?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Diospyros chloroxylon is the indigenous fruit of the Indian subcontinent and both unripe and ripe fruits are eaten by tribal people. The present study explores the nutritional status of Diospyros chloroxylon fruits. Unripe and ripe fruits were rich in fat, protein, carbohydrate, ash, fiber, and minerals. The energy levels of unripe, ripe fruits, and seeds were 93.78, 133.93, and 96.16 Kcal/100 g, respectively. Ripe fruits were also rich in phenolics (4.64 mg GAE/g DW) and flavonoids (1.62 mg QE/ g DW). Acetone, methanol, and water extracts of unripe, ripe fruits and seeds displayed potent free radical scavenging properties. The seeds of D. chloroxylon possess 3.16% oil and it contains oleic acid (39.91%) and palmitic acid (28.06%) as major fatty acids.
Introduction
Wild and underutilized fruits are rich sources of nutrients, vitamins, dietary fibers, and minerals (Li et al., Citation2016). Several underutilized tropical and subtropical fruits also contain polyphenolics, flavonoids, and other phytochemicals which are a richer source of antioxidants, helping to reduce the incidence of degenerative diseases, such as aging, arthritis, arteriosclerosis, brain dysfunction, cancer, heart disease, and inflammation (Ellong et al., Citation2015; Pereira-Netto, Citation2018). Studies on the nutritional and phytochemical status of underutilized fruits are very much useful for overcoming food security problems of tropics and subtropics (Mahapatra and Panda, Citation2012).
Diospyros chloroxylon (Green ebony persimmon) belongs to the family Ebenaceae and is a wild fruit-bearing plant indigenous to the Indian subcontinent. The unripe and ripe fruits have been eaten by tribal people. Diospyros chloroxylon is a small tree (), with bark dark brown; the wood is yellowish-gray which is used in the preparation of agricultural implements and musical instruments. The leaves of this plant are used as fodder (; Wealth of India, Citation1952). The unripe and ripe fruits are globose, the size of a cherry, purplish when ripe, are eaten, and are very palatable ( and d). In India, Diospyros chloroxylon is naturally distributed in Maharashtra, Andhra Pradesh, Karnataka, and Tamil Nadu states. The nutritional status of fruits of Diospyros chloroxylon is not known; therefore, in the present study, we analyzed the proximate composition, phytochemical status of fruits and seeds of Diospyros chloroxylon. We also assessed the physicochemical characteristics and fatty acid profile of seed oil of Diospyros chloroxylon.
Materials and Methods
Collection of Fruit Samples
Unripe, ripe fruits of Diospyros chloroxylon (Roxb.) were randomly collected from plants grown in Deva Deva Vana (Coordinates: 17.871061 N, 77.558572E), Bidar, India. The fruits were washed thoroughly with sterile distilled water; the pericarp and seeds () were separated from the fruits and samples were dried at 40°C in a hot air oven (ON-01E, Jeio Tech. Co. Ltd., Seoul, South Korea) for 48 h. All dried pericarp and seeds were powdered using a blender and used for nutritional composition analyses.
Chemicals and Reagents
Analytical grade chemicals were used throughout the experiment. Petroleum ether, acetone, ethanol, methanol, absolute alcohol, sodium carbonate, sodium nitrate, aluminum chloride, potassium hydroxide, potassium iodide, ninhydrin, sodium thiosulfate, iron sulfate, copper sulfate, sodium chloride, sodium hydroxide, ammonium thiocyanate, iron (II) chloride, phenolphthalein, hydrochloric acid, sulfuric acid, and Folin-Ciocalteu reagent were procured from Himedia Laboratories Pvt. Ltd. (Mumbai, India). Bovine serum albumin (BSA), gallic acid, quercetin, and Folin-Denis reagent were purchased from Sigma Aldrich Chemical Co. (USA).
Proximate Analysis
Dried and powdered pericarp and seed samples were analyzed for protein, fat, moisture, and ash content using official methods of Analysis of Association of Analytical Chemists (AOAC, Citation2000). The carbohydrate content in the samples was estimated as the difference between hundred and sum of ash, moisture, fat, and protein contents. The fiber content in the samples was determined by the method described by Sadasivam and Manickam (Citation1991). Ash content in the samples was determined by igniting the oven-dried samples in the muffle furnace at 750°C. The ash content is expressed as a percentage of the mass of the oven-dried samples.
Analysis of Minerals
A NOVA 400 atomic absorption spectrometer (Analytik Jena AG, Jena, Germany) with an air/acetylene flame and respective hollow-cathode lamps was used for absorbance measurements. Wavelengths, slits and lamp current used for the determination of elements were 766.5 nm, 0.8 nm, 4.0 mA (potassium); 213 nm, 1.0 nm, 10 mA (phosphorus); 589.0 nm, 0.8 nm, 3.0 mA (sodium); 422.7 nm, 1.2 nm, 4.0 mA (calcium); 285.2 nm, 0.7 nm, 4 mA (magnesium); 249.8 nm, 0.2 nm, 20 mA (boron); 213.9 nm, 0.5 nm, mA (zinc); 248.3 nm, 0.2 nm, 6.0 mA (iron); 258.0 nm, 0.2 nm, 10 mA (sulfur); 279.5 nm, 0.2 nm, 20 mA (manganese) and 324.8 nm, 1.2 nm, 3.0 mA (copper) respectively (AOAC, Citation2000; Fernandez-Hernandez et al., Citation2010). Nitrogen was determined using two stem digestion-UV spectrophotometric method at 220 nm (Liu et al., Citation2013). The results for mineral contents were expressed as mg/100 g DW.
Estimation of Phytochemicals
The 10 g of fruit and seed powder was extracted in 100 mL of different solvents viz. petroleum ether, acetone, methanol, and water in the increasing order of their polarity at 35–40°C, 45–50°C, 50–60°C, and 90–100°C respectively using Soxhlet apparatus for 8 h.
The quantitative estimation of phenolics was determined by the spectrophotometric method using the protocol of Ainsworth and Gillespie (Citation2007) with little modifications. 100 µL of methanolic extract was mixed with 2.5 mL of distilled water, then 0.1 mL of Folin Ciocalteu reagent and allowed to stand for 6 min and then 0.5 mL of 20% sodium carbonate reagent was added. After 30 min of incubation at room temperature, absorbance was measured at 760 nm using a spectrophotometer. The phenolic content in the sample was expressed as gallic acid equivalents (mg GAE g−1 of dry mass).
The flavonoid content of the fruit samples was determined spectrophotometrically by the method of Prior et al. (Citation2005). 0.25 mL of methanolic extract was mixed with 1.25 mL of distilled water, followed by the addition of 75 µL of 5% sodium nitrate solution. After 6 min of incubation, 0.15 mL of 10% aluminum chloride solution was added and then absorbance was measured using a spectrophotometer at 510 nm. The amount of flavonoid was expressed as quercetin equivalent (mg QE g−1 of dry mass).
The quantification of phytic acid was carried out by the spectrophotometric method (Agostinho et al., Citation2016). Aliquots of 1 mL of calcium chloride solution (10 mg L−1), 0.2 mL of glyoxal bis(2-hydroxyaniline) solution (1 mg L−1), 1.0 mL of borate buffer solution (pH 12.5) containing CTAB (1 mmol L−1), 10 mL of sample or phytic acid and 1.8 mL of ethanol/methanol mixture were taken in falcon tube and after 20 min absorbance was measured by using a spectrophotometer at 500 nm.
The amount of oxalic acid was determined using the method of Oke (Citation1966). 1 g of fruit samples with 6 M hydrochloric acid was heated at 90°C for 4 hours. The obtained supernatant was titrated with concentrated ammonia solution in presence of methyl orange until the color changed to faint yellow. The warm solution was precipitated with 5% calcium chloride solution. The precipitate was dissolved with 25% sulfuric acid and titrated against 0.05 M potassium permanganate till the appearance of persistent pale pink color.
Evaluation of Antioxidant Activity by 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Scavenging Activity
The antioxidant activity of un-ripened and ripened fruit and seed acetone, methanol, and water extracts was estimated as described by Chen et al. (Citation1999). Two milliliters of 100 µM DPPH solution in ethanol was mixed with 2 mL of 100 µg.mL−1 pulp and seed extracts. The reaction mixture was incubated in the dark for 15 min and the optical density was recorded at 517 nm against a blank. For the control, 2 mL of DPPH solution in ethanol was mixed with 2 mL of ethanol and the optical density of the solution was recorded after 15 min. The decrease in optical density of DPPH on the addition of test samples in relation to the control was used to calculate the antioxidant activity, as a percentage inhibition of DPPH radical. The percentage of the DPPH remaining was calculated as
The percentage of remaining DPPH* (DPPH*REM) is proportional to the antioxidant concentration, and the concentration that causes a decrease in the initial DPPH* concentration by 50% is defined as EC50 (Brand-Williams et al., Citation1995).
Estimation of Antioxidant Activity by Ferric Reducing Antioxidant Power (FRAP) Assay
The reducing power was determined by following the procedure of Oyaizu (Citation1986). Pulp and seed acetone, methanol, and water extracts of samples were mixed with 2.5 mL of 200 mM sodium phosphate buffer (pH 6.6) and 2.5 mL of 10 g.L−1 potassium ferrocyanide. The reaction mixtures were incubated at 50°C for 20 min, followed by the addition of 1 mL of 10% trichloroacetic acid. The mixtures were then centrifuged and 1 mL supernatant was treated with 1 mL distilled water and 200 µL of 0.1% ferric chloride. The absorbance of the reaction mixture was measured at 700 nm.
Estimation of Total Antioxidant Capacity by Phosphomolybdenum Method
The total antioxidant capacity of the fruit acetone, methanol, and water extracts was evaluated as described by Prieto et al. (Citation1999). An aliquot of 0.3 mL of the sample solution was mixed with 2.7 mL of the reagent solution consisting of 0.6 M sulfuric acid, 28 mM sodium phosphate, and 4 mM of ammonium molybdate. Ethanol of 0.3 mL was mixed with 2.7 mL of the reagent to prepare blank. The absorbance of the test sample was measured at 695 nm. Ascorbic acid and butylated hydroxyl anisole were used as standards.
Physico-chemical Characterization of Seed Oil
The seed oil () was extracted by a heat-reflux method using 100 ml of petroleum ether (b. p. 40–60°C). The percent of oil presented was determined gravimetrically after removal of the solvent. The specific gravity and refractive index were determined by using the pycnometer method and the Abbe refractometer using an analytical method (AOAC, Citation2000). Iodine, ester, peroxide, and saponification values were also determined by standard AOAC methods (AOAC, Citation2000).
Determination of Fatty Acid Profiles of Seed Oil
The fatty acid composition of seed oil was determined by gas chromatography using fatty acid methyl esters (FAMEs). FAMEs were prepared using catalyst boron trichloride according to the method of AOAC (Citation2000). Fatty acids were quantified using Varian GX 3400 flame ionization gas chromatograph (Labteknik, Hyderabad, Telangana, India), with a fused silica capillary column, Chromopack CPSIL 88 (100 m length, 0.25 μm ID, 0.2 μm film thickness). The temperatures of column, injector, and detector were maintained at 182°C, 182°C, and 225°C repetitively. Fatty acid methyl esters in hexane (1 μL) were injected into the column using a Varian 8200 CX auto-sampler with a split ratio of 100:1. Nitrogen was used as carrier gas at 45 psi. Identification of sample fatty acids was made by comparing the relative retention times fatty acid methyl ester peaks from samples with those of standards obtained from Sigma (Supelco 37 Component FAME mix; Sigma-Aldrich, Bangalore, India). Chromatograms were recorded with Varian Star chromatography software version 4.0 and the relative percentage composition of fatty acids quantified as ratios of peak areas.
Statistical Analysis
All experiments were conducted in triplicate using three different lots of fruits. Data are presented with standard errors. Analysis of variance was applied to all the data using Duncan’s multiple range test.
Results
Proximate Analysis of Fruits and Seeds
The proximate composition of unripe, ripe fruits (pulp), and seeds are presented in . The unripe fruits of Diospyros chloroxylon contained 49.22, 0.94, 4.03, 17.30, 2.73, 25.28 g/100 g of moisture, fat, protein, carbohydrate, ash, and fiber respectively. The amounts of moisture, fat, protein, carbohydrate, ash, the fiber in ripe fruits were 50.04, 1.29, 3.82, 26.76, 2.35, 14.80 g/100 g respectively. Whereas, seeds possessed 35.87, 3.16, 4.04, 12.89, 2.30, 41.46 g/100 g of moisture, fat, protein, carbohydrate, ash, fiber. As the Diospyros chloroxylon fruit involves in the ripening process the fiber content decreases considerably and there was an enhancement in the concentration of carbohydrates (). The energy levels of unripe, ripe fruits (pulp), and seeds were 93.78, 133.93, and 96.16 Kcal/100 g, respectively.
Table 1. Proximate composition of Diospyros chloroxylon Roxb. fruits
Mineral Composition of Fruits and Seeds
The fruits are a rich source of a large number of minerals and trace elements that need a human body. Diospyros chloroxylon fruits are rich in varied minerals (). The amount of nitrogen (N), phosphorous (P), and potassium (K) in the pulp of the unripe fruit were 794, 69, and 1540 mg/100 g, respectively. The concentration of sulphur (S), sodium (Na), calcium (Ca), magnesium (Mg), boron (B), zinc (Zn), iron (Fe), manganese (Mn) and copper (Cu) were 53.4, 1600, 1200, 960, 6.2, 1.1, 45.6, 3.7 and 0.1 mg/100 g in unripe fruits. The levels of N, P, K, S, Na, Ca, Mg, B, Zn, Fe, Mn, and Cu were 827, 80, 1430, 53.4, 1630, 1800, 840, 8.3, 17.1, 12, 0.4, 0.1 mg/100 g, respectively, in ripe fruits pulp, whereas their levels were 1390, 150, 1190, 30, 1690, 1600, 480, 10, 9.9, 10, 2.3, and 6.9 mg/100 g, respectively in seeds of Diospyros chloroxylon.
Table 2. Mineral concentration of Diospyros chloroxylon Roxb. fruits
Phytochemical Composition of Fruits and Seeds
Varied phytochemicals including phenolics, flavonoids, phytic acid, and oxalic acid were estimated in the unripe, ripe fruits and the seed of Diospyros chloroxylon extract of acetone, methanol, and water, and results are presented in . Phenolics and flavonoids were rich in unripe fruits and the levels of these phytochemicals were 15.98 mg of GAE/ g DW and 1.05 mg of QE/ g DW in methanol extract, whereas the amount of flavonoids was rich in acetone extract (2.97 mg QE/ g DW) of unripe fruits. The levels of phenolics (4.63 mg GAE/g DW), flavonoids (1.62 mg QE/ g DW) were optimal in the methanol extract of ripe fruits. Seeds were also abundant with these phytochemicals and 70.63 mg GAE/ g DW of phenolics and 13.21 mg QE/g DW of flavonoids were recorded in the methanol extract of seeds ().
Table 3. Phytochemical composition of Diospyros chloroxylon Roxb. fruits
Table 4. Amount of anti-nutrients in Diospyros chloroxylon Roxb. fruits
Anti-nutrient Composition of Fruits and Seeds
Phytic acid and oxalic acid are considered antinutrient factors and the levels of these phytochemicals were 25.59 and 23.40 mg/g DW, respectively in ripe fruits ().
Antioxidant Activities of Fruit and Seed Extracts
Antioxidant activities of acetone, methanol, and water extracts of unripe, ripe fruits, and seeds were analyzed by 2,2-diphenyl-1-picrylhydrazyl (DDPH) scavenging assay, ferric reducing antioxidant power (FRAP) assay, and phosphomolybdate assay (total antioxidant capacity), and the results are presented in . The acetone extract of pulp obtained from unripe and ripe fruits showed better DPPH scavenging activity compared to methanol and water extracts (). The highest DPPH scavenging activity was evident with acetone extract of unripe fruit pulp with an EC50 value of 0.38 mg/mL and it was lower than acetone extract of ripened fruit pulp (0.56 mg/ml). FRAP assay showed that acetone extract un-ripened fruit and seeds of Diospyros chloroxylon were good antioxidant agents (). These values were comparable to ascorbic acid and butylated hydroxyl anisole (standards) used for the assay. All the extracts showed optimal total antioxidant capacity and were dose-dependent increase with increasing concentration (). The antioxidant activity of extracts increased in the order water >methanol>acetone. The seed acetone extract was 539.49 µg ascorbic acid equivalents (AAE)/mL and it was higher than that of seed methanol extract (409.17 µg AAE/mL).
Table 5. Antioxidant activity of Diospyros chloroxylon fruits: DPPH scavenging activity of unripe fruit pulp extracts, ripe fruit pulp extracts and seed extracts compared with ascorbic acid (AA) and butylated hydroxyl anisole (BHA) standards
Table 6. Antioxidant activity of Diospyros chloroxylon fruits: antioxidant power (absorbance at 593 nm) of unripe fruit pulp extracts, ripe fruit pulp extracts and seed extracts by FRAP assay compared with ascorbic acid (AA) and butylated hydroxyl anisole (BHA) standards
Table 7. Antioxidant activity of Diospyros chloroxylon fruits: total antioxidant capacity (µg ascorbic acid equivalents per ml) of unripe fruit pulp extracts, ripe fruit pulp extracts and seed extracts by phosphomolybdate assay compared with ascorbic acid equivalent (AAE)
Physical, Chemical Properties and Fatty Acid Profile of Seed Oil
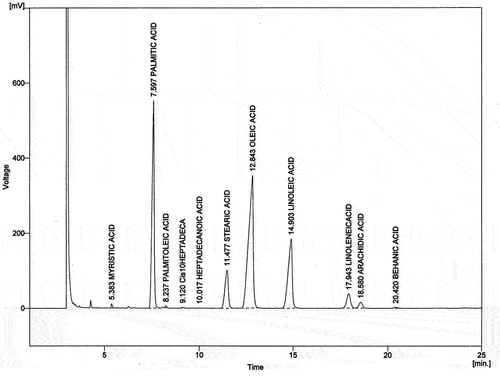
The seeds of D. chloroxylon contain 3.16% oil and data on the physical and chemical properties of the seed oil is presented in . The oil was liquid at room temperature and yellow. The specific gravity and refractive index of the oil were 0.88 g/cc and 1.48 respectively. The acid value, saponification value, iodine value, peroxide value, and ester value of seed oil were 5.61 mg of KOH/g, 70.12 mg of KOH/g, 74.87 g/ 100 g, 3.85 meq O2/kg, and 64.51 mg of KOH/g respectively. shows the fatty acid composition of seed oil. The most abundant fatty acids were oleic acid (39.91%) and palmitic acid (28.06%) respectively. The oil also possessed linoleic acid (18.01%), stearic acid (8.24%), and γ-linolenic acid (3.51%). The monounsaturated fatty acid (MUFA) and polyunsaturated fatty acid (PUFA) were 40.24% and 21.52% respectively, and saturated fatty acid (SAFA) content was 38.23% ().
Table 8. Physiochemical properties of Diospyros chloroxylon Roxb. seed oil
Table 9. Fatty acid compositions (%) of Diospyros chloroxylon Roxb. seed oil
Discussion
Proximate Analysis of Fruits and Seeds
Wild fruits are a vital part of the diet and are also a rich source of varied phytochemicals/bioactive compounds (Murthy and Bapat, Citation2020). Diospyros chloroxylon (Green Ebony persimmon) is one such wild fruit indigenous to the Indian subcontinent and widely used by local people, however, the nutritional status and biochemical composition of this fruit are not known. In contrast one of its akin, Diospyros kaki (persimmon) is a widespread fruit that is rich in nutrients, minerals, dietary fiber, and bioactive phytochemicals such as polyphenols, terpenoids, steroids (Yaqub et al., Citation2016). In the current study, we analyzed the proximate composition, the phytochemical status of fruits of Diospyros chloroxylon. Proximate analysis of Diospyros chloroxylon reveals that unripe, ripe fruits and seeds are rich in fat, protein, carbohydrate, ash, fiber (). The ripe fruits contained 1.29, 3.82, 26.76, 2.35, and 14.80 g/100 g of fat, protein, carbohydrate, ash, and fiber respectively. The energy levels of unripe, ripe fruits, and seeds were 93.78, 133.93, and 96.16 Kcal/100 g, respectively. The nutritional value of Diospyros kaki was reported to possess 18.59 g/100 g of carbohydrate, 0.19 g/100 g of fat, 0.38 g/100 g of protein (USDA, Citation1998). In comparison to Diospyros kaki, D. chloroxylon possesses higher levels of carbohydrate, fat, and protein. D. melonoxylon (commonly called tendu fruits) fruits are reported to accumulate higher carbohydrates when they in ripened condition compared to unripe fruits (Sailakshmi et al., Citation2018) and ripe fruits contain total sugar of 3.78 g/100 g. The ripened fruits of D. chloroxylon (2.35 g/100 g) contain comparatively lesser carbohydrates than D. melanoxylon (3.78 g/100 g). Both D. melanoxylon and D. chloroxylon fruits are commonly used in wine preparation by tribals of South India (Sailakshmi et al., Citation2018).
Mineral Composition of Fruits and Seeds
It was reported that the major minerals of Diospyros kaki were 8–17 mg/100 g of calcium, 0.15–2.50 mg/100 of iron, 17–26 mg/100 g of phosphorous, 161–310 mg/100 g of potassium, and 1 mg/100 g of sodium (Butta et al., Citation2015). The levels of N, P, K, S, Na, Ca, Mg, B, Zn, Fe, Mn, and Cu were 827, 80, 1430, 53.4, 1630, 1800, 840, 8.3, 17.1, 12, 0.4, 0.1 mg/100 g, respectively, in ripe fruits of D. chloroxylon (). Thus, D. chloroxylon fruits are rich in varied minerals and their concentration was comparable to fruits of Diospyros kaki (Butta et al., Citation2015).
Phytochemical Composition of Fruits and Seeds
Diospyros kaki fruits are a rich source of phytochemicals including naphthoquinones, anthraquinones, terpenoids, lignans, steroids, flavonoids, and phenolic acids (Rauf et al., Citation2017). In the current study, varied phytochemicals including phenolics, flavonoids, phytic acid, and oxalic acid were estimated in the unripe, ripe fruits and the seed of Diospyros chloroxylon extracted in acetone, methanol, and water, and results are presented in . The levels of phenolics (4.64 mg GAE/g DW) and flavonoids (1.62 mg QE/ g DW) were optimal in methanol extract of ripe fruits of D. chloroxylon. The levels of phenolics content in the pulp of D. chloroxylon fruit, decreased sharply when the fruit ripened. In unripe fruit, total phenolic content was 15.98 mg of GAE/ g DW and it has decreased to 4.63 mg GAE/ g DW (). More than 3 times decrease was observed in the phenolic content of the pulp. A decrease in phenolics after fruit ripening has also been observed in D. melonoxylon (Sailakshmi et al., Citation2018) and Mangifera indica (Ajila et al., Citation2007). Seeds of D. chloroxylon were also abundant with these phytochemicals and 70.63 mg GAE/ g DW of phenolics and 13.21 mg QE/g DW of flavonoids, were recorded in methanol extract of seeds ().The quantity of phenolics and flavonoids was highest in seeds compared to unripe and ripe fruits of D. chloroxylon (). In contrast to the current results, Inglett and Chen (Citation2011) reported that phenolic compounds in the skin (52.73 mg/g) was almost 3 times of that found in pulp (16.95 mg/g) and 4 times of that seeds (11.56 mg/g) of miracle fruit. They have reported a similar trend for flavonoid contents of miracle fruit.
Antioxidant Activities of Fruit and Seed Extracts
Antioxidant assay of acetone, methanol, and water extracts of unripe, ripe fruits and seeds of D. chloroxylon were analyzed by 2,2-diphenyl-1-picrylhydrazyl (DDPH) scavenging assay, ferric reducing antioxidant power (FRAP) assay and phosphomolybdate assay (total antioxidant capacity or TAC) and the results showed that varied fruit extracts depicted potent DPPH scavenging, ferric reduction activities (). TAC assay also showed a reduction of molybdenum in a concentration-dependent manner (). The highest DPPH scavenging activity was evident with acetone extract of unripe fruit pulp with an EC50 value of 0.38 g/ml (). The free antioxidant activities from seeds were higher in all three methods studied (DPPH, FRAP, and TAC methods; ) when compared to unripe and ripe pulp extracts. Comparison of unripe pulp extracts showed better antioxidant activity than ripe pulp extracts. The free antioxidant activity for seed was about two times higher when compared with ripe pulp extract and this trend was evident in all the three in vitro antioxidant assays (DPPH, FRAP, and TAC methods; ). Thus, the seeds could be a good resource to be utilized for antioxidant activity. The high antioxidant activity of D. chloroxylon seeds could be related to the high total phenolic and flavonoid contents. Similar, results were found in Diospyros kaki (persimmon) fruits where high content of phenolics and flavonoids found in various parts of pulp extracts had high antioxidant activities (Gorinstein et al., Citation2001; Jung et al., Citation2005). Further, it was reported that the differences in free antioxidant activity of different parts of the fruits might be because of various extraction methodologies. For example, antioxidant activities from pulp and skin using direct method were significantly lower than from pulp and skin using double extraction in miracle fruit (Inglett and Chen, Citation2011). The free antioxidant activity was possible not completed by single extraction using the direct method. In general, the antioxidant activity using sequential methods was higher than that using the direct method since bound antioxidant activities were added to total antioxidant activities. Therefore, the sequential extraction method might be suitable for studying the antioxidant activity of D. chloroxylon.
Physical, Chemical Properties and Fatty Acid Profile of Seed Oil
The seeds of D. chloroxylon possess 3.16% oil which was having low free fatty acid (2.82% as oleic acid) and low acid value (5.61 mg of KOH/g; ). It was reported that the oils with high peroxide values are unstable and can easily become rancid (Ojeh, Citation1981). The peroxide value of D. chloroxylon seed oil was 2.85 meq O2/kg), which could be stored for a longer duration. The iodine value of this oil, 74.87 g/100 g, placed it in the non drying oil. The fatty acid composition of D. chloroxylon seed oil reveals it contains oleic acid (39.91%) and palmitic acid (28.06%) as major fatty acids (). Palmitic acid, oleic acid, and linoleic acids are major fatty acids found in Diospyros kaki seeds, ranging from 70.4% to 78.3% of total fatty acids (Yaqub et al., Citation2016). Oils containing higher concentrations of oleic acid and linoleic acid were considered as good edible oils as they are good for health (Menendez et al., Citation2005; Willett, Citation2007). The monounsaturated fatty acid (MUFA) and polyunsaturated fatty acid (PUFA) were 40.24% and 21.52% respectively, and saturated fatty acid (SAFA) content was 38.23% (). Similar to these results, Diospyros virginiana seeds were reported to contain 80.24% unsaturated fatty acids with oleic acid and linoleic acids as major components and 16.25% saturated fatty acids with stearic acid and palmitic acids (Cloughly and Burlage, Citation1959).
Conclusions
In conclusion, proximate, phytochemical, and antioxidant analysis of unripe, ripe fruits, and seeds of Diospyros chloroxylon was assessed in the present study and results reveal that unripe, ripe fruits and seeds are rich in proteins, carbohydrates, fat/oil, and minerals. The fruits and seeds are also abundant with phytochemicals viz. phenolics, flavonoids and acetone, methanol and water extracts of fruits and seeds depicted strong free radical scavenging properties. Major fatty acids of seed oil were oleic acid (39.91%) and palmitic acid (28.06%). The above results showed that fruits and seed oil of Diospyros chloroxylon are useful for edible purposes.
Acknowledgments
Authors are thankful to the DST-PURSE Phase II program and UGC-BSR Mid-Career Award [No. F.19-223/2018(BSR)].
Disclosure Statement
No potential conflict of interest was reported by the author(s).
Literature Cited
- Agostinho, A.J., W.D. Oliveira, D.S. Anunciacao, and J.C.C. Santos. 2016. Simple and sensitive spectrophotometric method for phytic acid determination in grains. Food Anal. Methods. 19:2087–2096. doi: 10.1007/s12161-015-0387-0.
- Ainsworth, E.A., and K.M. Gillespie. 2007. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2:875–877. doi: 10.1038/nprot.2007.102.
- Ajila, C.M., S.G. Bhat, and U.J.S. Prasada Rao. 2007. Valuable components of raw and ripe peels from Indian Mango verities. Food Chem. 102:1006–10011. doi: 10.1016/j.foodchem.2006.06.036.
- AOAC. 2000. Official methods of analysis of the association of analytical chemists. 17th ed. Arlington VA, USA: Association of Official Agricultural Chemists International.
- Brand-Williams, W., M.E. Cuvelier, and C. Berset. 1995. Use of free radical method to evaluate antioxidant activity. LebensmWiss Technol. 28:25–30.
- Butta, M.S., M.T. Sultanb, M. Aziza, A. Nazc, W. Ahmeda, N. Kumard, and M. Imrane. 2015. Persimmon (Diospyros kaki) fruit: Hidden phytochemicals and health claims. EXCLI J. 14:542–561. doi: 10.17179/excli2015-159.
- Chen, Y., M. Wang, R.T. Rosen, and C.T. Ho. 1999. 2,2-Diphenyl-1-picrylhydrazyl radical-scavenging active components from Polygonum multiflorum thunb. J. Agric. Food Chem. 47:2226–2228. doi: 10.1021/jf990092f.
- Cloughly, C.P., and H.M. Burlage. 1959. An examination of the oil of the seeds of Persimmon (Diospyros virginiana L., Fam. Ebenuceue). J. Am. Pharm. Assoc. 48:449–451. doi: 10.1002/jps.3030480807.
- Ellong, E., C. Billard, S. Adenet, and K. Rochefort. 2015. Polyphenols, carotenoids, vitamin content in tropical fruits and vegetables and impact of processing methods. Food Nutrit. Sci. 6:299–313.
- Fernandez-Hernandez, A., R. Mateos, J.A. Garcia-Mesa, G. Beltran, and R. Fernandez-Escobar. 2010. Determination of mineral elements in fresh olive fruits by flame atomic spectrometry. Span. J. Agric. Res. 8:1183–1190. doi: 10.5424/sjar/2010084-1206.
- Gorinstein, S., Z. Zachwieja, M. Folta, H. Barton, J. Piotrowicz, M. Zemser, M. Weisz, S. Trakhtenberg, and O. Martin-Belloso. 2001. Comparative contents of dietary fiber, total phenolics, and minerals in persimmons and apples. J. Agric. Food Chem. 49:952–957. doi: 10.1021/jf000947k.
- Inglett, G.E., and D. Chen. 2011. Contents of phenolics and flavonoids and antioxidant activities in the skin, pulp, and seeds of Miracle fruit. J. Food Sci. 76:C479–C482. doi: 10.1111/j.1750-3841.2011.02106.x.
- Jung, S.T., Y.S. Park, Z. Zachwieja, M. Folta, H. Barton, J. Piotrowicz, E. Katrich, S. Trakhtenberg, and S. Gorinstein. 2005. Some essential phytochemicals and the antioxidant potential in fresh and dried persimmon. Int. J. Food Sci. Nutr. 56:105–113. doi: 10.1080/09637480500081571.
- Li, Y., J.J. Zhang, D.P. Xu, T. Zhou, Y. Zhou, S. Li, and H.B. Li. 2016. Bioactivities and health benefits of wild fruits. Int. J. Mol. Sci. 17:1258. doi: 10.3390/ijms17081258.
- Liu, W.J., F.X. Zeng, and H. Jiang. 2013. Determination of total nitrogen in solid samples by two-step digestion–ultraviolet spectrophotometry method. Commun. Soil Sci. Plant Anal. 44:1080–1091. doi: 10.1080/00103624.2012.750330.
- Mahapatra, A.K., and P.C. Panda. 2012. Wild edible fruit diversity and its significance in the livelihood of indigenous tribals: Evidence from eastern India. Food Sec. 4:219–234. doi: 10.1007/s12571-012-0186-z.
- Menendez, J.A., L. Vellon, R. Colomer, and R. Lupu. 2005. Oleic acid, the main monounsaturated fatty acid of olive oil, suppresses Her-2/neu (erbB-2) expression and synergistically enhances the growth inhibitory effects of trastuzumab (Herceptin) in breast cancer cells with Her-2/neu oncogene amplification. Ann. Oncol. 16:359–371. doi: 10.1093/annonc/mdi090.
- Murthy, H.N., and V.A. Bapat. 2020. Importance of underutilized fruits and nuts. In: H.N. Murthy, and V.A. Bapat (eds.). Bioactive compounds in underutilized fruits and nuts, reference series in phytochemistry. Switzerland AG: Springer Nature, pp 3–19. doi:10.1007/978-3-030-06120-3_1-1
- Ojeh, O. 1981. Effects of refining on the physical and chemicalproperties of cashew kernel oil. J. Fats Oils Technol. 16:513–517.
- Oke, O.L. 1966. Chemical studies on some Nigerian vegetables. Trop. Sci. Trop. Sci. 8:128–132.
- Oyaizu, M. 1986. Studies on product of browning reaction prepared from glucosamine. Japn. J. Nutr. Diet. 44:307–315. doi: 10.5264/eiyogakuzashi.44.307.
- Pereira-Netto, A.B. 2018. Tropical fruits as natural, exceptionally rich, sources of bioactive compounds. Int. J. Fruit Sci. 18:3, 231–242. doi: 10.1080/15538362.2018.1444532.
- Prieto, P., M. Pineda, and M. Aguilar. 1999. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of Vitamin E1. Anal. Biochem. 269:337–341. doi: 10.1006/abio.1999.4019.
- Prior, R., X. Wu, and K. Schaich. 2005. Standardized methods for the determination of antioxidant capacity and phenolic in foods and dietary supplements. J. Agric. Food Chem. 53:4290–4302. doi: 10.1021/jf0502698.
- Rauf, A.G., U.S. Patel, A. Khan, S.A. Halim, S. Bawazeer, K. Ahmad, N. Muhammad, and M.S. Mubarak. 2017. Diospyros, an under-utilized, multi-purpose plant genus: A review. Biomed. Pharmacother. 91:714–730. doi: 10.1016/j.biopha.2017.05.012.
- Sadasivam, S., and A. Manickam. 1991. Biochemical methods. 3rd edn ed. New Age International Publishers, New Delhi.
- Sailakshmi, A.S.R., A. Anand, K. Madhhusudhana, V.L. Nayak, A. Zehra, K. Suresh Babu, and A.K. Tiwari. 2018. Diospyros melanoxylon (Roxb.): A tribal fruit that maintains a euglycemic state after consumption and cools oxidative stress. Indian J. Nat. Prod. Res. 9:194–203.
- USDA. 1998. Agriculture research service, p. 47–88. Vol. 44. National Agricultural Library, Beltsville, Md, USA.
- Wealth of India. 1952. A dictionary of Indian raw materials and industrial products, p. 236. In: Raw materials, council of scientific and industrial research. Vol. 3. (New Delhi, India: Council of Scientific and Industrial Research).
- Willett, W.C. 2007. The role of dietary n-6 fatty acids in the prevention of cardiovascular disease.J. Cardiovasc. Med. 8:S42–S45. doi: 10.2459/01.JCM.0000289275.72556.13.
- Yaqub, S., U. Farooq, A. Shafi, K. Akram, M.A. Murtaza, T. Kausar, and F. Siddique. 2016. Chemistry and functionality of bioactive compounds present in Persimmon. J. Chem. ID 3424025:13.