ABSTRACT
Anthracnose fruit rot, caused by Colletotrichum spp. is a major disease of highbush blueberries. The inheritance of fruit rot resistance to C. fioriniae was investigated in crosses of parents with varying levels of susceptibility. Three cultivars with known resistance profiles (Bluecrop, Elliott, and Jersey) and progeny from 16 crosses of parents with varying levels of susceptibility were screened. Fruit of field-grown bushes was inoculated when immature, harvested when ripe, and rated for infection incidence after 5, 8, and 12 days of incubation at 100% RH and 22–23°C. Area under the disease progress curves (AUDPC) values were calculated for 2010 and 2011 and slightly higher disease pressure was observed in 2011. These values were then regressed against actual disease incidences of cultivars and predicted (midparent) values for cross families based on two previous studies in 2010 and 2011 and significant correlations with the proportion of fruit decayed and sporulation capacity were observed. These findings provide strong evidence that anthracnose resistance is heritable in highbush blueberries, which has important implications for anthracnose resistance breeding. Additionally, this research provides benchmark AUDPC values for evaluation of future breeding selections for their resistance to C. fioriniae.
Introduction
Anthracnose fruit rot on highbush blueberries is caused by the fungus Colletotrichum fioriniae (Marcelino & S. Gouli) R.G. Shivas & Y.P. Tan which is a member of the C. acutatum species complex (Damm et al., Citation2012). The main symptom is rotting of ripe fruit in the field before harvest and in storage after harvest (Milholland, Citation1995). Infections occur as early as fruit set, but remain latent until fruit ripening, which complicates detection of the disease. Initially, sunken areas develop on the fruit surface, followed by the formation of sporulating structures (acervuli) exuding salmon-colored spores (conidia). This disease can have a severe economic impact, with preharvest losses estimated at 10% to 20% and post-harvest losses as high as 100% in storage (Milholland, Citation1995). Most blueberry cultivars are susceptible to anthracnose fruit rot, including popular cultivars such as Bluecrop, Bluegold, Duke, Jersey, Nelson, and Ozarkblue. However, several resistant cultivars, including Elliott, Brigitta, and Legacy, display strong resistance in the field and in laboratory inoculation studies (Ehlenfeldt, Citation2003; Miles et al., Citation2009; Miles et al., Citation2012; Polashock et al., Citation2005).
The development of anthracnose resistant cultivars is necessary because resistant cultivars are not available in the early and mid-season part of the production season (Polashock et al., Citation2005). Knowledge about the inheritance of anthracnose resistance will facilitate breeding of new resistant cultivars. Currently, managing anthracnose relies almost entirely on prophylactic fungicide applications from pre-bloom until harvest (Wise et al., Citation2020). Some of these fungicides are suspected carcinogens (e.g., chlorothalonil), whereas others are prone to fungicide resistance development (e.g., azoxystrobin) (Forcelini et al., Citation2018). Resistant cultivars are necessary because despite some knowledge about the best cultural methods it is often challenging to optimize management practices. Also, high levels of fungicide resistance have been reported in some Colletotrichum-fruit pathosystems such as strawberry (Forcelini et al., Citation2018) and growers are usually unaware of this problem until a management failure occurs.
In other Colletotrichum-host pathosystems, resistance has been shown to be localized to a single gene, several genetic loci or a combination thereof. In the C. acutatum sensu lato-strawberry pathosystem, a single dominant gene (Rca2) is responsible for high-level resistance, and lower levels of resistance appeared to be quantitative and controlled by a number of minor genes (Denoyes-Rothan et al., Citation2005). In the C. acutatum sensu lato-chili pepper pathosystem, resistance was mapped to a single recessive gene in mature green fruit and a single dominant gene in ripe fruit (Mahasuk et al., Citation2009). In another species of pepper, resistance to C. capsici (Syd.) Butler & Bisby (now re-classified as C. truncatum) has been associated with a single recessive gene (Pakdeevaraporn et al., Citation2005). Mapping of host plant resistance in Phaseolus vulgaris L. has revealed two independent resistance genes within the same cluster that confer resistance to different strains of C. lindemuthianum (Sacc. & Magnus) Briosi & Cavara (Geffroy et al., Citation2008). Resistance to C. higginsianum Sacc. has been shown to be localized to a single genetic locus RCH1 in the Arabidopsis ecotype Eil-0 (Narusaka et al., Citation2004).
In previous studies, resistance to anthracnose fruit rot in blueberries was not significantly correlated with resistance to foliar infection (Ehlenfeldt et al., Citation2006) which departs from strawberries where Colletotrichum spp. can survive and reproduce asymptomatically on leaves and serve as a reservoir for specific susceptible cultivars (Leandro et al., Citation2001). Also, in blueberries resistance has not been correlated with the production of antimicrobial fruit volatiles (Polashock et al., Citation2007). However, resistance was associated with a change in infection strategy by C. fioriniae (reported as C. acutatum) (Wharton and Schilder, Citation2008). While we have information on the relative anthracnose fruit rot resistance of many cultivars based on previous studies of C. fioriniae in Michigan and those of Polashock et al. (Citation2005) in New Jersey, the susceptibility of many of the newer cultivars is largely unknown (Hancock et al., Citation2008). An understanding of how resistance to Colletotrichum species is inherited is important in making strategic breeding decisions as well as estimating durability of resistance. The objectives of this study were to: 1) determine the anthracnose fruit rot susceptibility of daughters from crosses of parents with varying susceptibility to study the inheritance and 2) correlate their susceptibility with actual and predicted resistance values from previous studies to determine heritability. Our hypothesis is that anthracnose resistance will be a heritable trait and that our results will correlate with previous screening efforts.
Materials and Methods
Plant Material
Ten to 20 individuals within a cross between parents varying in susceptibility to anthracnose were selected that were interspersed within two breeding selection blocks located at Michigan State University’s Southwest Michigan Research and Extension Center (Benton Harbor, MI). The crosses occurred prior to this study as it takes several years to establish bushes that bare fruit with sufficient quantity to screen for fruit rot resistance. The selected family blocks were randomized and planted together at 0.7-m spacing within rows and 3 m between rows. The plantings were established in 2006 and 2008. Parents with varying levels of susceptibility according to Polashock et al. (Citation2005) and Miles et al. (Citation2012) were crossed (). Sixteen crosses were evaluated including ‘Aurora’ × ‘Legacy,’ ‘Aurora’ × ‘Ozarkblue,’ ‘Bluegold’ × ‘Elliott,’ ‘Bluegold’ × ‘Nelson,’ ‘Brigitta’ × ‘Draper,’ ‘Brigitta’ × ‘Duke,’ ‘Brigitta’ × ‘Ozarkblue,’ ‘Draper’ × ‘Legacy,’ ‘Draper’ × ‘Nelson,’ ‘Draper’ × ‘Ozarkblue,’ ‘Liberty’ × ‘Legacy,’ ‘Liberty’ × ‘Nelson,’ ‘Liberty × Ozarkblue,’ ‘Nelson’ × ‘Ozarkblue,’ ‘Ozarkblue’ × ‘Elliott,’ and ‘Ozarkblue’ × ‘Legacy.’ Additionally, within the field, three reference cultivars (Bluecrop, Elliott, and Jersey) were present. Twenty individual daughter plants bearing pea-sized green fruit were selected at random within each family and the same plants were screened in 2010 and 2011 (n = 200).
Table 1. Resistance profiles based on artificial inoculation of the blueberry cultivars used in this study previously rated by Polashock et al. (Citation2005) and Miles et al. (Citation2012).
Fungal Material and Inoculation Methods
A single-conidium isolate of C. fioriniae isolated from blueberry fruit in Grand Junction, MI, USA in August 2006 was used for all inoculations. This isolate was the most virulent of 25 isolates in a preliminary test and was also used in two previous studies (Miles et al., Citation2011, Citation2012) and was originally referred to as C. acutatum strain #0001 but several significant phylogenetic studies have required that this isolate be assigned a new species name (Damm et al., Citation2012). Therefore in 2020, to confirm the current species identity of this isolate DNA was extracted using procedures described in Miles et al. (Citation2011) and the internal transcribed spacer regions were amplified (i.e. ITS1, 5.8S and ITS2) using ITS1 and ITS4 primers. A 502 bp fragment was subjected to Sanger sequencing at MSU’s Genomics – Research Technology Support Facility (East Lansing, MI, USA). BLASTn analysis revealed that this isolate shared 100% identity with the type strain of C. fioriniae (Seq ID: JQ948292) along with several other C. fioriniae strains but no other matches to members of the C. acutatum species complex. Due to the fact that, ITS was sufficient to distinguish C. fioriniae from any other species in the C. acutatum species complex, there should be no additional need to sequence other commonly used reference genes (i.e. ACT, CHS1, GADPH, HIS3, or TUB). Fungal cultures of this C. fioriniae strain were grown and stored in accordance with protocols used by Miles et al. (Citation2011). For inoculum production, sporulating cultures were flooded with 3 mL of sterile deionized water (SDW), and conidia were dislodged using a sterilized L-shaped glass rod. Conidia were counted using a hemocytometer and diluted to 1 × 106 conidia per milliliter with SDW.
Pea-sized green fruits were inoculated on each daughter plant by spraying the plant with a hand-pump sprayer until run-off, then covering the whole plant with a 114-liter-clear plastic bag for approximately 12 hours overnight. Bags were removed the following morning. In 2010, plants were inoculated on 21 and 22 of June with nighttime temperatures ranging from 20 to 27°C. In 2011, plants were inoculated on 16 and 17 of June when nighttime temperatures ranged from 15 to 24°C.
Disease Rating
All sound fruit from inoculated plants was harvested in individual 475-ml-sized clamshells when ripe (fully blue) on four different dates in 2010 (7, 14, 21, and 28 of July) and in 2011 (6, 13, 20, and 27 of July). Fruit was immediately cooled and transported to the laboratory. They were placed equidistantly on wire mesh over a layer of water in covered aluminum pans that acted as humidity chambers, and were incubated for 12 days at 22–24°C. Fruit was visually rated at 5, 8 and 12 days for incidence of C. fioriniae (i.e. number of infected fruit) in the various progeny populations and cultivars. Between 25 and 200 fruits were harvested from each individual daughter plant, and area-under–the-disease-progress curves (AUDPC) were calculated for individual berries by summing the area between 5 to 8 days and 8 to 12 days. Due to small plant size, only 25 fruit were sampled from each daughter; therefore, some families were sampled more extensively than others. Several daughters did not produce enough fruit in 2011 and were not sampled, this was attributed to twig dieback caused by the inoculation in 2010 and a subsequent loss of new fruit clusters the following year.
Statistical Analyses
Data were analyzed using an unbalanced analysis of variance and least square means differences by Tukey’s HSD’s multiple-range test for mean separation procedures of calculated AUDPC values using the statistical algorithms and GLIMMIX procedure of SAS version 6.04 (SAS Institute, Cary, NC) and SIGMAPLOT version 11 (SYSTAT Software, San Jose, CA). There was a significant effect of year (P = .008) and therefore the data from 2010 to 2011 were analyzed separately.
Average AUDPC values for each family and cultivar were regressed against previously reported resistance ratings from Polashock et al. (Citation2005) and Miles et al. (Citation2012). Based on the previous studies, an average value was calculated for the parents in a cross (mid-parent value). Midparent values were subjected to linear regression against the mean AUDPC of each cross family, and the R2 value was used to estimate heritability.
Results
Cross Family Susceptibility to Anthracnose Fruit Rot
Fruit rot caused by C. fioriniae significantly increased between 5 and 12 days after inoculation. (). Analysis of variance showed a statistically significant difference among the families and reference cultivars (P < .001 for both years). In 2010, AUDPC values across families and cultivars ranged from 117 to 417 with an average of 220. In 2011, AUDPC values ranged from 100 to 545 with an average of 262 (). Average AUDPC values were highest for ‘Bluecrop’ (417 in 2010 and 545 in 2011), moderate for ‘Jersey’ (281 in 2010 and 282 in 2011), and lowest for ‘Elliott’ (160 in 2010 and 138 in 2011).
Table 2. Area under the disease progress (AUDPC) values for the incidence of Colletotrichum fioriniae on various highbush blueberry cultivars and cross families screened by artificial inoculation in 2010 and 2011 at the Southwest Michigan Research and Extension Center (Benton Harbor, MI, USA). Fruit of 4- to 7-year-old bushes was inoculated when immature, harvested when ripe, incubated, and rated at 5, 8 and 12 days. Since the effect of year was statistically significant (P = .008), data from the two years were analyzed separately.
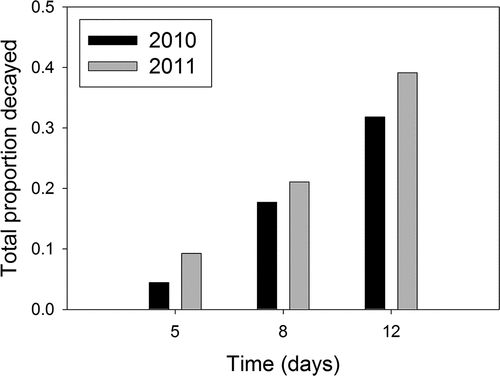
Figure 1. The proportion of all blueberry fruit decayed by Colletotrichum fioriniae after 5, 8 and 12 days of incubation at 100% RH and 22–23°C in 2010 and 2011. Fruit from the Southwest Michigan Research and Extension Center (Benton Harbor, MI) had been inoculated when immature and harvested when mature; data were averaged over all cross families and cultivars.
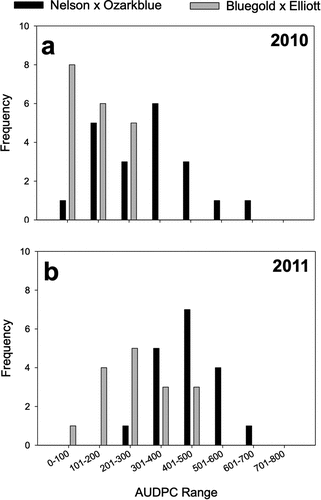
Families with the lowest average AUDPC values were ‘Aurora’ × ‘Legacy’ (118 in 2010 and 162 in 2011), ‘Bluegold’ × ‘Elliott’ (132 in 2010 and 184 in 2011), ‘Brigitta’ × ‘Draper’ (127 in 2010 and 186 in 2011), ‘Draper’ × ‘Legacy’ (189 in 2010 and 138 in 2011), and ‘Liberty’ × ‘Legacy’ (127 in 2010 and 100 in 2011). Those that showed relatively high overall AUDPC values included ‘Bluegold’ × ‘Nelson’ (343 in 2010 and 366 in 2011), ‘Draper’ × ‘Ozarkblue’ (226 in 2010 and 339 in 2011), and ‘Nelson’ × ‘Ozarkblue’ (312 in 2010 and 440 in 2011) ().
Figure 2. Frequency distribution of anthracnose fruit rot resistance, expressed as area under the disease progress curve (AUDPC), among daughters of A) a susceptible x moderate cross (‘Nelson’ x ‘Ozarkblue’) and B) a resistant x resistant cross (‘Bluegold’ x ‘Elliott’) in 2010 and 2011. Discrete levels were defined every 100 AUDPC values.
Correlation between Midparent Values and Previously Described Resistance Values
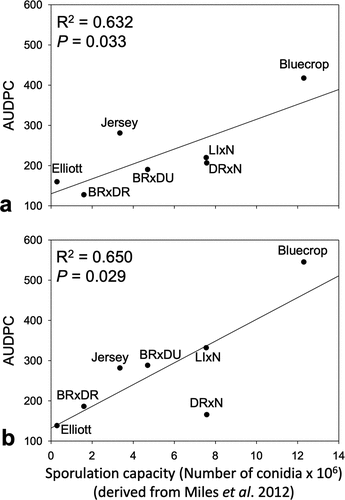
AUDPC values for families and cultivars were significantly correlated with the values derived from previous studies of anthracnose resistance including the proportion of fruit decayed (disease incidence) (R2 = 0.73, P = .001 for 2010, and R2 = 0.71, P = .004 for 2011) (Polashock et al., Citation2005) () and sporulation capacity (disease severity) (R2 = 0.63, P = .033 for 2010, and R2 = 0.65, P = .029 for 2011) (Miles et al., Citation2012) (). The predicted midparent values for the proportion of the fruit decayed for the various families averaged 0.39 and ranged from 0.31 to 0.53, using the parental values generated by Polashock et al. (Citation2005). The predicted midparent values for sporulation capacity of the various families ranged between 1.60 × 106 and 7.58 × 106 (average 5.36 × 106) conidia produced per fruit, based on the parental values generated from Miles et al. (Citation2012).
Figure 3. Correlation between the average anthracnose fruit rot incidence on various blueberry cultivars and cross families expressed as area under the disease progress curve (AUDPC) against actual and predicted proportion decayed values from Polashock et al. (Citation2005) in (A) 2010 and (B) 2011. Abbreviations for parent cultivars: BG = Bluegold; BR = Brigitta; DU = Duke; E = Elliott; LE = Legacy; N = Nelson;O = Ozarkblue.
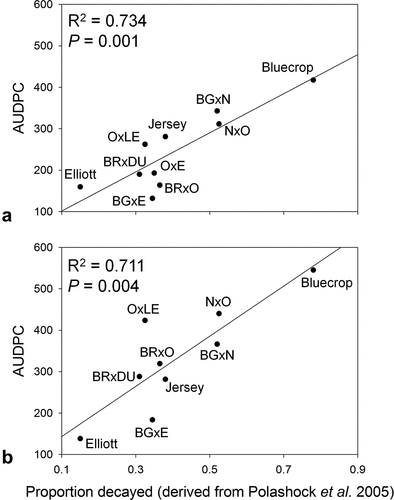
Figure 4. Correlation between the average anthracnose fruit rot incidence on various blueberry cultivars and cross families expressed as area under the disease progress curve (AUDPC) against actual and predicted sporulation capacity values from Miles et al. (Citation2012) in (A) 2010 and (B) 2011. Abbreviations for parent cultivars: BR = Brigitta; DU = Duke; DR = Draper; E = Elliott; LI = Liberty; N = Nelson.
Discussion
There were significant differences in average disease susceptibility among the cross families as well as the three reference cultivars. No family or cultivar exhibited complete resistance; however, this was not expected as previous studies have never identified a cultivar that was immune to anthracnose fruit rot. Additionally, for the three cultivars screened, our results mirrored two previous studies (Miles et al., Citation2012; Polashock et al., Citation2005) and identified ‘Bluecrop’ as highly susceptible, ‘Jersey’ as intermediate, and ‘Elliott’ as highly resistant to anthracnose fruit rot. Additionally, the overall AUDPC values for the families were significantly correlated with mid-parent values predicted from previous ratings of the parents for disease incidence and severity (Miles et al., Citation2012; Polashock et al., Citation2005). Also, in cases where both parents were strongly resistant (i.e. ‘Liberty’ and ‘Legacy’) the result was a resistant daughter phenotype. This suggests that anthracnose resistance is a heritable trait, which is strongly dependent on parental susceptibility. Furthermore, this inheritance appears to be quantitative in nature, as a continuous pattern of variability was observed within and between families. Our midparent regression values suggest that heritability (H2) for anthracnose fruit rot resistance in blueberry ranges between 63% to 73% (based on R2 regression values), depending on how resistance is measured. Such a high heritability estimate indicates that it should be relatively easy to breed for anthracnose resistance in blueberry. A study on C. graminicola resistance in corn reported values between 26 and 70% using a regression analysis (Carson and Hooker, Citation1981) meaning standard breeding efforts would be effective and transferring resistance phenotypes.
Quantitative resistance to plant pathogens is typically controlled by multiple loci and is common in many Colletotrichum plant pathosystems (Carson and Hooker, Citation1981; Denoyes-Rothan et al., Citation2005; Geffroy et al., Citation2000; Iamsupasit et al., Citation1993). While this phenomenon has been documented in many systems it is poorly understood. In strawberry different modes of inheritance of resistance to anthracnose fruit rot have been suggested in relation to pathogenicity groups of Colletotrichum (Denoyes and Baudry, Citation1995). Resistance to pathogenicity group 1 is quantitative, whereas a single dominant gene (Rca2) controls resistance to pathogenicity group 2, although minor genes may also contribute to this resistance in several cultivars (Denoyes-Rothan et al., Citation2005). Due to the high heritability values for anthracnose fruit rot of blueberry, resistance is likely more qualitative or if it is controlled by multiple genes they are likely closely linked at the genomic level. Recently published genomic resources may help elucidate these unique regions and identify unique breeding targets using QTL- or SNP-based analyses (Colle et al., Citation2019).
Understanding how resistance to anthracnose fruit rot is inherited might provide insight into the mechanism of resistance. Multiple mechanisms of blueberry anthracnose resistance have been identified such as increased sugar content within the fruit (Miles et al., Citation2012), production of pathogenesis-related proteins and reactive oxygen species (Miles et al., Citation2011), and antifungal compounds (Miles et al., Citation2013). A variety of defense mechanisms in other Colletotrichum–plant interactions has been observed, including the production of reactive oxygen species (Brown et al., Citation2008), host-derived cell wall-degrading enzymes (Casado-Diaz et al., Citation2006; Goodwin et al., Citation2004; Lafitte et al., Citation1993; Wijesundera et al., Citation1989) and preformed and induced antifungal compounds (Prusky et al., Citation2000).
Future work to study segregating populations of specific crosses will make it possible to identify loci that are involved in anthracnose fruit rot resistance in blueberries. In strawberries Sequenced Characterized Amplified Region (SCAR) markers have been developed for the Rca2 gene and have been demonstrated to be predictive of anthracnose resistance (Lerceteau-Köhler et al., Citation2005). Identification of quantitative trait loci (QTL) or associated SNPs could be a useful for developing a marker-assisted selection protocol, which will greatly facilitate future screening for anthracnose fruit rot resistance early in the breeding and selection process.
Acknowledgments
The authors gratefully acknowledge funding from Michigan State University Project GREEEN (Generating Research and Extension to meet Economic and Environmental Needs). The authors also would like to thank Christopher Woelk, Kevin Messing, and Chelsea Reynolds for technical assistance and Annemiek Schilder for critical reading of the manuscript.
Disclosure Statement
No potential conflict of interest was reported by the author(s).
Literature Cited
- Brown, S.H., O. Yarden, N. Gollop, S. Chen, A. Zveibil, E. Belausov, and S. Freeman. 2008. Differential protein expression in Colletotrichum acutatum: Changes associated with reactive oxygen species and nitrogen starvation implicated in pathogenicity on strawberry. Mol. Plant Pathol 9(2):171–190. doi: 10.1111/j.1364-3703.2007.00454.x.
- Carson, M., and A. Hooker. 1981. Inheritance of resistance to stalk rot of corn caused by Colletotrichum graminicola. Phytopathology 71:1190–1196.
- Casado-Diaz, A., S. Encinas-Villarejo, B. Santos, E. Schiliro, E.M. Yubero-Serrano, F. Amil-Ruiz, M.I. Pocovi, F. Pliego-Alfaro, G. Dorado, and M. Rey. 2006. Analysis of strawberry genes differentially expressed in response to Colletotrichum infection. Physiol. Plant 128(4):633–650. doi: 10.1111/j.1399-3054.2006.00798.x.
- Colle, M., C.P. Leisner, C.M. Wai, O. S., K.A. Bird, J. Wang, J.H. Wisecaver, A.E. Yocca, E.I. Alger, H. Tang, et al. 2019. Haplotype‐phased genome and evolution of phytonutrient pathways of tetraploid blueberry. GigaScience. 8(3):giz012. doi:10.1093/gigascience/giz012.
- Damm, U., P.F. Cannon, J.H.C. Woudenberg, and P.W. Crous. 2012. The Colletotrichum acutatum species complex. Stud. Mycol. 73:37–113. doi: 10.3114/sim0010.
- Denoyes, B., and A. Baudry. 1995. Species identification and pathogenicity study of French Colletotrichum strains isolated from strawberry using morphological and cultural characteristics. Phytopathology. 85:53–57. doi: 10.1094/Phyto-85-53.
- Denoyes-Rothan, B., G. Guérin, E. Lerceteau-Kohler, and G. Risser. 2005. Inheritance of resistance to Colletotrichum acutatum in Fragaria × ananassa. Phytopathology. 95:405–412. doi: 10.1094/PHYTO-95-0405.
- Ehlenfeldt, M.K. 2003. ‘Elliott’ highbush blueberry. J. Am. Pomol. Soc 57:2–6.
- Ehlenfeldt, M.K., J.J. Polashock, A.W. Stretch, and M. Kramer. 2006. Leaf disk infection by Colletotrichum acutatum and its relation to fruit rot in diverse blueberry germplasm. HortScience 41(1):270–271. doi: 10.21273/HORTSCI.41.1.270.
- Forcelini, B.B., C.S. Rebello, N. Wang, and N.A. Peres. 2018. Fitness, competitive ability, and mutation stability of isolates of Colletotrichum acutatum from strawberry resistant to QoI fungicides. Phytopathology. 108:462–468. doi: 10.1094/PHYTO-09-17-0296-R.
- Geffroy, V., M. Sévignac, P. Billant, M. Dron, and T. Langin. 2008. Resistance to Colletotrichum lindemuthianum in Phaseolus vulgaris: A case study for mapping two independent genes. Theor. Appl. Genet 116(3):407–415. doi: 10.1007/s00122-007-0678-y.
- Geffroy, V., M. Sévignac, J.C.F. De Oliveira, G. Fouilloux, P. Skroch, P. Thoquet, P. Gepts, T. Langin, and M. Dron. 2000. Inheritance of partial resistance against Colletotrichum lindemuthianum in Phaseolus vulgaris and co-localization of quantitative trait loci with genes involved in specific resistance. Mol. Plant-Microbe Interact. 13:287–296. doi: 10.1094/MPMI.2000.13.3.287.
- Goodwin, P.H., R.P. Oliver, and T. Hsiang. 2004. Comparative analysis of expressed sequence tags from Malva pusilla, Sorghum bicolor, and Medicago truncatula infected with Colletotrichum species. Plant Sci 167(3):481–489. doi: 10.1016/j.plantsci.2004.04.014.
- Hancock, J.F., P. Callow, S. Serce, E.J. Hanson, and R. Beaudry. 2008. Effect of cultivar, controlled atmosphere storage, and fruit ripeness on the long-term storage of highbush blueberries. HortTechnology 18(2):199–205. doi: 10.21273/HORTTECH.18.2.199.
- Iamsupasit, N., S. Chakraborty, D. Cameron, and S. Adkins. 1993. Components of quantitative resistance to anthracnose (Colletotrichum gloeosporioides) in tetraploid accessions of the pasture legume Stylosanthes hamata. Aust. J. Exp. Agric 33(7):855–860. doi: 10.1071/EA9930855.
- Lafitte, C., J. Barthe, X. Gansel, G. Dechamp-Guillaume, C. Faucher, D. Mazau, and M. Esquerré-Tugayé. 1993. Differential induction by endopolygalacturonase of β-1, 3-glucanases in Phaseolus vulgaris isoline susceptible and resistant to Colletotrichum lindemuthianum race β. Mol. Plant-Microbe Interact. 6:628–634. doi: 10.1094/MPMI-6-628.
- Leandro, L.F.S., M.L. Gleason, F.W. Nutter Jr, S.N. Wegulo, and P.M. Dixon. 2001. Germination and sporulation of Colletotrichum acutatum on symptomless strawberry leaves. Phytopathology. 91:659–664. doi: 10.1094/PHYTO.2001.91.7.659.
- Lerceteau-Köhler, E., G. Guérin, and B. Denoyes-Rothan. 2005. Identification of SCAR markers linked to Rca2 anthracnose resistance gene and their assessment in strawberry germplasm. Theor. Appl. Genet. 111:862–870. doi: 10.1007/s00122-005-0008-1.
- Mahasuk, P., P.W.J. Taylor, and O. Mongkolporn. 2009. Identification of two new genes conferring resistance to Colletotrichum acutatum in Capsicum baccatum. Phytopathology. 99:1100–1104. doi: 10.1094/PHYTO-99-9-1100.
- Miles, T., M. Nair, C. Vandervoort, and A.M.C. Schilder. 2013. Characterization and biological activity of flavonoids from ripe fruit of an anthracnose resistant blueberry cultivar. Physiol. Mol. Plant Pathol. 83:8–16. doi: 10.1016/j.pmpp.2013.02.004.
- Miles, T.D., B. Day, and A.M.C. Schilder. 2011. Identification of differentially expressed genes in a resistant versus a susceptible blueberry cultivar after infection by Colletotrichum acutatum. Mol. Plant Pathol 12(5):463–477. doi: 10.1111/j.1364-3703.2010.00687.x.
- Miles, T.D., J. Hancock, P. Callow, and A.M.C. Schilder. 2012. Evaluation of screening methods and fruit composition in relation to anthracnose fruit rot resistance in blueberries. Plant Pathol 61(3):555–566. doi: 10.1111/j.1365-3059.2011.02541.x.
- Miles, T.D., P.S. Wharton, and A.M.C. Schilder. 2009. Cytological and chemical evidence for an active resistance response to infection by Colletotrichum acutatum in ‘Elliott’ blueberries. Acta Hortic. 810:361–368. doi: 10.17660/ActaHortic.2009.810.47.
- Milholland, R.D. 1995. Anthracnose fruit rot (ripe rot), p. 17. In: F.L. Caruso and D.C. Ramsdell (eds.). Compendium of Blueberry and Cranberry diseases. APS Press, St. Paul, MN, USA.
- Narusaka, Y., M. Narusaka, P. Park, Y. Kubo, T. Hirayama, M. Seki, T. Shiraishi, J. Ishida, M. Nakashima, A. Enju, et al. 2004. RCH1, a locus in Arabidopsis that confers resistance to the hemibiotrophic fungal pathogen Colletotrichum higginsianum. Mol. Plant-Microbe Interact. 17:749–762. doi: 10.1094/MPMI.2004.17.7.749.
- Pakdeevaraporn, P., S. Wasee, P.W.J. Taylor, and O. Mongkolporn. 2005. Inheritance of resistance to anthracnose caused by Colletotrichum capsici in Capsicum. Plant Breeding 124(2):206–208. doi: 10.1111/j.1439-0523.2004.01065.x.
- Polashock, J.J., M.K. Ehlenfeldt, A.W. Stretch, and M. Kramer. 2005. Anthracnose fruit rot resistance in blueberry cultivars. Plant Dis. 89(1):33–38. doi: 10.1094/PD-89-0033.
- Polashock, J.J., R. Saftner, and M. Kramer. 2007. Postharvest highbush blueberry fruit antimicrobial volatile profiles in relation to anthracnose fruit rot resistance. J. Am. Soc. Hortic. Sci 132(6):859–868. doi: 10.21273/JASHS.132.6.859.
- Prusky, D., I. Koblier, R. Ardi, D. Beno-Moalem, N. Yakoby, and N. Keen. 2000. Resistance mechanisms of subtropical fruits to Colletotrichum gloeosporioides, p. 232–244. In: D. Prusky, S. Freeman, and M.B. Dickman (eds.). Colletotrichum: Host specificity, pathology, and host-pathogen interaction. APS Press, St. Paul, MN, USA.
- Wharton, P.S., and A.M.C. Schilder. 2008. Novel infection strategies of Colletotrichum acutatum on ripe blueberry fruit. Plant Pathol 57:122–134.
- Wijesundera, R., J. Bailey, R. Byrde, and A. Fielding. 1989. Cell wall degrading enzymes of Colletotrichum lindemuthianum: Their role in the development of bean anthracnose. Physiol. Mol. Plant Pathol. 34:403–413. doi: 10.1016/0885-5765(89)90067-2.
- Wise, J., L.A. Miles, D. Acimovic, C. Vandervoort, R. Isaacs, T.D. Miles, and A.M.C. Schilder. 2020. Sprayer type and water volume influence spatial patterns of pesticide deposition and control of diseases and insect pests of highbush blueberries. Int. J. Fruit Sci. 20(sup3):S1805–S1818. (in press). doi:10.1080/15538362.2020.1834895.
