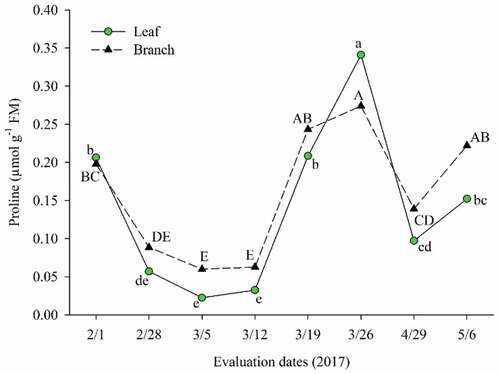
The increase in proline levels on the first evaluation date (after applying PBZ) corresponds to the beginning of the reduction in the irrigation depth and consequent water stress. This stage is inserted in the sprout maturation phase of the aerial part, necessary for the cultivation of the mango tree, with the intuition of maintaining the metabolic activities and accumulating reserves of plant, aiming the floral induction (Cavalcante et al., Citation2018).
Proline is responsible for performing osmotic adjustment of cells under abiotic stress (Taiz et al., Citation2017), acting on plants with low water potential in the leaf, as verified by Helaly et al. (Citation2017) who reported an increase in proline levels in leaves of four mango cultivars under water stress. In mango cv. Tommy Atkins grown in semiarid conditions, Mudo et al. (2020) also observed an increase in the levels of leaf proline in plants under water stress during the shoot maturation phase.
There was also an increase in proline leave (PL) before and after the 3rd floral induction, which coincides, besides the water stress, with the application of Naturamin® compound fertilizer (2.5%) containing 80% amino acids and calcium nitrate (2.5%), constituting an exogenous source of proline. The increase, therefore, could be due to its high content in the fertilizer formulation, in addition to it, an increase in biosynthesis of proline from the other amino acids present may have occurred.
The period in which there was a reduction in the levels of PL and PB coincided with the occurrence of precipitations in the region (), referring to the months from February to May 2017, and this factor can have been an attenuating of water stress, reducing the need of its accumulation and promoting its degradation by the action of proline dehydrogenase when the water status returned to normal (Taiz et al., Citation2017).
Regarding soluble carbohydrates, the results indicated a greater presence of these organic solutes in the leaves compared with the contents in branches, attaining a maximum peak of 428.1 µmol g−1 FM on the day 02/01/2017 (50 days after PBZ) (), corresponding to the start of water supply reduction.
Figure 9. Total soluble carbohydrates in leaves and branches in ‘Tommy Atkins’ Mango on different evaluation dates in the production cycle
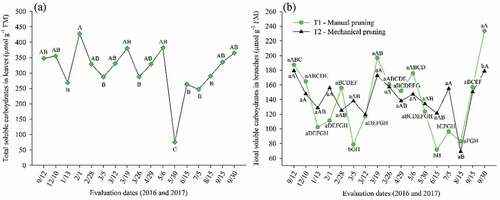
According to Marijuan and Bosch (Citation2013), plants under abiotic stresses activate defense mechanisms such as the accumulation of carbohydrates in the tissues. This reduces the plant energy consumption and maintains optimal levels of carbohydrates for flowering.
In the present study, a reduction of 77.05% was recorded from the beginning of flowering (day 04/29/2017) until full flowering (day 05/30/2017) (). For Prasad et al. (Citation2014), high levels of carbohydrates occur in the pre-flowering phase due to the intense activity of hydrolytic enzymes and the mobilization of leaf metabolites to form panicles, followed by a decrease in full flowering. A similar response was observed in ‘Kent’ mango grown in a semiarid environment, with an average reduction of 21.83% in the levels of leaf soluble carbohydrates between the referred phases (Lobo et al., Citation2019a).
When evaluating the levels of soluble carbohydrates in the branches (), it is verified that there was an oscillation in the values in the initial phase with reduction until the 3rd evaluation date, followed by an increase and a subsequent decrease as the seasons for both types of pruning advance, and then, an accumulation in the repose after pruning (day 9/30/2017).
The distribution of carbohydrates in the branches data confirms the importance of carbohydrates in metabolism, physiological events, and plant development, by importing carbon into regions considered to be metabolically active drains such as vegetative sprouts after pruning, branches during maturation, in the process of breaking the buds and formation of panicles at flowering (Prasad et al., Citation2014).
According to the result of the ANOVA it is possible to state that the type of pruning significantly affected the floral malformation (FM) and fruit yield (FY), but had no effect on the number of malformed panicles per plant (NMPP) ().
Table 2. ANOVA F-value for floral malformation (FM), number of malformed panicles per plant (NMPP) and fruit yield (FY) of ‘Tommy Atkins’ Mango as a function of manual and mechanical pruning
The percentage of floral malformation (FM) in plants mechanically pruned was 50% lower than plants manually pruned ().
Figure 10. Floral malformation and number of malformed panicles per plant in ‘Tommy Atkins’ Mango subjected to manual and mechanical pruning
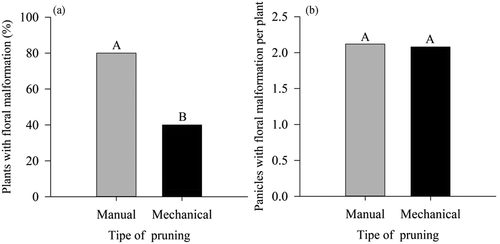
This disease is common in mango trees, mainly in ‘Tommy Atkins,’ as it is susceptible. Dias et al. (Citation2003) who studied FM in several mango cultivars, obtained 100% incidence for the cultivar Tommy Atkins in the state of Bahia.
Singh et al. (Citation2010), studying different pruning intensities on mango varieties and evaluating its influences on characteristics such as flowering and incidence of floral malformation, could observe that moderate pruning stood out from the treatment without pruning, increasing the number of panicles per branch and prolonging the period of flowering, in addition to drastically reducing malformed panicles, as observed in the present work in mechanically pruned plants.
These results can be related to those of the present study for the lowest percentages of plants with FM and number of malformed panicles per plant for plants subjected to T2 (), in which, possibly, there was greater removal of plant material from the apex of the branches that could be a source of inoculum.
The yields of the two treatments were higher than the average yield of 15.6 t ha−1 of the mango in the Bahia state (IBGE, Citation2018), which indicates that both types of pruning are efficient to guarantee good yield. In contrast, plants pruned manually obtained 9.2 t ha−1 more than those pruned mechanically ().
Figure 11. Fruit yield in ‘Tommy Atkins’ Mango subjected to manual and mechanical pruning
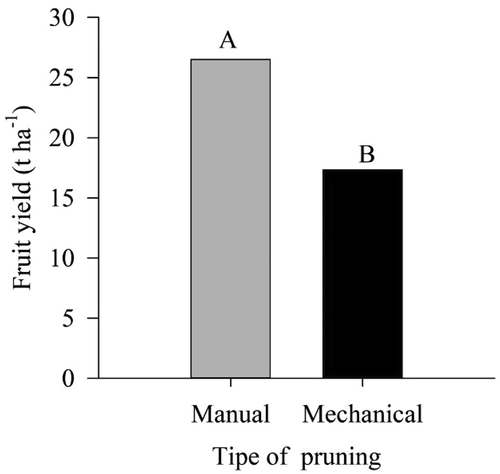
Barraza et al. (Citation2016) evaluated the effects of types and intensities of pruning in relation to plants not pruned in ‘Ataulfo’ mango trees in Mexico, and their results demonstrated that fruit yield of pruned trees decreased during the first year compared with non-pruned trees, but there was an increase during the second year.
The same occurred in the study by Asrey et al. (Citation2013) with ‘Amrapali’ mango in India, where it was verified that pruning resulted in stress for the plant, through assessments of the contents of the phenolic compounds, which was minimized in the following harvest.
Results that corroborate with those obtained in the present study, once the plants were mechanically pruned for the first time, which possibly resulted in a more intense stress condition compared with T1, justifying the lower yield, because, despite the manual pruning be slower, it is more careful and, therefore less intense, which resulted in less stress for the plant in the first year of implantation.
Manual and mechanical pruning do not affect the concentrations of nitrogen, proline and total soluble carbohydrates. However, mechanical pruning increases the length of sprouts and decreases the percentage of plants with symptoms of floral malformation. Mechanical pruning decreases the fruit yield in the first evaluation cycle after continuous manual pruning, suggesting an initial impact, but with a potential adaptation of the plants in the next productive cycles.
Therefore, more studies are needed to be able to assess the real contribution of mechanized pruning for the cultivation of mango trees, since there were promising effects that should be observed for more consecutive cycles.
Disclosure Statement
No potential conflict of interest was reported.
References
- Albarracín, V., A.J. Hall, P.S. Searles, and M.C. Rousseaux. 2018. Impact of simulated mechanical hedge pruning and wood age on new shoot demography and return flowering in olive trees. Trees 32(6):1767–1777. doi: https://doi.org/10.1007/s00468-018-1749-1.
- Albuquerque, J.A.S., V.D. Medina, and M.A.C. Mouco. 2002. Sistemas de poda, p. 243–258. In: P.J.C. Genú and C.A.Q. Pinto (eds.). A cultura da mangueira. Embrapa Informação Tecnológica, Brasília, Brazil.
- Asrey, R., V.B. Patel, K. Barman, and R.K. Pal. 2013. Pruning affects fruit yield and postharvest quality in mango (Mangifera indica L.) cv. Amrapali. Fruits 68(5):367–380. doi: https://doi.org/10.1051/fruits/2013082.
- Barbosa, L.F.S., Í.H.L. Cavalcante, and A.M.N. Lima. 2016. Desordem fisiológica e produtividade de mangueira cv. Palmer associada à nutrição de boro. Rev. Bras. Frutic. 38(1):1–9. doi: https://doi.org/10.1590/0100-2945-273/14.
- Barraza, M.H.P., M.A.U. Lópes, J.A.O. García, A.I.P. Luna, Y.N. González, and N.C.G. Álvarez. 2016. Efecto de poda en escama blanca y producción de mango ‘Ataulfo.’ Rev. Mex. Cienc. Agríc. 7:1841–1853. doi: https://doi.org/10.29312/remexca.v7i8.96.
- Bates, L.S., R.P. Waldren, and I.D. Teare. 1973. Rapid determination of free proline for water-stress studies. Short communication. Plant Soil. 39(1):205–207. doi: https://doi.org/10.1007/BF00018060.
- Cavalcante, Í.H.L., G.N.F. dos Santos, M.A. Da Silva, R. Dos Santos. Martins, A.M.N. Lima, P.I.R. Modesto, and M.Z. Beckmann-Cavalcante. 2018. A new approach to induce Mango shoot maturation in Brazilian semi-arid environment. J. Appl. Bot. Food Qual. 91:281–286. doi: https://doi.org/10.5073/JABFQ.2018.091.036.
- Cavalcante, Í.H.L., G.J.N. E Silva, J.A. Cavacini, R.A. E Amariz, S.T. de Freitas, K.A.O. De Sousa, M.A. Da Silva, and J.G. da Cunha. 2020. Metconazole on inhibition of gibberellin biosynthesis and flowering management in mango. Erwerbs-Obstbau. 62(1):89–95. doi: https://doi.org/10.1007/s10341-019-00466-w.
- Dias, N.O., M.T.R. Vila, A.E. Viana, T.N.H. Rebouças, A.R. São José, M.A.C. Boaretto, M.P. Bomfim, and A.E.L. Ribeiro. 2003. Incidência e severidade da malformação floral em seis cultivares de Mangueira. Rev. Bras. Frutic. 25(1):179–180. doi: https://doi.org/10.1590/S0100-29452003000100049.
- Dubois, M., K.A. Gilles, J.K. Hamilton, P.T. Rebers, and F. Smith. 1956. Colorimetric method for determination of sugars and related substances. Anal. Chem. 28(3):350–356. doi: https://doi.org/10.1021/ac60111a017.
- Farias, W.C., F.M.M. Câmara, E.C. Pereira, J.M. Costa, G.A. Pereira, and V. Mendonça. 2019. Phenology and production of guava tree cv. “Paluma” pruned at different seasons in Mossoro–RN, Brazil. Comum. Sci. 10(3):376–383. doi: https://doi.org/10.14295/cs.v10i3.2202.
- Fernandes, F.M., and V.N. Nascimento. 2004. Fertilidade do solo e nutrição da mangueira, p. 179–198. In: D.E. Rozane, R.J. Darezzo, R.L. Aguiar, G.H.A. Aguilera, and L. Zambolim (eds.). Manga: Produção integrada, industrialização e comercialização. UFV, Viçosa, Brazil.
- Genú, P.J.C., and A.C.Q. Pinto. 2002. A cultura da mangueira. Embrapa Informação Tecnológica. Brasília, Brazil.
- Helaly, M.N., H. El-Hoseiny, N.I. El-Sheery, A. Rastogi, and H.M. Kalaji. 2017. Regulation and physiological role of silicon in alleviating drought stress of mango. Plant Physiol. Biochem. 18:31–44. doi: https://doi.org/10.1016/j.plaphy.2017.05.021.
- IBGE. 2018. Produção agrícola municipal: Lavouras permanentes. 8 Mar. 2020. https://sidra.ibge.gov.br/tabela/1613#resultado
- Lobo, J.T., Í.H.L. Cavalcante, A.M.N. Lima, Y.A.C. Vieira, P.I.R. Modesto, and J.G. da Cunha. 2019a. Biostimulants on nutritional status and fruit production of mango ‘Kent’ in the Brazilian semiarid region. HortScience. 54(9):1501–1508. doi: https://doi.org/10.21273/HORTSCI13753-18.
- Lobo, J.T., K.D.S.M. De Sousa, V.B. de Paiva Neto, R.N. Pereira, L.D.S. Silva, and Í.H.L. Cavalcante. 2019b. Biostimulaibgents on fruit yield and quality of mango cv. Kent grown in semiarid. J. Am. Pomol. Soc. 73:52–160. http://www.pubhort.org/aps/73/v73_n3_a2.htm
- MAPA. 2019. Estatísticas de Comércio Exterior do Agronegócio Brasileiro. 12 Mar. 2020. http://indicadores.agricultura.gov.br/agrostat/index.htm
- Marijuan, M.P., and S.M. Bosch. 2013. Ecophysiology of invasive plants: Osmotic adjustment and antioxidants. Trends Plant Sci. 18(12):660–666. doi: https://doi.org/10.1016/j.tplants.2013.08.006.
- Mayande, V.M. 2019. Status and strategies for mechanization of mango crop. Adv. Agri. Res. Tech. J. 3:89–91. http://www.isasat.org/Vol-3-issue-1-Jan-019/AARJ_III_1_2019_10_Mayande_pp89-91.pdf
- Medina-Urrutia, V.M., and R. Nuñez-Elisea. 1997. Mechanical pruning to control tree size, flowering, and yield of mature (Tommy Atkins) mango trees. Acta Hortic. 455(455):305–314. doi: https://doi.org/10.17660/ActaHortic.1997.455.40.
- Mika, A., Z. Buler, and W. Treder. 2016. Mechanical pruning of apple trees as an alternative to manual pruning. Acta Sci. Pol-Hortorum. 15:113–121. http://www.acta.media.pl/pl/full/7/2016/000070201600015000010011300121.pdf
- Mouco, M.A.C., and J.A.S. Albuquerque. 2004. Sistemas de Poda, p. 32–36. In: M.A.C. Mouco (ed.). Cultivo da Mangueira. Embrapa Semi-Árido, Petrolina.
- Mudo, L.E.D., J.T. Lobo, D.A. Carreiro, J.A. Cavacini, L.D.S. Silva, and Í.H.L. Cavalcante. 2020. Leaf gas exchange and flowering of mango sprayed with biostimulant in semi-arid region. Rev. Caatinga.33(2):332-340. https://doi.org/https://doi.org/10.1590/1983-21252020v33n206rc.
- Nafees, M., R. Anwar, M. Jameel, M.N. Aslam, S. Ahmad, F.Z. Akhtar, and N. Memon. 2010. Flushing pattern of mango (Mangifera indica L.) Cultivars in response to pruning of panicles and its effect on carry over effect of floral malformation. Pakistan J. Agric. Sci. 47:13–18. https://pdfs.semanticscholar.org/a9db/9b0fc998bc96fd296a9e70f1b2d7b70105f1.pdf?_ga=2.58648938.1760006922.1586359384-1357155029.1586359384
- Oldoni, F.C.A., A.M.N. Lima, Í.H.L. Cavalcante, K.D.S.M.D. Sousa, M.A. Carneiro, and I.R.B. de Carvalho. 2018. Boron fertilizing management on fruit production and quality of mango cv. Palmer in semiarid. Rev. Bras. Frutic 40(3):1–8. doi: https://doi.org/10.1590/0100-29452018622.
- Pinto, A.C.Q., D.J. Silva, and P.A.C. Pinto. 2009. Mangueira, p. 125–145. In: L.A. Crisóstomo and A. Naumov (eds.). Adubando para alta produtividade e qualidade: Fruteiras tropicais do Brasil. Embrapa Agroindústria Tropical, Fortaleza, Brazil.
- Poni, S., S. Tombesi, A. Palliotti, V. Ughini, and M. Gatti. 2016. Mechanical winter pruning of grapevine: Physiological bases and applications. Sci. Hortic. 204:88–98. doi: https://doi.org/10.1016/j.scienta.2016.03.046.
- Prasad, S.R.S., Y.T.N. Reddy, K.K. Upreti, and A.N. Rajeshwara. 2014. Studies on changes in carbohydrate metabolism in regular bearing and “Off” season bearing cultivars of mango (Mangifera indica L.) during flowering. Int. J. Fruit Sci 14(4):437–459. doi: https://doi.org/10.1080/15538362.2014.897891.
- Quaggio, J.A., B.V. Raij, and C.T. Piza Junior. 1996. Frutíferas, p. 121–153. In: B.V. Raij, H. Cantarella, J.A. Quaggio, and A.M.C. Furlani (eds.). Recomendações de adubação e calagem para o Estado de São Paulo. Instituto Agronômico - Fundação IAC, Campinas, Brazil.
- R CORE TEAM. 2018. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 18 Dec. 2019. https://www.R-project.org
- Sarkhosh, A., C. McConchieb, and A. Khadivic. 2018. The effects of different tip-pruning times on flowering, yield, and maturity of two mango cultivars in subtropical climate of Northern territory (Katherine region) from Australia. Sci. Hortic. 234:140–145. doi: https://doi.org/10.1016/j.scienta.2018.02.039.
- Silva, K.K.A., E.O. Ono, M.D.C. Mouco, G.J.N. Silva, R.J.M. de Souza, N.C. Da Silva, and R. Silva. 2014. Uniconazole no florescimento e produção da mangueira (Mangifera indica L.) cv. Palmer. Magistra. 26:505–514. https://ainfo.cnptia.embrapa.br/digital/bitstream/item/124932/1/Mouco-2014.pdf
- Singh, S.K., S.K. Singh, R.R. Sharma, and V.B. Patel. 2010. Influence of pruning intensity on flowering, fruit yields and floral malformation in three Mango cultivars planted under high density. Indian J. Hortic. 67:84–89. https://www.indianjournals.com/ijor.aspx?target=ijor:ijh&volume=67&issue=4&article=019
- Solanki, P.D., N. Shah, D. Prajapati, and H.R. Patel. 2016. Response of Mango (Mangifera indica l.) to different pruning time and intensity for vegetative, flowering and fruiting parameters. The Bioscan. 11:2317–2322. https://thebioscan.com/supplements/07_7256_P.%20D.%20SOLANKI_Agro.pdf
- Taiz, L., E. Zeiger, I.M. Møller, and A. Murphy. 2017. Fisiologia e desenvolvimento vegetal (trans). 6th ed. Artmed, Porto Alegre, Brazil.
- Tedesco, M.J., C. Gianello, C.A. Bissani, H. Bohnen, and S.J. Wolkweiss. 1995. Análises de solo, plantas e outros materiais. 2nd ed. UFRGS, Porto Alegre, Brazil.
- Wongsrisakulkaew, Y., U. Boonprakob, R. Sethpakdee, and N. Juntawong. 2017. Effect of paclobutrazol concentrations and time of foliar application on flowering of ‘Namdokmai-sitong’ Mango. Int. J. Geomate. 12(30):41–45. doi: https://doi.org/10.21660/2017.30.96545.
