ABSTRACT
One major problem with cacao crops is the variability of plantings obtained from seeds. This has led to considering somatic embryogenesis (SE), a means of obtaining stable clones with minimal somaclonal variation; however, achieving this has not been possible with many genotypes. In this work, we used IMC67 genotype (rootstock in the traditional grafting process) to evaluated various SE protocols; four culture media for induction and expression primary embryogenesis (PSE): induction medium (INDI), expression medium (INDIEXP), primary callus induction medium (PCG), and secondary callus growth medium (SCG), as well as two explants (petal and staminode). Different culture times for PSE (15, 25, 35, 45, 55, 65 days) and SSE (10, 20, 30, 40, 50, 70, 80 days) were evaluated in terms of callus formation, PSE, and SSE. For acclimation, seven substrates were evaluated for plantlet survival. One hundred percent callogenesis was achieved at 50 days with the staminode in INDI, and the most PSE per explant (8) in INDIexp was achieved at 45 days. Secondary somatic embryogenesis (SSE) obtained the most globular and cotyledonary embryos (30) at 30 days in callus multiplication (CM2). The embryos achieved conversion and development into plantlets in maturation medium (MM6). Embryogenic efficiency from explants was 11.7 for PSE, and 15.2 for SSE. Acclimation obtained a 42.1% survival rate in the 70% sand- 30% soil substrate. This work reports a complete regeneration protocol via somatic embryogenesis up to plantlet development in a nursey for the IMC67 rootstock genotype; contributing to standardizing a high-quality plant production system.
Introduction
Theobroma cacao L. grows in humid tropical areas, is part of the Malvacea family, and is an important species (Sánchez, Citation2018). Cacao beans have nutritional, therapeutic, and cosmetic attributes and are the main raw material for the chocolate industry. Cacao is also a functional product because of its beneficial effects on cardiovascular diseases and high levels of antioxidants (Badrie et al., Citation2015). Colombia has the right agro-environmental conditions to be a net exporter of cacao. Its cacao beans are cataloged as fine and aromatic (Abbott et al., Citation2019), with awards at the “Paris Chocolate Show” in 2010, 2011, 2015, and 2019 for samples from the departments of Arauca, Nariño, Antioquia and Santander (Bucaramanga, Citation2019). Cacao production is increasing in Colombia, moving from 36.7 thousand MT in 2000 to 59.7 thousand MT in 2019. As of November 2019, Colombia recorded 8.33 thousand MT in exports, an 18% increase with respect to the 7.06 thousand MT exported in 2018. Imports (402 MT in November 2019) decreased by 32% with respect to November, 2018 (Fedecacao, Citation2019). Colombia needs strategies to maintain this production and export rate in order to continue and strengthen this trend, which would further benefit cacao farmers and production areas where cacao is the economies’ axis.
Sufficient high-quality planting material is required to establish new crops and renew old ones. Cacao has various propagation systems, such as seeds, which is considered a simple, traditional, inexpensive, and common means. However, it is highly variable unless the seeds were obtained through controlled pollination in certified nurseries (Palencia et al., Citation2007). Grafting and root cuttings have provided plant material to establish plantations. Nevertheless, these techniques require large clonal gardens, are completely manual processes and have not been industrialized. Therefore, developing quick, highly efficient systems to facilitate cacao’s vegetative propagation is considered necessary. Asexual propagation has been advised for commercial crops in order to increase propagation levels, decrease multiplication time and ensure constant high-quality production (Fernandez, Citation2018).
Somatic embryogenesis (SE) was described by Williams and Maheswaran (Citation1986) as “the process by which haploid or diploid somatic cells develop into differentiated plants through characteristic embryological stages without fusion of gametes.” SE is a common process present in nature that occurs during both embryonic and post-embryonic plant development, known as a type of apomixis (Smertenko and Bozhkov, Citation2014). On an international level, somatic embryogenesis has successfully been established as a biotechnological propagation system, achieving massive plantlet multiplication in little time. This has allowed streamlining genetic improvement work on the species, in addition to conserving and multiplying it and exchanging its genetic material (Guillou et al., Citation2018; Solano, Citation2008). Somatic embryos are similar to zygotic embryos in terms of morphology and development, but their genetic structure is identical to the plant of origin (Mata, Citation2013). Plants obtained from SE require an ex vitro adaptation, in most cases, to a difficult phase in the process. For ex vitro acclimation, it is necessary to provide some optimal environmental factors in order to gradually transition the plantlets from the in vitro environment to the natural environment’s conditions. These factors are air humidity, adequate pH, ventilation, and adequate substrate (crop soil, sand, perlite, peat, or various mixes). For cacao, substrates, such as soil from the field, sand and coconut coir, have been used in successful previous ex vitro acclimatization experiments (Henao et al., Citation2018; Urrea et al., Citation2011).
Companies such as Nestlé, currently use SE to propagate their cacao crops in countries such as Ecuador, Indonesia, Puerto Rico, Brazil, Ghana and Ivory Coast (Guillou et al., Citation2015). These propagation methodologies will be more efficient as long as there are optimized protocols that decrease malformations and somaclonal variations to efficiently germinate plants (Ajijah et al., Citation2016). Promising results have been obtained in Colombia for cacao propagation through somatic embryogenesis in solid media for commercial genotypes CCN51, TSH565, EET8, ICS1, ICS39, ICS60, ICS95 and Colombian genotypes CNCh12, CNCh13, CNCh16, CNCh24, CNCh4 (Henao et al., Citation2018). Genotype IMC67 is among the genotypes of interest for propagation. It is considered the most commercially used rootstock in countries such as Colombia, Peru and, Ecuador (Delgadillo, Citation2017). According to Echeverri (Citation2006), a rootstock must have a high tolerance to soil and climate conditions, various plagues and radicular diseases, and a good vegetative vigor. Clones IMC67, PA121-46-150, Pound7-12, EET 399–400-96, SPA9, and CAU37-39-42, which have these main traits, are the rootstocks recommended for use (Giraldo et al., Citation2018).
Some studies on agronomic performance in Costa Rica and Colombia have demonstrated that IMC67 has significant attributes in the nursery phase, with a larger stem diameter and resistance to diseases (Cardenas, Citation2017; Sarmiento et al., Citation2011). In addition, it is moderately susceptible to bud-rot (Phytophthora palmivora) and moniliasis (Moniliophthora roreri) and moderately resistant to witches’ broom disease (Crinipellis perniciosa) (Gamboa, Citation2015; Gamboa et al., Citation2017).
Plants are propagated without sexual crossing through grafting, ensuring the plant’s crown has genetic and phenotypic characteristics identical to the donor plant. However, the rootstock, which normally originates from a seed, and is therefore heterogeneous, provides the root and a short segment of the stem (Palencia et al., Citation2007). Since propagating the rootstock and crown via somatic embryogenesis can guarantee a genetic identity with the donor plants while conserving the crop’s traits of interest (Rodríguez et al. (Citation2004), Rodríguez et al. (Citation2010) and Ajijah et al. (Citation2016)), this ensures producing plants without pathogens. This helps reduce space in clonal gardens and obtain a greater amount of plant material. This work’s aim was to evaluate two protocols for PSE and SSE induction in the IMC67 genotype and evaluate different substrates in the acclimation processes of plantlets developed in a nursery. This is the first report of a complete regeneration protocol via somatic embryogenesis up to plantlet development in a nursery for the IMC67 rootstock genotype.
Materials and Methods
Plant Material
The plant material consisted of staminodes and petals derived from closed flower buds of the IMC67 rootstock genotype. The immature closed flower buds were collected from field-grown plants of Yariguíes farms owned by Compañía Nacional de Chocolates in Barrancabermeja’s municipality in the department of Santander – Colombia. The flowers were transported to Universidad de Antioquia’s Laboratory of Plant Physiology in the city of Medellín, in the department of Antioquia – Colombia. The acclimation phase was performed in a nursery at the “La Nacional” farm owned by Compañia Nacional de Chocolates in the municipality of Támesis in the department of Antioquia – Colombia.
The Flower Buds collection and disinfection process was performed in accordance with the methodology described by Henao et al. (Citation2018).
Explant Preparation and Culture
Petals and staminodes were extracted from flower buds using sterile scalpels. Twenty to 25 explants were placed on the culture media (induction medium) per Petri dish. The effect of the detection protocol for the percentage of petal and staminode contamination and necrosis was evaluated in the first experimentstaminode. Contamination by fungi and bacteria (%) was calculated 5 days after in vitro introduction and necrosis at 10 days. Each Petri dish was considered an experimental unit. This experiment had a total of 225 repetitions. The experiment was laid out in a completely randomized design (CRD) with one factor: explant type (staminode and petal).
In vitro Stage: Callogenesis, primary and secondary somatic embryogenesis
Callus induction starts off cacao’s primary somatic embryogenesis process. Callus was induced from petals and staminodesin the second experiment. These explants were cultivated in two semi-solid culture media to evaluate induced embryogenic calli, the first named PCG by Maximova et al. (Citation2002) and the second named INDI by Fontanel et al. (Citation2002) (Appendix 1). Callus percentage (%) was calculated at different cultivation times (10, 20, 30, 40, 50, 60 days) and for two explant types (petals and staminodes) and two culture media (PCG and INDI). A total of 225 repetitions were performed for callogenesis. The experiment was laid out in a CRD with three factors: explant type, culture time and culture media.
In the third experiment, explants were subsequently transferred to evaluate the PSE expression stage in two culture media: SCG (Maximova et al., Citation2002) and INDIexp (Fontanel et al., Citation2002) (Appendix 1). Different cultivation times were evaluated for PSE (15, 25, 35, 45, 55, 65, 75 days), as well as two explant types (petals and staminodes). All primary embryo counts were in the globular and cotyledonary development stages. One hundred and ten repetitions were performed for PSE. The experiment was laid out in a CRD with three factors: culture media, culture time, and explant type.
In the fourth experiment, once the primary somatic embryos developed, they were fragmented and moved to the CM2 medium for SSE induction (Fontanel et al., Citation2002). Different cultivation times were evaluated for SSE (5, 10, 20, 30, 40, 50, 70, 80 days), and all embryo counts were in the globular and cotyledonary development stages. One hundred and ten repetitions were performed for SSE induction. The experiment was laid out in a CRD with one factor: culture time. Embryogenic efficiency (EE) related to average number of embryos per explant was calculated in accordance with the protocol of Garcia et al. (Citation2016). Embryogenic efficiency for PSE and SSE was determined through the relationship between the total number of produced somatic embryos and the total number of initial explants for primary and secondary somatic embryogenesis independently.
Once the SSE developed, they were transferred to the MM6 maturation medium (Henao et al., Citation2018) (Appendix 1). For the maturation stage, two embryo development experiments were performed from the globular to plantlet stage with 30 repetitions, with an average of 67 explants per Petri dish.
In the fifth experiment, different cultivation times (8, 16, 24, 32 days) were evaluated for abnormal embryo development, and the sixth experiment evaluated the effect of embryo stage on abnormal development. The considered embryo stages were the immature stage (IM) with globular embryos, transition stage (TS) with heart-torpedo/early cotyledonary embryos and mature stage (MA) with mature cotyledonary embryos. The experiments were laid out in a CRD with one factor in each experiment: culture time and developmental stage, respectively.
Culture Conditions
All the cultures were randomly placed in a growth chamber in continuous darkness for callogenesis, PSE and SSE, at an average temperature of 26°C ± 2°C and 70% relative humidity. The plantlets were cultured in 500 ml vessels and placed in a growth chamber under light with a 16-hour photoperiod and a photosynthetic photon flux density (PPFD) of 50 μmol m − 2 per second, at an average temperature of 26°C ± 2°C and 70% relative humidity.
Ex vitro Stage: Acclimation
In the seventh experiment, preliminary tests for ex vitro adaptation were performed in a nursery at the “La Nacional” farm owned by Compañia Nacional de Chocolates. Approximately 130 plants regenerated by somatic embryogenesis were used and the effects of different substrates on plantlets’ development and survival were evaluated: (1) 100% soil from the field, (2) 100% sand, (3) 100% coconut coir, (4) 50% soil-50% sand, (5) 70% soil-30% sand, (6) 30% soil-70% sand, (7) 30% soil- 70% coconut coir. All substrates were previously sterilized in the laboratory and subsequently taken to the farm. Plantlets with a minimum of 3 cm-long stems and some level of rooting were kept in covered seedbeds for 2–3 days and subsequently transferred to plastic bags (30x14cm). The variable responses were stem length (cm), total number of leaves and survival (%) at 20 days after cultivation. Humidity was controlled every 4 hours by an automatic irrigation system by nebulization.
Statistical Analysis
Results were subjected to an analysis of variance (ANOVA), and a comparison test was performed based on residuals to test normality. If they were not normal, the Kruskal-Wallis and Mann Whitney test was used. For unequal numbers, an analysis of variance across the generalized linear model (GLM) was performed for some stages with a Poisson variable response distribution. Dispersion, box and whisker diagrams were used. A significance of 95% was determined for all comparisons using the R project v3.6.1 software.
Results and Discussion
Explants’ responses to somatic embryogenesis in cacao are genotype-dependent. In order to develop a reliable and applicable protocol for the somatic embryogenesis of cacao genotypes, IMC67 was tested in differing culture conditions during this study, from in vitro establishment to acclimation. In the first experiment of the study, microbial contamination by fungi (Supplement, Table 1) and bacteria (Supplement, Table 2) was found not to be related to the type of explant. However, it should be noted that different climate conditions during flower collection lead to changes in the abundance of microorganisms on the crops, and fungi emergence is higher during rainy seasons than in dry seasons (data not shown). The percentage of tissue necrosis was found to be significantly higher for petals (Supplement, Table 3), possibly because petals are more external on the floral structure and have a larger surface area exposed to disinfectants, which can cause more oxidative damage. Flowers were collected during the afternoon and floral tissues were kept between 10–15°C during transportation to reduce the percentage of necrosis during experiments (data not shown).
In vitro Stage: Callogenesis, PSE and SSE
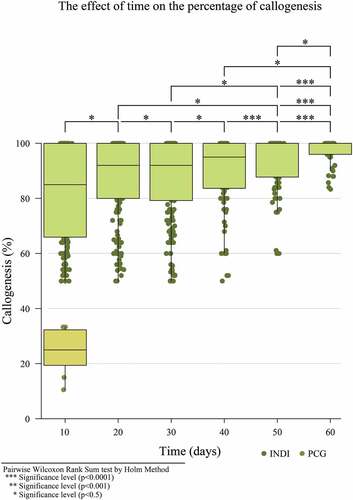
In the second experiment, callogenesis induction in the PCG medium with both explants (petals and staminodes) was a median 25%, while it surpassed 87% for both explants in the INDI medium, with 94.7% callus formation for the staminode explant and 88% for the petal explant (Supplement, Figure S1). In addition, it was observed that callus formation occurred at a median of 85% at 10 days and up to 100% at 50 days. Therefore, the best response to callus formation was with the INDI medium with a staminode explant 10 days after cultivation (). The results are in accordance with that which Monsalve et al. (Citation2005) reported for IMC67, where the staminode explant demonstrated a greater callogenic response. The response can be explained because staminodes are adjacent to the meristematic area on a flower’s structure. In addition, in a culture medium, the entire staminode’s surface is in contact with it, which is not the petal’s case due to its curved structure (Gallego et al., Citation2016).
Figure 1. Dispersion diagram for the effect of time on the percentage of callogenesis on petal and staminode explants with INDI and PCG culture media.
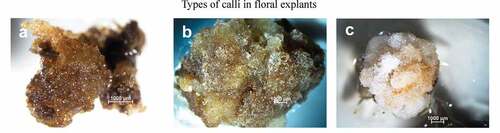
In accordance with Gallego et al. (Citation2016), three types of calli were also recognized: a vitreous granular-white callus (TC), waxy granular-brown callus (WBC) and filamentous white callus (FC) (). Embryos always originated from brown granular calli (WBC), which is considered an embryogenic callus for cacao. The presence of polyphenols in cacao is always associated with embryogenic development and, depending on their position inside or outside of the proembryogenic tissue, they can act as process inductors or repressors (Chanatásig and Aguilar, Citation2004; Kouassi et al., Citation2017). Furthermore, Kouassi et al. (Citation2018) found that embryogenic differentiation can be promoted through phenols, which, as antioxidants, are better substrates for oxidative enzymes, allowing auxin release. In addition, the results are consistent with Daouda et al. (Citation2019), in which the combination of 2,4-D and KIN is suitable for inducing callogenesis and, subsequently, primary embryo formation. PSE formation came about from the WBC embryogenic callus (), although two other clearly recognizable types of calli were present. The first consisted of round, translucent glassy-white cells (TC) () and the second type consisted of waxy-yellow cells (WC) (), which did not complete the full embryogenic process. These results were comparable to those obtained by Gallego et al. (Citation2016) for genotype ICS95 and Henao et al. (Citation2018) for genotypes EET8, ICS1, ICS39, ICS60, ICS95.
Figure 2. Different types of calli developed in floral explants. Embryogenic (a) granular, waxy-yellow calli (WBC), a mixed callus of waxy-yellow cells (WC) (b), a nodular (c) callus of round, translucent, glassy-white cells (TC).
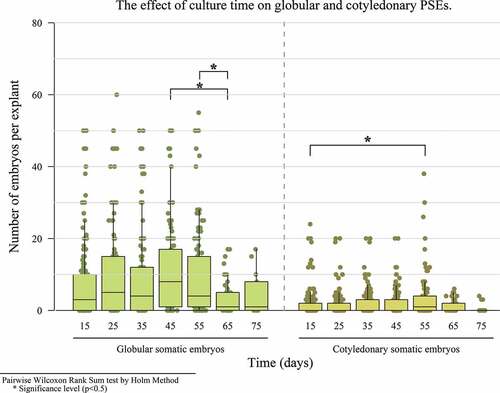
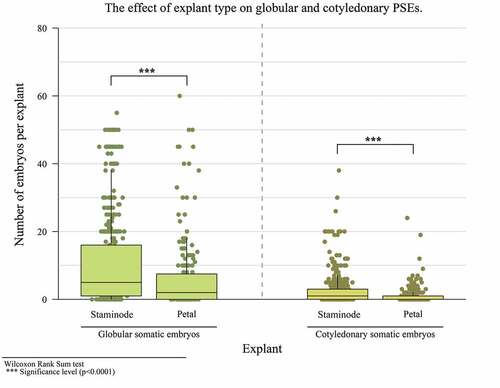
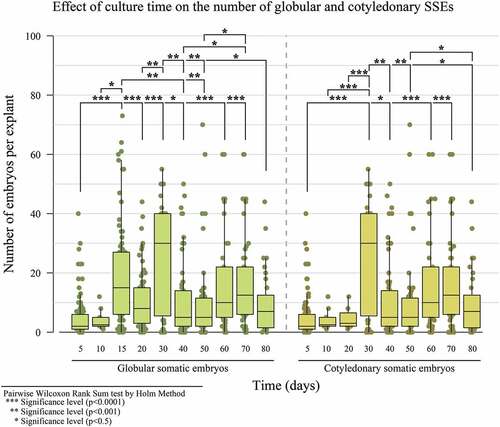
In the third experiment, a relationship between the type of medium, type of explant and different culture times was found in PSE induction in a globular state. PSE showed a significant increase at 45 days of cultivation, with a median of 8 embryos. In terms of cotyledonary embryos, there was a maximum production of 38 embryos at 55 days (). A significant difference with respect to the explant and culture media was observed for both types of embryos, obtaining more embryos with the staminode explant (). The same was true for the INDIexp medium ().
Various authors such as Minyaka et al. (Citation2017), Ajijah and Hartati (Citation2019) and Iracheta et al. (Citation2019) affirm that the callogenic and embryogenic response in cacao is a dependent genotype, and culture conditions and type of explant influence it. Monsalve et al. (2005) performed a study with the IMC67 genotype. Despite achieving a high frequency of callus formation, they only obtained primary embryos up to the heart stage. Minyaka et al. (Citation2008), using IMC67, also obtained a larger percentage of embryo formation with the staminode explant. However, PCG and SCG mediums with 6.0 mM MgSO4 concentrations were used for this work. The differences in embryogenic responses between these studies can be due to the different growth regulators, culture medium components and type and physiological state of the used explant (Boutchouang et al., Citation2016). Various authors have confirmed that DKW basal salts are more appropriate for the somatic embryogenesis of cacao than MS basal salts (Ajijah et al., Citation2016; Li et al., Citation1998). The positive response to the combination of nutrients from DKW basal salts can be the result of a higher concentration of calcium, sulfur and magnesium. These macronutrients can be crucial during the first induction stages and expression of proembryogenic structures and embryos (Ajijah et al., Citation2016).
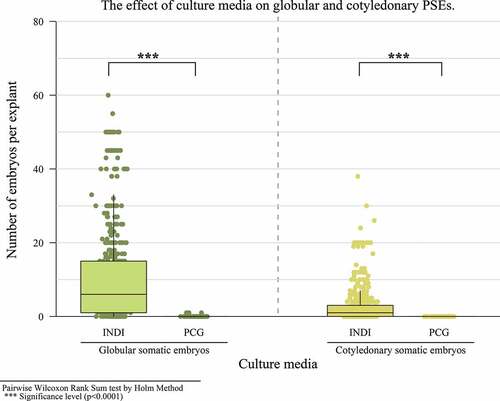
Somatic embryogenesis is begun in the INDI induction medium, which contains auxin and cytokinin growth regulators (with auxin (2,4 D) and cytokinin (KIN)). Cells respond to different stress factors, changing their development program to a specific physiological condition by reprogramming gene expression toward acquiring an embryonic competence (Fehér et al., Citation2016; Méndez et al., Citation2019). For cacao, it has been identified that stress factors, such as lesions, salt content and growth regulators like TDZ, KIN and NAA can trigger floral tissue somatic cell conversion into competent embryonic callus and embryo formation (Ajijah and Hartati, Citation2019). This embryogenic response in tissues occurs, not only because of the external addition of growth regulators, but also due to the result of interactions between exogenous regulators and endogenous contents from other regulators in the plant tissue. The presence of KIN in the culture medium can specifically favor embryogenic tissue induction and regeneration. This entire process is a coordinated action with auxins and amino acids, which offer a hormonal balance that can lead to regenerating various organs (Guillermo et al., Citation2003).
Releasing the hormonal stimulus of the explants from the INDI medium, which are moved to the INDexp medium, enables their embryogenic potential’s expression and development (Guillou et al., Citation2018; Iracheta et al., Citation2019). The INDexp culture medium, which does not have growth regulators, responds well. This may be because of the presence of various amino acids, such as arginine, glycine, leucine, lysine and tryptophan. These amino acids are an effective and immediate source of nitrogen for the cells and are considered essential for embryogenesis (Méndez et al., Citation2019). The negative responses obtained from the SCG medium may have occurred by using high concentrations of auxin, which can suppress organized growth and nullify the embryogenic mass’ polarity, inhibiting the embryogenic process begun by endogenous auxins. Although it has theoretically been said that somatic embryogenesis begins with a culture medium with high auxin levels, especially 2,4-D (Smertenko and Bozhkov, Citation2014), it is important to note that the correct balance between internal and external stimuli is required. The obtained results could suggest that the concentration of 2,4-D affects embryogenic development in this genotype. The SCG culture medium also contains TDZ, which normally increases purine supply, assisting cell development and metabolite accumulation (Victor et al., Citation1999). However, the presence of TDZ in the SCG induction medium may have limited embryogenic responses. According to Guillermo et al. (Citation2003), this regulator is highly resistant to cytokinin oxidase’s actions, which suggests it can remain active for prolonged periods of time, inhibiting the expected response.
In the fourth experiment, SSE ()) was achieved in the CM2 culture medium drawing from PSE fragments () originated from the INDexp medium. The most SSE was obtained with the staminode explant (Supplement, Figure S2), as well as a significant difference at 30 days, with a median of 30 globular and cotyledonary embryos (). According to Daouda et al. (Citation2019), using auxins, in this case 2,4,5-T, is common for inducing SSE in cacao. It has been used for numerous genotypes with positive results. Embryogenic efficiency by explant was 11.7 for PSE and 15.2 for SSE. A 29.8% increase was observed for each explant between PSE and SSE. This result was similar to the one reported by Garcia et al. (Citation2016) for the TSH1188 genotype, with 12% EE.
Figure 6. Primary somatic embryos (a – c), Secondary somatic embryos (d – f), Malformed embryos (G – I).
Figure 7. The effect of culture time on the number of globular and cotyledonary SSEs formed for IMC67 in the CM2 culture medium.
In the fifth experiment, 52.1% of embryos reached the cotyledonary stage and developed into plantlets during SSE development and conversion in the MM6 medium. The remaining percentage includes necrotic embryos and malformations such as embryonic fusion, embryos with a single cotyledon, multicotyledonary embryos and embryos without apical and radicular development or atrophied development ()). The lowest number of malformed embryos in the MM6 medium was obtained at 24 and 32 days (), with a median of 2 embryos and no significant difference between them. The most malformations were 37 embryos at 8 days. In the sixth experiment, no significant difference was observed between the IM stage and TS stage, in which the most malformations were obtained. This coincides with the first 8 days of the experiment. Upon comparing this with the MA stage, it had significant differences with respect to the IM and TS stages. The MA stage had the least malformed embryos and, therefore, the embryos must be transferred from the CM2 medium to MM6 in the MA ().
Figure 8. Malformed secondary cotyledonary somatic embryos with respect to culture time in MM6 maturation medium.
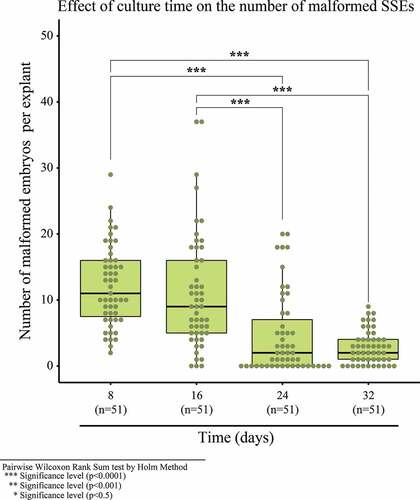
Figure 9. Malformed secondary cotyledonary somatic embryos with respect to the development stage in which they were transferred to the MM6 maturation medium. IM (immature), TS (transition) and MA (mature).
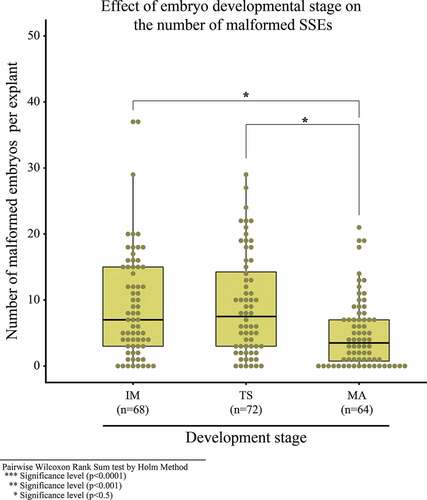
Malformations are typical in cacao during the embryogenic process and have been reported by various authors (Garate et al., Citation2017; Garcia et al., Citation2019). They have tried solving this issue using liquid media (Guillou et al., Citation2018). Having tested the IMC67 genotype and with researchers’ experience with other genotypes such as CCN51 and TSH565, it has been determined that malformations increase upon prolonging the SSE’s individuation process. Therefore, they must be separated from the globular stage. In addition, prolonged time under the effect of growth regulators can trigger these malformations (using 2,4,5-T has been reported to be a trigger) (Kahia et al., Citation2017; Lázaro et al., Citation2015).
Embryo development and conversion in the MM6 medium are the result of the effect of ANA and GA3 growth regulators, activated carbon and other culture medium components, such as amino acids, salts, vitamins, high concentrations of macroelements, especially nitrogen as NH4NO3, and high carbon concentrations originating from glucose. ANA and GA3 have also been used by Iracheta et al. (Citation2019) to favor cotyledonary embryo development. However, they add 3.7 µM of abscisic acid. Moreover, various authors have been able to develop somatic embryos from Juglans regia (Ali et al., Citation2010) and Cucurbita pepo (Urbanek et al., Citation2004) in the culture medium with MS salts, supplemented with NAA and GA3. In addition, GA3 has also been used to elongate and develop embryos and plantlets in other species (Kordestani and Karami, Citation2008). NH4NO3 also favors embryo conversion to plantlets as a source of inorganic nitrogen (Menke and Zimny, Citation2001). Activated carbon can have a significant effect on embryo development because it can absorb substances, such as toxic metabolites and phenolic exudates, which inhibit the process, preventing correct maturation. Additionally, when the culture medium becomes darker, it allows accumulating auxins and/or cofactors due to the generated environment (López et al., Citation2005).
Ex vitro Stage: Acclimation
In the last experiment, no significant difference was found between treatments 20 days after the ex vitro adaptation in number of leaves (Supplemment, Table 4) and stem length (Supplement, Table 5). A higher survival rate was obtained in 70% sand – 30% soil from the area (42.1%). The lack of nutrients in sand can be compensated by the cacao crop’s soil, which generally favors plantlet growth. (Supplemment, Table 6). The treatment with the lowest survival rate was in the 70% sand – 30% coconut coir substrate (22.2%). This is contrary to other reports, which have shown that this mixture has a significant effect on survival, growth expressed as stem length and the number of formed leaves. Coconut coir offers high porosity, aeration capacity and water retention capacity, and sand offers good drainage, consistency and porosity, which could reduce the risk of flooding and favor good rooting (Vasquez, Citation2005) ().
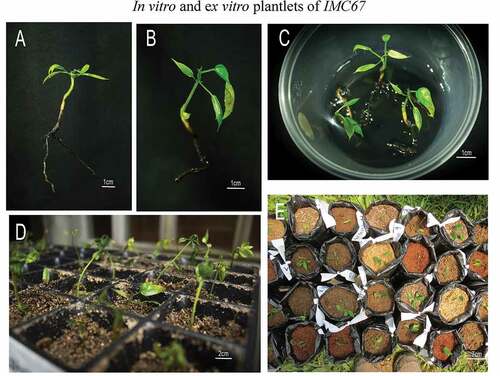
Figure 10. Complete IMC67 plantlets to be transferred to ex vitro conditions (a and b), Elongating and developing plantlets in a maturation medium (c), Plantlets in nurseries during the first days of acclimation (d) and plants transferred in growth bags for preliminary substrate evaluation in the ex vitro adaptation of nursery conditions (e).
Conclusion
This article offers a study for propagating genotype IMC67 cacao (T. cacao L.) by somatic embryogenesis, achieving 100% callogenesis in 50 days with the INDI staminode, the most PSE’s per explant in the globular stage (8) in INDIexp at 45 days to be fragmented and work as the basis for SSE induction and the most globular and cotyledonary SSE’s (30) at 30 days in CM2. An embryogenic efficiency of 11.7 embryo per explant was obtained for PSE and 15.2 for SSE. Cacao regeneration, maturation and somatic embryo conversion into plants were finally achieved.
In perspective, using a hormone-free medium between secondary embryogenesis and the embryo maturation stage has been proposed for future tests, which would favor the embryogenic potential’s expression, prematuration and a reduction of malformations. Evaluating a germination medium that focuses on developing roots to obtain a more robust plant has also been proposed. We recommend evaluating the treatment matrix again with more experimental units and repetitions for ex vitro adaptation.
Supplemental Material
Download MS Word (108.8 KB)Acknowledgments
We would like to thank professional Juan Diego Villa Franco for his statistical and technical advice, as well as the laboratory of plant physiology and plant tissue culture of Universidad de Antioquia. Special thanks to Universidad de Antioquia’s Research Development Committee (CODI) and Granja Yariguíes – Compañia Nacional de Chocolates.
Disclosure Statement
No potential conflict of interest was reported by the author(s).
Supplementary Material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Abbott, P., T. Benjamin, G. Burniske, M. Croft, M. Fenton, C. Kelly, M. Lundy, F. Rodriguez, and M. Wilcox. 2019. An analysis of the supply chain of Cacao in Colombia. Colombia: United States Agency for International Development-USAID.
- Ajijah, N., and R. Hartati. 2019. Primary and secondary somatic embryogenesis of cacao: The effect of explant types and plant growth regulators. Indones J Agric Sci 20(2):69–76. doi: 10.21082/ijas.v20n2.2019.p69-76.
- Ajijah, N., R. Hartati, R. Rubiyo, D. Sukma, and D. Sudarsono. 2016. Effective cacao somatic embryo regeneration on kinetin supplemented DKW medium and somaclonal variation assessment using SSRs markers. Agrivita 38(1):80–92. doi: 10.17503/agrivita.v38i1.619.
- Ali, S., V. Kourosh, B. Hassan, K. Siamak, and L. Charles. 2010. Enhancement of maturation and germination of somatic embryos in Persian walnut (Juglans regia L.) using osmolites, hormones and cold treatments. African J Food Sci 4(12):735–743.
- Badrie, N., F. Bekele, E. Sikora, and M. Sikora. 2015. Cocoa agronomy, quality, nutritional, and health aspects. Crit Rev Food Sci Nutr 55(5):620–659. doi: 10.1080/10408398.2012.669428.
- Boutchouang, R., O. Zebaze, A. Ngouambe, and N. Niemenak. 2016. Influence of the position of flowers buds on the tree on somatic embryogenesis of cocoa (Theobroma cacao L.). Int J Plant Physiol Biochem 8(2):7–16. doi: 10.5897/IJGMB2016.0247.
- Bucaramanga, C. 2019. Destacan en París al cacao de Santander Colombia. El Tiempo, Colombia. 24 Mar 2020. https://www.eltiempo.com/colombia/otras-ciudades/destacan-en-paris-al-cacao-de-santander-429588.
- Cardenas, N. 2017. Efecto de la simbiosis de Rhizophagus irregularis con plántulas de cacao (Theobroma cacao L.) en el tizón de plántulas (Phytophthora palmivora (Butler)). Universidad Nacional De Colombia, Colombia.
- Chanatásig, C., and M. Aguilar 2004. Inducción de la embriogénesis somática en clones superiores de cacao (Theobroma cacao L.), con resistencia a enfermedades fungosas. Centro Agronómico Tropical de Investigación y Enseñanza, Costa Rica.
- Daouda, K., K. Modeste, N. Oulo, and K. Edmond. 2019. Induction of somatic embryos of recalcitrant genotypes of Theobroma cacao L. J Appl Biosci 133(1):13552. doi: 10.4314/jab.v133i1.7.
- Delgadillo, P. 2017. Estudio de la respuesta de defensa de la especie Theobroma cacao L. a la infección de Phytophthora palmivora (Butler). Universidad Nacional de Colombia, Colombia.
- Driver, J., and A. Kuniyuki. 1984. In vitro propagation of Paradox walnut rootstock. HortScience 19:507–509.
- Echeverri, J. 2006. El injerto en la producción de cacao orgánico. Manejo Integr Plagas y Agroecol 78(53):21–23.
- Fedecacao. 2019. Producción de cacao en Colombia. Fed Nac Cacaoteros. Colombia. 24 Mar 2020. https://www.fedecacao.com.co/portal/index.php/es.
- Fehér, A., D. Bernula, and K. Gémes. 2016. The Many Ways of Somatic Embryo Initiation, p. 1–506. In: V.M. Loyola-Vargas and N. Ochoa-Alejo (eds.). Somat Embryog Fundam Asp Appl. Springer, Cham, Switzerland.
- Fernandez, F. 2018. Efecto de reguladores de crecimiento en la inducción de callo embriogénico en láminas foliares de cacao (Theobroma cacao L.) variedad CCN51 establecidas in vitro. Escuela Agrícola Panamericana, Honduras.
- Fontanel, A., S. Gire, G. Labbe, P. Favereau, M. Alvarez, S. Von, and V. Petiard. 2002. In vitro multiplication and plant regeneration of Theobroma cacao L. via stable embryogenic calli. IAPTC Congr Plant Biotechnol 1: 23–28. beyond.
- Gallego, A., A. Henao, A. Urrea, and L. Atehortúa. 2016. Polyphenols distribution and reserve substances analysis in cocoa somatic embryogenesis. Acta Biol Colomb 21(2):335–345. doi: 10.15446/abc.v21n2.50196.
- Gamboa, R., R. Borjas, D. Saravia, G. Alarcon, L. Alvarado, and A. Julca. 2017. Comportamiento en Vivero de Diferentes Patrones y Plantas Injertadas De Cacao (Theobroma cacao L.) en Rio Negro, Satipo, Junín, Perú. Rev Pakamuros 5(1):34–42.
- Gamboa, R. 2015. Comportamiento en vivero de cuatro clones de cacao (Theobroma cacao L.) sobre diferentes patrones ensatipo. Universidad Nacional Agraria La Molina, Peru.
- Garate, M., E. Arévalo, L. Do Bomfim, and D. Da Costa. 2017. Pro-embrionary somatic structure of three Cacao Genotypes (Theobroma Cacao L.) using staminode s. Int Ann Sci 2(1):28–32. doi: 10.21467/ias.2.1.28-32.
- Garcia, C., A. Furtado de Almeida, M. Costa, D. Britto, R. Valle, S. Royaert, and J. Marelli. 2019. Abnormalities in somatic embryogenesis caused by 2,4-D: An overview. Plant Cell Tissue Organ Cult. 137(2):193–212. doi: 10.1007/s11240-019-01569-8.
- Garcia, C., F. Corrêa, F. Seth, A. Alex, C. Marcio, C. Motamayor, R. Schnell, and M. Jean. 2016. Optimization of somatic embryogenesis procedure for commercial clones of Theobroma cacao L. African J Biotechnol 15(36):1936–1951. doi: 10.5897/AJB2016.15513.
- Giraldo, D., D. Valencia, and D. Mesa. 2018. Análisis comparativo del estudio fisicoquímico de suelos con plantación de Theobroma cacao L. en zonas específicas de los municipios de Belén de Umbría- Risaralda y Belalcázar-Caldas. Suelos Ecuatoriales 48:57–63.
- Guillermo, I., A. Angarita, and T. Mosquera. 2003. Inducción de embriogénesis somática en Alstroemeria spp. Agron Colomb 21(3):121–128.
- Guillou, C., A. Fillodeau, E. Brulard, D. Breton, S. De Faria, D. Verdier, M. Simon, and J. Ducos. 2018. Indirect somatic embryogenesis of Theobroma cacao L. in liquid medium and improvement of embryo-to-plantlet conversion rate. Vitr Cell Dev Biol - Plant 54(4):377–391. doi: 10.1007/s11627-018-9909-y.
- Guillou, C., A. Fillodeau, E. Brulard, D. Verdier, M. Simon, A. Landmann, F. Lausanne, A. Fontanel, J. Ducos, A. Buchwalder, et al.2015. Nestlé Cocoa plan: Cocoa propagation by somatic embryogenesis. p. 75–80. In: eds. Y.S. Park and J.M. Bonga. Proc Third Int Conf IUFRO “Woody Plant Prod Integr Genet Veg Propag Technol. Vol. 1, IUFRO, Spain.
- Henao, A., T. De-La-Hoz, T. Ospina, L. Atehortúa, and A. Urrea. 2018. Evaluation of the potential of regeneration of different Colombian and commercial genotypes of cocoa (Theobroma cacao L.) via somatic embryogenesis. Sci. Hortic. 229:148–156. doi: 10.1016/j.scienta.2017.10.040.
- Iracheta, L., L. Cruz, P. Lopez, C. Avendaño, and S. Ortiz. 2019. 2iP and brasinosteroids promote somatic embryogenesis induction in Theobroma cacao L. Agroproductividad 12(1):65–70.
- Kahia, J., S. Kone, L. Diby, G. Ngoran, C. Dadjo, and C. Kouame. 2017. Enhanced plantlet regeneration in two cacao (Theobroma cacao) clones from immature inflorescence explants. HortScience 52(6):892–895. doi: 10.21273/HORTSCI11844-17.
- Kordestani, G., and O. Karami. 2008. Picloram-induced somatic embryogenesis in leaves of strawberry (fragaria ananassa l.). Acta Biol Cracoviensia Ser Bot 50(1):69–72.
- Kouassi, M., E. Koffi, O. Silue, M. Tahi, M. Toure, and E. Konan. 2018. Comparison of systems combining auxins with thidiazuron or kinetin supplemented with polyvinylpirrolidone during embryogenic callus induction in three Theobroma cacao L. genotypes. Int J Biol Chem Sci 12(2):804. doi: 10.4314/ijbcs.v12i2.15.
- Kouassi, M., E. Manlé, D. Koné, B. Soumahoro, T. Koné, K. Koffi, and M. Koné. 2017. Effect of antioxidants on the callus induction and the development of somatic embryogenesis of cocoa (Theobroma cacao L.). Aust J Crop Sci 11(1):25–31. doi: 10.21475/ajcs.2017.11.01.pne174.
- Lázaro, A., L. Azpeitia, S. Sáenz, and F. Mirafuentes. 2015. Somatic secondary embryogenesis in the genotype of cocoa (Theobroma cocoa L.) inifap 1 and his histologycal description. Nov Sci 7(14):398–417.
- Li, Z., A. Traore, S. Maximova, and M. Guiltinan. 1998. Somatic embryogenesis and plant regeneration from floral explants of cacao (Theobroma cacao L.) using thidiazuron. Vitr Cell Dev Biol 34:293–299. doi: 10.1007/BF02822737.
- Lloyd, G., and B. McCown. 1981. Commercially feasible micropropagation of Mountain Laurel, Kalmia latifolia, by use of shoot tip culture. Comb. Proc. –Int. Plant Propag. Soc. 30:421–427.
- López, A., J. Carreño, A. Martínez, and M. Dabauza. 2005. High embryogenic ability and plant regeneration of table grapevine cultivars (Vitis vinifera L.) induced by activated charcoal. Vitis - J Grapevine Res 44(2):79–85.
- Mata, A. 2013. Evaluación de dos protocolos para la inducción de embriogénesis somática en clones de cacao (Theobroma cacao L.) seleccionados por el Programa de Mejoramiento Genético de Cacao del CATIE. Centro Agronómico Tropical de Investigación y Enseñanza, Costa Rica.
- Maximova, S., L. Alemanno, A. Young, N. Ferriere, A. Traore, and M. Guiltinan. 2002. Efficiency, genotypic variability, and cellular origin of primary and secondary somatic embryogenesis of Theobroma Cacao L. Vitr Cell Dev Biol - Plant 38(3):252–259. doi: 10.1079/IVP2001257.
- Méndez, H., M. Ledezma, R. Avilez, Y. Juárez, A. Skeete, J. Avilez, C. De-La-Peña, and V. Loyola. 2019. Signaling overview of plant somatic embryogenesis. Front Plant Sci 10:1–15. doi: 10.3389/fpls.2019.00001.
- Menke, I., and J. Zimny. 2001. NH4+ and NO3- requirement for wheat somatic embryogenesis. Acta Physiol. Plant. 23(1):37–42. doi: 10.1007/s11738-001-0020-2.
- Minyaka, E., N. Niemenak, D. Ndoumou, D. Ndoumou, and D. Ndoumou Omokolo. 2008. Effect of MgSO4 and K2SO4 on somatic embryo differentiation in Theobroma cacao L. Plant Cell Tissue Organ Cult. 94(2):149–160. doi: 10.1007/s11240-008-9398-5.
- Minyaka, E., N. Niemenak, L. Ngangue, B. Madina, J. Bahoya, and N. Omokolo. 2017. Peroxidase and polyphenol oxidase activities associated to somatic embryogenesis potential in an elite hybrid genotype of Theobroma cacao L. African J Biotechnol 16(49):2278–2288. doi: 10.5897/AJB2017.16157.
- Monsalve, L., C. García, and A. Sigarroa. 2005. Obtención de embriones somáticos primarios de theobroma cacao en clones de interés regional para el departamento norte de santander, colombia. Respuestas 10(1):21–29.
- Murashige, T., and F. Skoog. 1962. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 15(3):473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x.
- Palencia, G., R. Gomez, and L. Mejia 2007. Patrones para cacao. Bucaramanga – Santander, Colombia.
- Rodríguez, C., A. Wetten, and M. Wilkinson. 2004. Detection and quantification of in vitro-culture induced chimerism using simple sequence repeat (SSR) analysis in Theobroma cacao (L.). Theor. Appl. Genet. 110(1):157–166. doi: 10.1007/s00122-004-1823-5.
- Rodríguez, C.M., A.C. Wetten, and M.J. Wilkinson. 2010. Progressive erosion of genetic and epigenetic variation in callus-derived cocoa (Theobroma cacao) plants. New Phytol. 186(4):856–868. doi: 10.1111/j.1469-8137.2010.03242.x.
- Sánchez, C. 2018. Alternativas para el control de malezas en el cultivo de cacao (theobroma cacao) en el cantón Montalvo. Universidad Técnica de Babahoyo, Ecuador.
- Sarmiento, S., J. Gamboa, and J. Velasquez. 2011. Desempeño agronómico de tres clones de cacao en fase de vivero en la amazonia colombiana. Ing Amaz 4(1):39–47.
- Smertenko, A., and P. Bozhkov. 2014. Somatic embryogenesis: Life and death processes during apical-basal patterning. J. Exp. Bot. 65(5):1343–1360. doi: 10.1093/jxb/eru005.
- Solano, W. 2008. Embriogénesis Somática en Clones Superiores de Cacao (Theobroma Cacao L.) Obtenidos en el Programa de Mejoramiento Genético CATIE. Centro Agronómico Tropical de Investigación y Enseñanza, Costa Rica.
- Urbanek, A., B. Zechmann, and M. Müller. 2004. Plant regeneration via somatic embryogenesis in Styrian pumpkin: Cytological and biochemical investigations. Plant Cell Tissue Organ Cult. 79(3):329–340. doi: 10.1007/s11240-004-5177-0.
- Urrea, I., L. Atehortúa, and A. Gallego. 2011. Regeneration through somatic embryogenesis of an elite colombian Theobroma cacao L. variety. Rev Colomb Biotecnol XIII(2):39–50.
- Vasquez, J. 2005. Aclimatación de plántulas de Epidendrum schomburgkii (Lindl.) C. Shweinf (Orchidaceae) propagadas in vitro. Universidad Nacional de San Martín, Tarapoto. Perú.
- Victor, J., S. Murch, S. Krishnaraj, and P. Saxena. 1999. Somatic embryogenesis and organogenesis in peanut: The role of thidiazuron and N 6 -benzylaminopurine in the induction of plant morphogenesis. Plant Growth Regul. 28(1):9–15. doi: 10.1023/A:1006274615736.
- Williams, E., and G. Maheswaran. 1986. Somatic embryogenesis: Factors influencing coordinated behaviour of cells as an embryogenic group. Ann. Bot. 57(4):443–462. doi: 10.1093/oxfordjournals.aob.a087127.