 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Anthocyanins are present in blackberries and can be used in the food industry as visual indicators that allow understanding the color variations related to the quality of a food. The purpose of this study was evaluated castilla blackberry (Rubus glaucus Benth) and wild blackberry (Rubus adenotrichos) were assessed considering the type of raw material; fresh and lyophilized (22°C, 4.3 mbar, 7 days), pretreatment with microwaves (2450 MHz, 30s) and extraction method; conventional and assisted by ultrasound (300 W, 10 min) in the extraction of monomeric anthocyanins. The lyophilized raw material, the use of microwaves and ultrasound-assisted extraction extract more anthocyanins. The extracts changed color accordingly to pH, presenting colorations from red to green.
Introduction
The consumption of red fruits has increased during the last years. According to the Food and Agriculture Organization of the United Nations (FAO), the production of such fruits has remained above 3 million tons since 2011, without including the strawberry, whose figures ascended to more than 9 million tons in 2016 (FAO, Citation2018). The blackberry is part of the group of red fruits, which in Colombia is produced in an amount of more than 100 thousand tons per year since 2012 (Agronet, Citation2018), being the castilla blackberry (Rubus glaucus Benth) variety the most cultivated (Dane, Citation2013). However, there are other wild varieties such as Rubus adenotrichos, which grows between 2500 and 3000 meters above sea level (Martínez et al., Citation2007). These fruits have shown antioxidant capacity (Aguirre et al., Citation2019; Alarcón-Barrera et al., Citation2018), raising interest in their use in products with high added value.
The anthocyanins stand out among the compounds that represent antioxidant capacity; they are water-soluble pigments that provide colors from red to blue (Sigurdson et al., Citation2017). Additionally, therapeutic properties for diseases such as cancer (Mazewski et al., Citation2018) and obesity (Wu et al., Citation2016), as well as the protective effects against cardiovascular disorders (Warner et al., Citation2018), have been attributed to these compounds. This is the reason why we have seen the use of raw materials rich in anthocyanins, either for the formulation of functional foods (Hornedo-Ortega et al., Citation2016) or the generation of natural dyes (Gordillo et al., Citation2018). Furthermore, since different factors such as light, pH and temperature can affect the stability of anthocyanins (Laleh et al., Citation2006; Liu et al., Citation2018), there has been a growing interest in the use of such compounds as visual indicators (Gras et al., Citation2017; Prietto et al., Citation2017), providing information useful in understanding the process conditions or variations in the quality of a product.
In addition, there is currently a growing interest in the design of packaging for the food industry, which allows direct visual information on the quality of the product. For example, smart packaging shows through a color scale the deterioration of the product within the packaging. This type of packaging allows to increase the time of permanence in the product displays, improve the rotation, monitoring, traceability and food safety. These smart packages have biosensors or electronic devices that can identify pH changes related to food degradation. They can recognize the color changes that occur in compounds such as anthocyanins when they come into contact with food in a package. That is why a practical application of this research work could be the use of anthocyanin extracts from blackberry, as a pH indicator using a biosensor that allows predicting the quality of the food, using a color scale so that the consumer has more information in their purchase decision process.
Anthocyanin extraction with the use of microwaves and ultrasound has been evaluated (López et al., Citation2018; Xue et al., Citation2018). However, there are no studies that use both techniques contiguously to improve the extraction of blackberry anthocyanins. Therefore, the present study evaluated the effect of microwave pretreatment and ultrasound-assisted extraction in obtaining anthocyanins from two varieties of blackberry (Rubus glaucus Benth and Rubus adenotrichos), both fresh and lyophilized. Subsequently, the extract rich in anthocyanins of both varieties of blackberry was characterized by its antioxidant capacity and color at different pH conditions. Likewise, the extract was added to some commercial products with variable pH, in order to demonstrate its possible use as a visual indicator.
Materials and Methods
Plant Material
The plant material was composed of 10 Kg of Castilla blackberry (Rubus glaucus Benth) and 10 Kg of wild blackberry (Rubus adenotrichos). Castilla blackberry was provided by a fruit marketer (Freskifruta) located in the municipality of Chía, Cundinamarca (4°51ʹ48 “N 74°03ʹ10”W). The wild blackberry was purchased at a local market in the municipality of Guasca, Cundinamarca (4°51ʹ57”N 73°52ʹ38” W). In both cases, the fruit was stored at 4–6°C and transported to the laboratory of the University Jorge Tadeo Lozano in Bogotá. The storage conditions were maintained for both varieties until their analysis and processing.
Characterization of Raw Material
Thirty grams of each variety of blackberry were taken. First, the polar and equatorial diameter was determined with a steel 200 mm king foot (Digital Caliber T304B.W-1220, Oregon, USA). Following this, the total soluble solids (°Brix) were quantified with a refractometer (ATAGO pocket refractometer, Tokyo, Japan), the pH was measured with a potentiometer (Seven Easy TM pH Meter S20, Switzerland, Switzerland) and titratable acidity with 0.1 N NaOH. These parameters were evaluated in triplicate, under the section Experimental design and statistical analysis.
Anthocyanin’s Extraction
Type of Raw Material: Fresh and Lyophilized
A longitudinal cut was made along the polar diameter of each fruit. Subsequently, three cuts were made along the equatorial diameter in sections of 1 cm. Finally, the blackberry was maintained at 4°C for later use, being this the fresh raw material.
To obtain lyophilized blackberry, 250 g of fresh blackberry was taken for each variety and frozen at −34°C for 24 h. Then, the samples were taken to 22°C and 4.3 mbar for 7 days in a lyophilizer (Drycol, Bogotá, Colombia), until obtaining a moisture of 10 ± 2%. Next, the dried product was milled and sieved in a paddle mill with a 0.75 mm screen (Retsch sk100, Hann, Germany). Finally, the samples obtained for each blackberry variety were vacuum packed (Multivac chamber machine c200, Bremen, Germany) and stored at room temperature (20 ± 2°C).
Pretreatments with Microwaves
This procedure was performed as described by Flores (Citation2017), modified to laboratory conditions. Ten grams of fresh sample, with a moisture content of 85.25% for wild blackberry and 87.68% for Castilla blackberry (), were placed in a crucible and taken to a microwave of 2450 MHz (Haceb, Medellín, Colombia) for 30s; subsequently, the sample was cooled to room temperature (20 ± 2°C) and kept protected from light. The tests were carried out in triplicate.
Table 1. Factorial design 24 to the extraction of anthocyanin from Castilla and Wild blackberry.
Table 2. Raw material characterization.
Conventional and Ultrasound-assisted Extraction
The conventional extraction method consisted of 10 g of sample were weighed into falcon tubes with 30 mL of 60% ethanol (v/v) acidified with 1% citric acid. Three extractions were performed for a period of 10 min each, as stipulated by de Vargas et al. (Citation2017). Afterward the falcon tubes were placed on an orbital shaker (Elements Chemicals Ltda., Bogotá, Colombia) at 120 x g for 10 min at 20°C.
The ultrasound-assisted extraction was performed with an ultrasonic bath (Elmasonic S 300, Singen, Germany) at a power of 300 W, frequency of 37 kHz, pulse intensity 1 and room temperature (20 ± 2°C). Analogous to conventional extraction, 10 g of the sample were mixed with 30 mL of solvent (diluted 1:3 w/v) in a falcon tube. Subsequently, the tube was immersed in the ultrasonic bath for 10 min. The tests were carried out in triplicate.
Finally, all samples were centrifuged at 4000 x g for 30 min at 20°C in a centrifuge (Helttich rotofix 32 zentrifugen, Schwerin, Germany). The supernatant (extract) was stored in an amber bottle at 4°C for the subsequent quantification of anthocyanins.
Quantification of Monomeric Anthocyanins, Differential pH Method
The determination of monomeric anthocyanin content was performed by the method of Giusti and Wrolstad (Citation2001) described as follows: two aliquots of the same volume of extract were diluted to appropriate volume using pH 1.0 buffer (potassium chloride, 0.025 M) and pH 4.5 buffer (sodium acetate, 0.4 M), respectively. Subsequently, its absorbance at 510 and 700 nm was measured in a spectrophotometer (Thermo Scientific 300 Evolution spectrophotometers, Matlock, England), using 1 mL of distiller water as the blank.
The net absorbance was calculated according to Equationequation 1(1)
(1) :
With the net absorbance, the monomeric total anthocyanin content expressed as mg cyanidin-3-O-glucoside was determined for every 100 g of raw material (Equationequation 2(2)
(2) ).
Where:
CF = Content of total monomeric anthocyanins (mg cyanidin-3-O-glucoside/100 g of raw material), A = absorbance, M = molecular weight (449.2 g per mole of cyanidin-3-O-glucoside), FD = dilution factor (ratio between the total volume (buffer + aliquot) and the volume of the aliquot subjected to the buffer), Vs = volume of the extract (L), ε = Molar extinction coefficient (29600 L mol−1 cm−1 of cyanidin-3-O-glucoside), MMP = mass of the raw material subjected to extraction (g).
Experimental Design and Statistical Analysis
The experimental design was executed with the test program Design-Expert 9.0.6 (Stat-Ease, Delaware, USA Echip, 2014) whereby a factorial design of four variables and two levels (24) was proposed to observe the effect between: the variety of blackberry (X1), the type of raw material (fresh/lyophilized) (X2), the pretreatment with microwaves (X3) and the extraction method (X4). The design consisted of 16 combinations () with three replicates, with the response variable being the content of monomeric anthocyanins. For the statistical analysis, an ANOVA (p < .05) was performed after the Box-Cox transformation of the response variable with lambda λ = 0. Subsequently, the interaction graphs were generated, and the levels were selected for each factor in which the best extraction conditions were given.
Characterization of Extracts Rich in Anthocyanins
The characterization of the extracts was based on the determination of their antioxidant capacity and the color parameters at different pH. These characteristics were measured for the samples that had the highest anthocyanin content for each variety ().
Antioxidant Capacity according to FRAP Assay
This method was carried out according to Benzie and Strain (Citation1996), which consist in the reduction of the ferric tripyridyltriazine complex (F +3-TPTZ) to the ferrous complex (Fe +2-TPTZ) in an acid medium. This change develops a blue color with maximum absorbance at 593 nm. Initially, the FRAP reagent was prepared from 300 mM sodium acetate buffer, pH 3.6, with 20 mM ferric chloride and 0.01 M TPTZ in a 10:1:1 ratio. Following this, 30 μL of diluted extract (with 96% ethanol) were taken and then mixed with 30 μL of 80% ethanol and 940 μL of FRAP reagent. The sample was then incubated for 1 h at 37°C in the absence of light to finally homogenize and read its absorbance at 593 nm in the spectrophotometer (Thermo Scientific 300 Evolution spectrophotometers, Matlock, England). The absorbances obtained were compared with a Trolox standard curve with R2 = 0.99 between 0.10–1.34 mM Trolox. This quantification was carried out in triplicate, with antioxidant capacity reported as μmol Trolox per g of sample (μmol Trolox/g sample).
Antioxidant Capacity according to DPPH Assay
This method is based on the acceptance of a hydrogen transforming to a DPPH2 which is colorless. This procedure was carried out according to Brand-Williams et al. (Citation1995) in triplicate as described here: An aliquot of 75 μL of diluted extract (with 96% ethanol) was mixed with 1435 μL of DPPH (6.29 × 10–5 M in 96% ethanol). This mixture was incubated for 1 h at 37°C in the absence of light. Following this, the sample was homogenized, and its absorbance was read at 515 nm in the spectrophotometer (Thermo Scientific 300 Evolution spectrophotometers, Matlock, England). The results were compared with a standard curve of Trolox with R2 = 0.99 between 0.03–0.6 mM Trolox, to then report the antioxidant capacity as μmol Trolox per g of sample (μmol Trolox/g sample).
Determination of Color and Use of the Extract as Visual Indicator
Twelve buffer solutions were prepared from pH 1.0 to 13, which 2 M phosphoric acid (H3PO4) and 2 M sodium hydroxide (NaOH) was used, covering the required pH spectrum as described by Casas et al. (Citation2009). Additionally, the following products were available: 70% antiseptic alcohol (JGB Bogotá DC, Colombia), bleach (4.5% sodium hypochlorite) (Clorox Bogotá D.C, Colombia), white vinegar (Unilever Bogotá D.C, Colombia), 0.2 g/ml of sodium bicarbonate (Cimpa, Bogotá DC, Colombia), peach syrup, potable water, and lemon, lime, melon and passion fruit juice (Passiflora edulis Sims). Following this, 2 ml of each substance were taken and 200 μL of blackberry extract was added, determining the color and pH of the mixture.
The color of the pure extract and the extract-buffer mixtures were quantified with a colorimeter (Konica Minolta, Chroma-Meter CR-410, Tokyo, Japan), which provided the parameters L* (brightness), a * (balance between red and green) and b * (balance between yellow and blue) of the CIELAB space. To determine the color difference (), The color values of the pure extract of the castilla and wild blackberry variety from
(46,17;52.32),
(57.19;58.53) and
(28.64;29.43) were taken as a standard. Color difference was obtained through EquationEquation (3)
(3)
(3) (Aguirre et al., Citation2019).
Where:
(brightness difference between the standard and the sample buffer-extract),
(difference in position between red and green between the standard and the sample buffer-extract),
(difference in position between yellow and blue between the standard and the sample buffer-extract).
Finally, the pH of these mixtures was determined with a potentiometer (Seven Easy TM pH Meter S20, Switzerland, Switzerland), in order to obtain a color table with respect to pH.
Subsequently, the pH of the product-extract mixtures was determined from the color table previously prepared. In addition, the pH of these samples was quantified with a potentiometer (Seven Easy TM pH Meter S20, Switzerland, Switzerland) and with universal indicator paper (Macherey-Nagel 902 04, Dϋren, Germany), identifying the differences between determinations and their associated error percentages, taking the quantification of the potentiometer as a reference.
Results and Discussion
Characterization of the Raw Material
Aside from pH, all the parameters evaluated were significantly different according to the variety (); being the Castilla blackberry the raw material with the highest content of total soluble solids, titratable acidity, maturity index and size. This last characteristic could be a factor that explains the preference of cultivation between the two varieties of blackberry.
The total soluble solids of the Castilla blackberry are lower than those reported by Horvitz et al. (Citation2017) for this variety in stage 3 of maturity (9.17 ± 0.23 ° Brix stored at 8°C), whereas for the wild blackberry, the result obtained resembles what was determined by Acosta-Montoya et al. (Citation2010) for such variety between state 1 (5.03 ± 0.05 °Brix) and state 2 (6.4 ± 0.2 °Brix). In contrast, the pH of both types of blackberry is higher than that reported in the literature, whose highest values are shown by Mertz et al. (Citation2007) for fully mature fruits (2.98 ± 0.01 for Rubus glaucus and 2.83 ± 0.01 for Rubus adenotrichus) and below that reported in blackberry byproducts (3.4 for Rubus glaucus Benth) (Aguirre et al., Citation2019). The titratable acidity for the samples studied was lower compared to Rubus glaucus (2.55 ± 0.01% citric acid) and Rubus adenotrichos (2.67 ± 0.01% citric acid) (Mertz et al., Citation2007).
Extraction of Anthocyanins
According to , it was observed that in the fresh samples of the two varieties (Castilla and wild) treated with microwaves and ultrasound, the anthocyanin content increased compared to the samples that were not treated with microwaves. This is because the absorption of energy from microwaves involves two mechanisms; ionic interaction and bipolar rotation. In the case of the water molecules in the blackberry, they present a polar behavior since they do not have a symmetric charge and present strong bipolar movements, therefore, the water in the food matrix is responsible for the polar rotation. These molecules exposed to electromagnetic waves try to orient themselves in the direction of the field, they collide randomly with their neighbors, causing thermal agitation and therefore heating. However, the extraction was better in lyophilized samples than in fresh samples, this is because when lyophilizing the components are concentrated, in addition, it is a method that allows to maintain the compounds of interest due to the applied process conditions such as low temperature (22°C) and vacuum (4.3 mbar). In addition, cavitation induces mechanical, physical, and biochemical changes that allow the breakdown of cell walls and greater extraction of anthocyanins.
According to ANOVA (); all variables significantly affect the content of monomeric anthocyanins in blackberry extracts. The variety, the type of raw material and the extraction method had a joint effect (); the wild blackberry, the lyophilized raw material and the ultrasonic assisted extraction method presented the highest value according to the prediction of the best fit model (). Additionally, although microwave pretreatment was not related to any variable, its use improves extraction (). Horvitz et al. (Citation2017), reported 228.0 ± 14.7 mg anthocyanins/100 g for Andean blackberries (Rubus glaucus Benth) from Ecuador at maturity 5 (dark purple), determining that the higher the maturity stage, the higher the anthocyanin content and the better the sugar/acid balance; similar to what was quantified for the Castilla blackberry under the best extraction conditions (), and to what has been determined for other fruit’s extract, such as purple passion fruit peel anthocyanin extract (487 ± 27 mg cyanidin-3-O-glucoside/100 g) (Meneses-Marentes et al., Citation2019).
Table 3. ANOVA and regression coefficients for the transformed model of the total monomeric anthocyanin content in blackberries extracts.
Figure 1. Total monomeric anthocyanin content from blackberries extracts and its best conditions. A. Effect of variety, type of raw material and extraction method. B. Effect of microwave pretreatment.
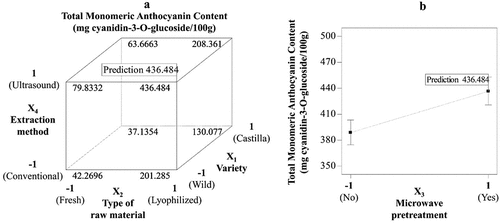
The results show that the wild blackberry has a greater potential for obtaining anthocyanins than the Castilla blackberry, raising interest in this crop to obtain these bioactive compounds. However, the foregoing differs from that reported by Mertz et al. (Citation2007) who determined 1010 and 720 g anthocyanins/100 g dry matter for Rubus glaucus and Rubus adenotrichus respectively. Although the varieties are the same, the differences in anthocyanin content may be due to four factors: First, the country of origin is different, the authors report blackberry values from Ecuador and Costa Rica. Second, it is due to soil conditions such as the composition and nature of the soil, climatic conditions such as weather phenomena like air temperature, atmospheric pressure or air weight, winds and humidity that characterize the average state of the atmosphere at one point on the earth’s surface, conditions that are different for each country. Third, the type of extraction of the sample, which was performed with 70% aqueous acetone containing 2% formic acid. In our case, an extraction was performed using 60% ethanol as solvent acidified to 1% citric acid, because the purpose is to be able to use this extract in the food industry. Fourth, the quantification was done by LC-DAD/M being this a more precise method than by spectrophotometry.
The use of ultrasound, also improved the extraction of anthocyanins compared to the conventional method. This behavior can be explained by the phenomenon of cavitation, which generates violent implosions that fragment the surface of the solid matrix, improving the mass transfer (Chemat et al., Citation2017).
Such a trend resembles that described by Backes et al. (Citation2018) for the extraction of anthocyanins from fig bark (Ficus carica L.), who determined an overall optimum of 3.82 mg cyanidin 3-rutinoside/g bark under the ultrasonic assisted extraction method versus the use of microwave and heat. In contrast, López et al. (Citation2018) stated that heat assisted extraction was more efficient than using ultrasound-assisted extraction to obtain anthocyanins from Arbutus unedo L. Fruits (Portugal) determining that the most effective technique was heat assisted extraction at 5 min, 90°C and 80% of ethanol, yielding 51.2% of the extract, with a total anthocyanin content of 38.24 mg/100 g dried fruit, and 74.46 mg/g extract. Anthocyanins are sensitive to heat and both the extraction time, the solvents and mixtures between them (water, methanol, ethanol and acetone) and the food matrix affect the yield.
Therefore, it can be inferred that the choice of the extraction technology depends on the solid matrix to be extracted, being the ultrasound-assisted extraction method the one recommended for obtaining anthocyanins from blackberries. Ultrasonic waves due to the force, pressure and temperature that are created in the process, improve the mass and energy transfer processes. Therefore, they cause rapid changes in the food matrix that allow cell destruction. Due to the breakdown of molecular structures and therefore generates an improvement in the interaction of the substrates with which it reacts. It should be noted that the effectiveness of this process depends on the intensity, amplitude, pressure and time of exposure of the samples to ultrasonic waves. In addition, ultrasound treatment has the advantage of reducing time and temperature gradients in the process, greater extraction and production of compounds of interest, as well as being a clean and environmentally friendly technology.
Microwave pretreatment increases the monomeric anthocyanin content of the extract regardless of the conditions of the other factors (). This is established because there is no significant effect between this factor and any of the other variables analyzed (). Therefore, the results show that the use of microwaves improves the extraction of anthocyanins from both varieties of blackberry (fresh or lyophilized) under the two extraction methods studied. The previous statement coincides with that reported by Romero-Díez et al. (Citation2019), who affirm that the microwave pretreatment for wine sludge improves the extraction of anthocyanins (6.20 mg malvidin equivalents/g dry sludge) and decreases the extraction time (from 15 min to 90s).
Microwave extraction is a clean technology that requires less extraction time, quick heat penetration, saves energy, water, is friendly to the environment and better extract performance compared to conventional extraction treatments. The microwave energy allows rapid heating with a uniform increase in the temperature of the food matrix.
In addition, microwave pretreatment increases the monomeric anthocyanin content due to tissue breakdown and therefore, greater interaction surface between the solvent used for the extraction of anthocyanins (KCl and sodium acetate) and the matrix (Castilla blackberry and wild blackberry). In addition, the composition of the food matrix affects the dielectric properties such as the dielectric constant and the loss factor that occurs in this type of electromagnetic treatments such as the microwave. For example, the water contained in the blackberry increases the dielectric properties due to bipolar rotation; molecules such as water are polar because they have no symmetrical charge and exhibit strong bipolar movements, with water in food being the first component responsible for polar rotation. On the other hand, the sugars present in the food matrix modify the dielectric behavior of water as a function of temperature and composition. Therefore, according to the results obtained, it can be inferred that the composition of Castilla blackberry and wild blackberry favored the behavior of anthocyanin extraction.
Characterization of the Extract
Antioxidant Capacity
The wild blackberry extract showed a higher antioxidant capacity (4.295 ± 0.009 and 3.763 ± 0.006 μmol Trolox/g according to the DPPH and FRAP methods) than what was determined for the extraction of the Castilla blackberry (3.763 ± 0.006 and 3.439 ± 0.003 μmol Trolox/g per DPPH and FRAP respectively) according to Tukey’s test (p < .05). This confirms what was previously evidenced; the wild blackberry has a greater potential for obtaining compounds with antioxidant capacity, among which the anthocyanins stand out. However, the results are similar to what was reported in purple passion fruit peel anthocyanin extract (3.66 ± 0.07 and 4.64 ± 19 µmol Trolox/g per DPPH and FRAP methods) (Meneses-Marentes et al., Citation2019), but lower than what was determined by Alarcón-Barrera et al. (Citation2018) for cranberries and Andean blackberries according to the DPPH (246 and 152 μmol Trolox/g, respectively) and FRAP method (226 and 102 μmol Trolox/g, respectively), as well as that found by Horvitz et al. (Citation2017) for the Rubus glaucus blackberry Beth on stage 5 of maturity (51.7 ± 3.28 μmol Trolox/g for DPPH).
Color of the Extract as a Visual Indicator of pH
According to , the anthocyanin-rich extracts of both varieties of blackberry provided red colorations at acidic pH; the presence of the flavylium cations can be the cause of such color. From pH 6.07, the samples showed violet colorations, typical of the quinoid forms of anthocyanins (Sigurdson et al., Citation2017); however, at basic pH (from 12.04) the leachates exhibited greenish colorations. In context, such color changes have been evidenced in films enriched with purple sweet potato (Choi et al., Citation2017), where red is shown as pH 2 and green at pH 10, similar to that reported in the present study. Nevertheless, although a red coloration appears at pH 1.0 in films with red cabbage anthocyanins, at pH 10 the sample is blue (Prietto et al., Citation2017). Therefore, such differences may lie in the raw material, the profile of anthocyanins being different for each case.
Table 4. Blackberries extracts’ color in different pH.
In terms of the color parameters, the highest value of L* was given at pH 4.98 for both extracts; on the other hand, the terms a* and b* were decreasing as the pH increased. However, there was an increase of b* under pH conditions higher than 12.04. In general, the change in the color parameters showed differences (ΔE) of up to 98.02 CIELAB units for the wild blackberry extract with respect to the pure extract. Additionally, the main changes in color difference appeared from pH 3.1 to 7.97, as well as for the pH interval 11.06–12.94, generating colorations that could work as pH indicators.
The pH determination of the products (); was carried out with universal indicator paper, as well as the two blackberry extracts. The quantification that presented the highest percentage error range in the pH with respect to the potentiometric reading was the universal indicator (0.43–20.63% error), followed by the blackberry’s extraction (1.87–19.84%). The above shows that the use of extracts can estimate the pH of different substances at plain sight, analogous to the universal indicator role. In addition, these extracts can also be useful as a vehicle in a food matrix to indicate changes in pH directly associated with food deterioration.
Table 5. pH determination of different substances from visual indicators.
Conclusions
The wild blackberry presented a greater number of monomeric anthocyanins and a larger antioxidant capacity than the Castilla blackberry, being the lyophilized raw material, the use of microwave pretreatment and ultrasound extraction the best extraction conditions for both cases. Additionally, the present study showed that the extract of both fruits changes with respect to pH, generating color tables that could be used as a visual guide, providing similar results to what was determined with universal indicator paper. This shows the potential of blackberry varieties for obtaining anthocyanins and being used as quality indicators.
Disclosure Statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Acosta-Montoya, Ó., F. Vaillant, S. Cozzano, C. Mertz, A.M. Pérez, and M.V. Castro. 2010. Phenolic content and antioxidant capacity of tropical highland blackberry (Rubus adenotrichus Schltdl.) during three edible maturity stages. Food Chem. 119(4):1497–1501. doi: 10.1016/j.foodchem.2009.09.032.
- Agronet 2018. Agronet Red de información y Comunicación del Sector Agropecuario Colombiano. Agronet., Bogotá D.C. 12 Sep. 2019. <http://www.agronet.gov.co/>
- Aguirre, L., L. Cubillos, M. Tarazona-Díaz, and L. Rodriguez. 2019. Efecto del tratamiento y tiempo de almacenamiento sobre los compuestos funcionales de subproductos de mora y fresa. Rev. U.D.C.A. Actual. Divulg. Científica 22(1):e1169. doi: 10.31910/rudca.v22.n1.2019.1169.
- Alarcón-Barrera, K.S., D.S. Armijos-Montesinos, M. García-Tenesaca, G. Iturralde, T. Jaramilo-Vivanco, M.G. Granda-Albuja, F. Giampieri, and J.M. Alvarez-Suarez. 2018. Wild Andean blackberry (Rubus glaucus Benth) and Andean blueberry (Vaccinium floribundum Kunth) from the Highlands of Ecuador: Nutritional composition and protective effect on human dermal fibroblasts against cytotoxic oxidative damage. J. Berry Res 8(3):223–236. doi: 10.3233/jbr-180316.
- Backes, E., C. Pereira, L. Barros, M.A. Prieto, A.K. Genena, M.F. Barreiro, and I.C.F.R. Ferreira. 2018. Recovery of bioactive anthocyanin pigments from Ficus carica L. peel by heat, microwave, and ultrasound-based extraction techniques. Food Res. Int. 113:197–209. doi: 10.1016/j.foodres.2018.07.016.
- Benzie, I.F.F., and J.J. Strain. 1996. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “‘Antioxidant Power’”: The FRAP Assay. Anal. Biochem 239(1):70–76. doi: 10.1006/abio.1996.0292.
- Brand-Williams, W., M.E. Cuvelier, and C. Berset. 1995. Use of a free radical method to evaluate antioxidant activity. LWT - Food Sci. Technol 28(1):25–30. doi: 10.1016/S0023-6438(95)80008-5.
- Casas, J.A.M., H.J.D. Castillo, J.M.H. Noy, A.N.P. Palomares, and R.L.V. Rodríguez. 2009. Elaboración de un papel indicador a base de extractos naturales: Una alternativa fundamentada en experiencias de laboratorio para el aprendizaje del concepto de pH. Rev. Eureka Sobre Enseñanza Y Divulg. Las Ciencias 6(2):302–314. doi: 10.25267/Rev_Eureka_ensen_divulg_cienc.
- Chemat, F., N. Rombaut, A. Meullemiestre, M. Turk, S. Perino, A.-S. Fabiano-Tixier, and M. Abert-Vian. 2017. Review of Green Food Processing techniques. Preservation, transformation, and extraction. Innov. Food Sci. Emerg. Technol. 41:357–377. doi: 10.1016/j.ifset.2017.04.016.
- Choi, I., J.Y. Lee, M. Lacroix, and J. Han. 2017. Intelligent pH indicator film composed of agar/potato starch and anthocyanin extracts from purple sweet potato. Food Chem. 218:122–128. doi: 10.1016/j.foodchem.2016.09.050.
- Dane 2013. El cultivo de la mora de Castilla (Rubus glucus Benth) frutal de clima frío moderado, con propiedades curativas para la salud humana. DANE., Bogotá D.C. 11 Sep. 2019. <https://www.dane.gov.co/files/investigaciones/agropecuario/sipsa/insumos_factores_de_produccion_nov_2013.pdf>
- de Vargas, E.F., A. Jablonski, S.H. Flôres, and A.D.O. Rios. 2017. Obtention of Natural Dyes from Industrial Blackberry Pulp Residues (Rubus sp). J. Food Process. Preserv 41(1):e12777. doi: 10.1111/jfpp.12777.
- FAO. 2018. FAOSTAT - Food and agriculture data. FAO., Rome. 12 Sep. 2019. <h ttp://w ww.fao.org/faostat/en/#home>
- Flores, E. 2017. Extracción de Antioxidantes de las Bayas del Sauco (Sambucus nigra L. subsp. peruviana) con Ultrasonido, Microondas, Enzimas y Maceración para la obtención de Zumos Funcionales. Inf. Tecnol 28(1):121–132. doi: 10.4067/S0718-07642017000100012.
- Giusti, M.M., and R.E. Wrolstad. 2001. Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy. Curr. Protoc. Food Anal. Chem 00(1):F1.2.1-:F1.2.13. doi: 10.1002/0471142913.faf0102s00.
- Gordillo, B., G.T. Sigurdson, F. Lao, M.L. González-Miret, F.J. Heredia, and M.M. Giusti. 2018. Assessment of the color modulation and stability of naturally copigmented anthocyanin-grape colorants with different levels of purification. Food Res. Int. 106:791–799. doi: 10.1016/j.foodres.2018.01.057.
- Gras, C.C., N. Nemetz, R. Carle, and R.M. Schweiggert. 2017. Anthocyanins from purple sweet potato (Ipomoea batatas (L.) Lam.) and their color modulation by the addition of phenolic acids and food-grade phenolic plant extracts. Food Chem. 235:265–274. doi: 10.1016/j.foodchem.2017.04.169.
- Hornedo-Ortega, R., M.A. Álvarez-Fernández, A.B. Cerezo, A.M. Troncoso, and M.C. García-Parrilla. 2016. Influence of storage conditions on the anthocyanin profile and colour of an innovative beverage elaborated by gluconic fermentation of strawberry. J. Funct. Foods. 23:198–209. doi: 10.1016/j.jff.2016.02.014.
- Horvitz, S., D. Chanaguano, and I. Arozarena. 2017. Andean blackberries (Rubus glaucus Benth) quality as affected by harvest maturity and storage conditions. Sci. Hortic. 226:293–301. doi: 10.1016/j.scienta.2017.09.002.
- Laleh, G.H., G. Frydoonfar, R. Heidary, R. Jameei, and S. Zare. 2006. The Effect of Light, Temperature, pH and Species on Stability of Anthocyanin Pigments in Four Berberis Species. Pakistan J. Nutr 5(1):90–92. doi: 10.3923/pjn.2006.90.92.
- Liu, Y., Y. Liu, C. Tao, Y. Liu, and Z. Lv. 2018. Effect of temperature and pH on stability of anthocyanin obtained from blueberry. J. Food Meas. Charact 12(3):1744–1753. doi: 10.1007/s11694-018-9789-1.
- López, C.J., C. Caleja, M.A. Prieto, M.F. Barreiro, L. Barros, and I.C.F.R. Ferreira. 2018. Optimization and comparison of heat and ultrasound assisted extraction techniques to obtain anthocyanin compounds from Arbutus unedo L. Fruits, Food Chem. 264:81–91. doi: 10.1016/j.foodchem.2018.04.103.
- Martínez, A., O. Beltrán, G. Velastegui, G. Ayala, R. Jácome, and W. Yánez. 2007. Manual Del Cultivo de la Mora de Castilla. Convenio INIAP-UTA, Ambato.
- Mazewski, C., K. Liang, and E. Gonzalez de Mejia. 2018. Comparison of the effect of chemical composition of anthocyanin-rich plant extracts on colon cancer cell proliferation and their potential mechanism of action using in vitro, in silico, and biochemical assays. Food Chem. 242:378–388. doi: 10.1016/j.foodchem.2017.09.086.
- Meneses-Marentes, N.A., E.J. Herrera-Ramírez, and M.P. Tarazona-Díaz. 2019. Caracterización y estabilidad de un extracto rico en antocianinas a partir del epicarpio de gulupa (Passiflora edulis f. edulis Sims). Rev. Colomb. Química 48(2):27–32. doi: 10.15446/rev.colomb.quim.v48n2.76682.
- Mertz, C., V. Cheynier, Z. Günata, and P. Brat. 2007. Analysis of Phenolic Compounds in Two Blackberry Species (Rubus glaucus and Rubus adenotrichus) by High-Performance Liquid Chromatography with Diode Array Detection and Electrospray Ion Trap Mass Spectrometry. J. Agric. Food Chem 55(21):8616–8624. doi: 10.1021/jf071475d.
- Prietto, L., T.C. Mirapalhete, V.Z. Pinto, J.F. Hoffmann, N.L. Vanier, L.-T. Lim, A.R.G. Dias, and E.D.R. Zavareze. 2017. pH-sensitive films containing anthocyanins extracted from black bean seed coat and red cabbage. LWT - Food Sci. Technol. 80:492–500. doi: 10.1016/j.lwt.2017.03.006.
- Romero-Díez, R., M. Matos, L. Rodrigues, M.R. Bronze, S. Rodríguez-Rojo, M.J. Cocero, and A.A. Matias. 2019. Microwave and ultrasound pre-treatments to enhance anthocyanins extraction from different wine lees. Food Chem. 272:258–266. doi: 10.1016/j.foodchem.2018.08.016.
- Sigurdson, G.T., P. Tang, and M.M. Giusti. 2017. Natural Colorants: Food Colorants from Natural Sources, Annu. Rev. Food Sci. Technol 8(1):261–280. doi: 10.1146/annurev-food-030216-025923.
- Warner, E.F., I. Rodriguez-Ramiro, M.A. O’Conell, and C.D. Kay. 2018. Cardiovascular Mechanisms of Action of Anthocyanins May Be Associated with the Impact of Microbial Metabolites on Heme Oxygenase-1 in Vascular Smooth Muscle Cells. Molecules 23(4):898. doi: 10.3390/molecules23040898.
- Wu, T., Z. Jiang, J. Yin, H. Long, and X. Zheng. 2016. Anti-obesity effects of artificial planting blueberry (Vaccinium ashei) anthocyanin in high-fat diet-treated mice. Int. J. Food Sci. Nutr 67(3):257–264. doi: 10.3109/09637486.2016.1146235.
- Xue, H., H. Xu, X. Wang, L. Shen, H. Liu, C. Liu, Q. Qin, X. Zheng, and Q. Li. 2018. Effects of Microwave Power on Extraction Kinetic of Anthocyanin from Blueberry Powder considering Absorption of Microwave Energy. J. Food Qual 1–13. doi: 10.1155/2018/9680184.
