ABSTRACT
The present study was conducted to evaluate the effect of ultrasound on the functional properties of citrus juices. Citrus fruits and juices were analyzed for physico-chemical properties. The ‘Pamelo’ excelled in terms of average fruit weight (451.83 ± 7.1 g), fruit volume (543.33 ± 9.1 cm3) and rag percentage (68.64 ± 1.1%). ‘Valencia late’ (48.67 ± 0.3%), ‘Flame’ (48.38 ± 1.3%) and ‘Kinnow’ (35.95 ± 4.9%) were good in juice %. The juice was subjected to sonication treatment at 15°C for 5 min keeping pulse duration 5 s on and 5 s off (70% amplitude level and 20 kHz frequency). Total phenol, flavonoids, free radical scavenging activity, antioxidant capacity, and reducing power increased in fruit juices as a result of sonication. The total phenolic contents in the juices of all citrus cultivars ranged from 223.49 ± 4.5 to 590.47 ± 5.5 µg gallic acid equivalent/g and increased from 315.18 ± 6.1 to 645.44 ± 7.1 µg GAE/g after ultrasonic processing. The DPPH values of juices of all citrus cultivars ranged from 791.54 to 1251.93 µmol equivalent of trolox/mL and increased from 1001.54 to 1336.77 µmol equivalent of trolox/mL after applying ultrasound. The sonication is highly feasible for practical use in the industry for the production of citrus fruit juices with improved quality in terms of nutrients.
Introduction
The Citrus (Rutaceae) family includes oranges, limes, lemons, mandarins, grapefruits, and sour orange. On the basis of area under citrus production, Pakistan is among the top 10 countries in the world. In Punjab province, the Sargodha district has been found to possess the largest share in the area as well as the production of citrus fruit (Sabir et al., Citation2010). Various agro-climatic conditions have different influences on citrus fruit yield and quality (Ishfaq et al., Citation2007). In other words, different cultivars show different behavior in diverse climates, but fruits cultivated in the specific environment exhibit more or less common quality characteristics (Fucik and Norwine, Citation1979; Ranjha et al., Citation2021a; Roobab et al., Citation2022).
Citrus fruits are an important fruit crop due to their special aroma and ability to fight against chronic diseases (Milind and Dev, Citation2012). Citrus juices such as ascorbic acid, flavonoids, phenolic compounds, and pectins are considered to be important sources of antioxidants (Asjad et al., Citation2013; Liu et al., Citation2012; Rekha et al., Citation2012). Owing to the presence of phytochemicals such as phytosterols, carotenoids, flavonoids, and phenols, citrus fruits have anti-inflammatory, anticancer, and antioxidant properties (Mathew et al., Citation2012).
Products from natural sources are being used from centuries (Nayik et al., Citation2021; Ranjha et al., Citation2022). The demand for healthy food is increasing because consumers are becoming health conscious as they like minimal processing of fresh food commodities (Walkling-Ribeiro et al., Citation2009). Less aggressive new techniques are now introduced to save the nutritional quality of fruits (Caminiti et al., Citation2011). Researchers are now working on processing techniques that not only retain but also can improve the nutritional quality of fruits and other products to meet the increasing demand by consumers for a healthy diet (Bhat et al., Citation2011; Nadeem et al., Citation2021a). Conventional heating is used to inactivate the enzymes and reduce microorganisms in fruit juices, which results in improved shelf life, but loss of color, flavor, and nutrients occurs. Therefore, non-thermal techniques are the best solution to solve these problems (Ranjha et al., Citation2021b; Zhang et al., Citation2013). Some of these non-thermal technologies have drawn the attention of researchers due to their specific effect on biological materials (Knorr et al., Citation2004; Ranjha et al., Citation2021c). Ultrasound is one of the important techniques used as an alternative to heat treatment due to less processing time and less energy inputs (Mehmood et al., Citation2019; Nadeem et al., Citation2018; Ranjha et al., Citation2022). Food processing by the use of ultrasound may retain nutritional value and microbiological safety of orange juice, guava juice, and tomato juice (Cheng et al., Citation2007; Valero et al., Citation2007; Wu et al., Citation2008).
Fruits, vegetables, cereals, legumes, spices, animal products, and beverages are a few sources of natural antioxidants (vitamin A, vitamin C, vitamin E, and beta carotene, selenium, phenolic, and flavonoid compounds) (Aliakbarian et al., Citation2010; Latoui et al., Citation2012; Nadeem et al., Citation2021b; Sikora et al., Citation2008). Fruit juices or pulps from mango, apple, banana, apricot, pear, plum, bilberry, kiwiberry, strawberry, raspberry, cherry, etc., have been investigated for their antioxidant potential (Batiston et al., Citation2013; Latocha et al., Citation2015; Skrede et al., Citation2004). The antioxidant potential of citrus fruit juice as well as peel from different countries such as Malaysia, Greece, Italy, Turkey, India, and Taiwan have been investigated (Anagnostopoulou et al., Citation2006; Ghafar et al., Citation2010; Herken and Guzel, Citation2010; Kumar et al., Citation2013; Monica et al., Citation2011; Tsai et al., Citation2007). Enzymes have also been applied to increase juice yield (Mohamed et al., Citation2009). As the nutritional quality and antioxidant potential of fruits vary depending on different climatic conditions, soil characteristics, and geographical locations (Gull et al., Citation2012; Khan et al., Citation2011; Lee and Kader, Citation2000; Mohamed et al., Citation2016; Siddiq et al., Citation2011), it was interesting to investigate some peculiar citrus cultivars of Pakistan. Moreover, no comprehensive study was done on the effect of ultrasound on the antioxidant potential of juices from citrus fruit cultivars. Hence, the main objective of the present study was to investigate and compare the antioxidant potential of juices from different citrus fruit cultivars by quantifying their total phenolic content and total flavonoid content with the use of ultrasound.
Materials and Methods
Materials
All the analytical-grade chemicals and reagents were purchased from Fluka Chemical Co. (Buchs, Switzerland) and Merck (Darmstadt, Germany). Citrus cultivars like Pigmented oranges (Citrus sinensis) that include ‘Red Blood’ and ‘Moro Blood’; Grapefruits (Citrus paradisi) ‘Pamelo,’ ‘Shamber’, and ‘Flame’; Mandrins (Citrus reticulata) ‘Kinnow,’ ‘Feutrell’s Early,’ and ‘Murocott’; and Common orange (Citrus sinensis) ‘Valencia late’ were collected from the Citrus Research Institute, Sargodha, Pakistan. One hundred fruits of each cultivar were picked at the optimum maturity. The samples of fruit were collected from all sides of the tree, i.e., from the top and inside the canopy.
Preparation of Juice and Ultrasound Treatment
The juice from each fruit was extracted by using a rotary citrus squeezer (MJ-M176P, Panasonic Manufacturing, Berhad, Malaysia), weighed, and filtered through a 0.8 mm pore size sieve. Juice of each cultivar (300 mL) was divided into two equal parts (150 mL each). Ultrasonic processor (UP400S, Hielscher Ultrasonics GmbH Hielscher USA, Inc.) with 0.5-in. probe was used for sonication. One portion of the juice was subjected to ultrasound treatment (24 kHz Frequency, amplitude level of 70% (400 W power) and pulse duration 5 sec on and 5 sec off) for 5 min at 15°C. In the present study, different parameters such as duty cycle, amplitude (%), time, and temperature were optimized by preliminary trials to obtain the maximum antioxidant activity. The temperature was maintained by using an automatic control system. The sonication was performed immediately after fresh juice was extracted. To avoid light interference, all experiments were performed in the dark. The fresh sample without sonication was used as a control. All the sonicated and control samples were stored at −18°C for further analysis. The juice percentage was expressed by the following expression.
Juice % = Juice weight (g)/Fruit weight with peel (g) × 100
Physical Analysis of Citrus Fruits
A representative sample of 20 fruits from the bulk sample was taken to measure physical quality characteristics such as fruit weight (g), fruit volume (cm3), peel thickness (mm), peel weight (%), and rag weight (%); after juice extraction, the fibrous material is called rag – and number of seeds (seediness).
Chemical Analysis of Citrus Fruits
The pH, total soluble solids (TSS), total titratable acidity, and vitamin C (ascorbic acid) contents of the juice were determined according to AOAC (Citation2016). TSS-Acid ratio that indicates the ripeness (also called Maturity Index) was also determined (Lacey et al., Citation2009). Total sugars, reducing sugars, and non-reducing sugars were determined by following the procedure of Lane and Eynon (AOAC, Citation2016).
Determination of Total Phenolics and Total Flavonoids
Spectrophotometer was used to determine the total phenolic contents by using Folin-Ciocalteu reagent with some modifications (Singleton et al., Citation1999). Diluted citrus fruit juice sample (500 μL each; 1 mL juice and 99 mL distilled water) was used for analysis, and absorbance was measured at 760 nm. Gallic acid was used to make a standard solution for calibration curve. The results of total phenolics were expressed as µg of gallic acid equivalents (GAE) per mL of sample. The tests were performed in triplicate
Flavonoid contents were determined by the described method of Kim et al. (Citation2003). Reagent solutions were prepared as 5% sodium nitrite, 10% aluminum chloride, and 1 M sodium hydroxide. Diluted citrus fruit juice sample (250 μL each; 1 mL juice and 99 mL distilled water) was used for analysis, and absorbance was measured by spectrophotometer at 510 nm wavelength. The results were expressed as µg of (+)-catechin equivalent per mL of sample. The tests were performed in triplicate.
Determination of DPPH Free Radical Scavenging Activity and Total Antioxidant Capacity (TAC)
Citrus cultivars were assessed for their DPPH free radical scavenging activity by the previous method of Yi et al. (Citation2008) with some minor modifications. Decrease in absorbance was measured by the following equation:
DPPH radical scavenging activity (%) = (A0 – A1/A0) × 100
Where A0 is the absorbance of the control and A1 is the absorbance of the extracts. The same procedure was adopted by taking trolox as a standard for the determination of DPPH radical scavenging activity and the results were expressed as μmol equivalent of trolox/mL juice.
For antioxidant capacity determination, citrus fruit juices of all three cultivars were analyzed by using the method described by Prieto et al. (Citation1999). Ascorbic acid was used to make a standard calibration curve, and the results were expressed as mg ascorbic acid equivalent/100 mL juice. The tests were performed in triplicate
Reducing Power Ability
The reducing power of citrus fruit juices was determined by the method as described by Hegazy and Ibrahium (Citation2012) with some modifications. The increased absorbance of the sample mixture indicated increased reducing power. Ascorbic acid was used to make a standard calibration curve. The tests were performed in triplicate.
Statistical Analysis
The data obtained from physico-chemical and phytonutrient assay was presented as mean value ± SD. For statistical analysis, cultivar was considered as one factor and second factor was treatment (sonication). The statistical analysis of these parameters was conducted with two-way ANOVA at a significance level of p ≤ .05, and significant differences between mean values were determined by LSD pairwise comparison test. Different alphabet letters represent significant differences (p ≤ .05) among cultivars and citrus groups. The statistical analyses were performed by using Statistix 8.1 software (Analytical Software, Tallahassee, FL, USA).
Results and Discussion
The research was conducted to find out the best cultivar for direct consumption and processing to get valuable products and by products. Along with the continuous increase in global consumption of citrus fruit and the demand of consumers to purchase high quality and tasty fruit, it became a high priority to further investigate the citrus cultivars both in external and internal characteristics.
Physical Properties of Citrus Cultivars
The mean values of ‘Pamelo’ average fruit weight (451.83 ± 7.1 g) followed by ‘Shamber’ (297.05 ± 6.3 g) and ‘Flame’ (284.0 ± 6.8 g). The fruit volume of ‘Pamelo’ was also highest (543.25 ± 9.07 cm3). The minimum fruit weight (93.20 ± 4.3 g) and fruit volume (121.67 ± 3.29 cm3) were recorded in ‘Murocott’ (). Both ‘Shamber’ and ‘Flame’ were close in terms of average weight (297.1 ± 6.3 g and 284.0 ± 6.8 g) and size (354.5 ± 7.5 and 336.8 ± 7.4 cm3), whereas peel thickness showed significant difference among varieties. Seediness was found higher in ‘Kinnow.’ ‘Pamelo’ and ‘Valencia late’ were also of good size. The maximum peel weight (44.03 ± 1.8%) was found in ‘Valencia late’ followed by ‘Murocott’ (37.20 ± 1.6%) and ‘Feutrell’s Early’ (36.73 ± 3.1%). ‘Pamelo’ exhibited the minimum peel weight (3.89 ± 0.3%) which was also the lowest in thickness 2.4 mm. The ‘Pamelo’ remained at par having 68.64 ± 1.1% rag weight followed by 39.25 ± 2.6% ‘Moro Blood’ and 38.68 ± 4.9% ‘Kinnow,’ while the lowest rag weight (7.30 ± 0.9%) was recorded in Valencia Late. Here, it is imperative to note that the rag percentage was more in ‘Pamelo’ fruit, which clearly depicts that the fruit of ‘Pamelo’ has more fiber contents, having tremendous health benefits.
Table 1. Physical properties of different citrus cultivars.
The number of seeds per fruit was significantly higher in ‘Kinnow’ (~20.33 ± 4.6), ‘Pamelo’ (~17.00 ± 2.5) and ‘Feutrell’s Early’ (~13.00 ± 1.3) as compared to other cultivars. ‘Valencia late’ (3.75 ± 0.5), ‘Flame’ (4.0 ± 1.8) and ‘Red Blood’ (4.67 ± 1.3) remained very less seedy or virtually seedless. Due to the low seed count in these types, they are classified as less seeded cultivars, a much wanted and distinctive character for the value addition sector besides the direct consumption as fresh fruit. The results of physical characteristics of citrus fruits are in line with (Din et al., Citation2012).
Juice percentage is an important measurement of internal quality. Under or overripe fruit tends to be less juicy, which directly affects eating quality (Mohamed et al., Citation2016). The ultimate demand of customer is higher juice percentage in the fruit. Results regarding the juice percentage revealed significant differences among cultivars selected for study. ‘Valencia late’ (48.7 ± 0.3%) and ‘Flame’ (48.4 ± 1.3%) were found to be the superior in juice percentage followed by ‘Shamber’ (36.6 ± 2.0%). Some other cultivars were also good in juice % like ‘Kinnow’ (35.95 ± 4.9bc %), ‘Red Blood’ (34.25 ± 5.9b %), and ‘Moro Blood’ (33.75b ± 1.8%). The juice recovery was least in ‘Feutrell’s Early’ (26.78 ± 2.8%) and ‘Pamelo’ (27.47 ± 1.9%). Juice recovery is a qualitative parameter and “Flame’ proved’ itself as a commercially important cultivar.
Chemical Properties of Citrus Cultivars
The results regarding chemical properties are presented in . The TSS (°Brix) is not only a measure of sugars, but it also indicates all the soluble solids that include acids and vitamins. The TSS provides a reliable indication for total sugar levels since sugars consist of approximately 80–85% TSS (Moshonas et al., Citation1991). The maximum TSS was observed in ‘Kinnow’ (12.4 ± 1.1%) followed by ‘Murocott’ (10.7 ± 0.6%) and ‘Valencia late’ (10.33 ± 0.4%), respectively. ‘Pamelo’ (9.9 ± 0.9%), ‘Shamber’ (9.7 ± 1.1%), and ‘Flame’ (9.42 ± 0.4 b %) did not differ significantly in terms of TSS.
Table 2. Chemical properties of pulp of different citrus cultivars.
The data pertaining to pH values clearly showed that the ‘Feutrell’s Early’ possesses the maximum pH value (4.4 ± 0.2) followed by ‘Morocott’ (4.24 ± 0.3) and ‘Red Blood’ (4.14 ± 0.01). The minimum pH values were recorded in ‘Pamelo’ (3.34) and ‘Flame’ (3.48). The titratable acidity was the highest in ‘Flame’ (1.38 ± 0.29%) followed by ‘Valencia late’ (0.92 ± 0.04%) and ‘Moro Blood’ (0.5 ± 0.02%). The titratable acidity of ‘Red Blood’ (0.5 ± 0.03%), ‘Pamelo’ (0.4 ± 0.03%), ‘Shamber’ (0.43 ± 0.03%), ‘Kinnow’ (0.4 ± 0.01%), and Morocott (0.5 ± 0.01%) was found almost the same, while the acidity of ‘Feutrell’s Early’ was the lowest (0.32 ± 0.02%). All other cultivars had a mild acid content that is less than 1.00%.
The taste of citrus fruit is principally governed by the levels of sugars and acids in the juice and the relative ratio between them also termed as the fruit Maturation Index. A good tasty fruit requires high levels of sugars and moderate levels of acidity rather than any other combination, resulting in a similar ripening ratio. In this context, although ‘Flame’ had a good TSS value (9.4 ± 0.4%) but with high acid (1.4 ± 0.3%), it resulted in the lowest Brix:Acid Ratio (7.04 ± 1.4), while ‘Feutrell’s Early’ (0.3 ± 0.02%) and ‘Kinnow’ (0.4 ± 0.01%) having mild acid contents resulted in comparatively good Brix:Acid Ratio, i.e., 29.8 ± 2.9 and 29.5 ± 3.3, respectively, imparting their good flavor, taste, and sensory acceptability.
The cultivar ‘Moro Blood,’ ‘Kinnow,’ ‘Pamelo,’ and ‘Feutrell’s Early’ exhibited higher vitamin C content (68.7 ± 1.05 mg/100 mL), (65.8 ± 3.47 mg/100 mL), (65.8 ± 2.46 mg/100 mL), and (65.6 ± 4.8 mg/100 mL), respectively. Liu et al. (Citation2012) stated that vitamin C in different citrus cultivars like oranges and grapefruits were in the range from 26.7 to 53.2 mg/100 mL juice, which are in agreement with our present findings. Another study (Rekha et al., Citation2012) found that orange juice contained 17.4 mg/100 mL of vitamin C, which was lower than investigated orange juice (65.8 mg/100 mL) in the present study. Similarly, Simona et al. (Citation2011) also found 56.5 mg/100 mL juice of vitamin C contents in orange (from Romania), whereas 40.8 mg/100 mL juice in ‘Pamelo’ (from Romania) which were lower than aforementioned citrus cultivars in the present study. The maximum total sugars (11.4 ± 1.4%) and reducing sugars (8.54 ± 1.3%) were found in ‘Kinnow’ and’Murocott’ (10.65 ± 1.6%), (8.19 ± 1.7%), whereas ‘Feutrell’s Early’ (3.28 ± 0.2%) showed the maximum value of non-reducing sugar. Our findings (of some cultivars) regarding total sugars and reducing and non-reducing sugars are concurrent with the findings of Liu et al. (Citation2012).
Effect of Ultrasound on the Total Phenolic and Flavonoids Contents of Citrus Juices
The results regarding the total phenolic contents of citrus fruit juices are presented in . Generally, citrus fruits have been investigated to have different phenolic compounds such as gallic acid, chlorogenic acid, sinapic acid, ferulic acid, quercetin, myricetin, naringenin, kaempferol, vanillic acid, syringic acid, rosmarinic acid, and naringin (Marzouk, Citation2013; Zulkifli et al., Citation2012). The total phenolics of citrus cultivars were in the range 223.49 ± 4.5–590.47 ± 5.5 μg GAE/mL but ‘Pamelo’ had the highest (590.47 ± 5.5 μg GAE/mL) phenolic contents among all the investigated citrus cultivars. The phenolic contents of Malaysian orange (135.3 mg GAE/100 mL) as investigated by (Anagnostopoulou et al., Citation2006) were higher than those of ‘Kinnow’ (orange) (24.37 mg GAE/100 mL) monitored in the present study. However, in another study (Kumar et al., Citation2013), total phenolic contents of orange (21.20 mg GAE/100 mL) were almost similar to those of our study (24.37 mg GAE/100 mL). In our study, the total phenolic contents were found significantly higher in the juices treated with ultrasound as compared to control. The total phenolic contents in the juices of citrus cultivars ranged from 223.49 ± 4.5 to 590.47 ± 5.5 µg GAE/mL without sonication and increased from 315.18 ± 6.1 to 645.44 ± 7.1 µg GAE/mL after applying ultrasound. Without sonication, the total phenolic content in ‘Pamelo’ juice was the highest followed by ‘Shamber’ and ‘Red Blood.’ The total phenolic contents after application of ultrasound were highest in ‘Pamelo,’ followed by ‘Moro Blood’ and ‘Shamber.’ Bhat et al. (Citation2011) observed a similar increasing trend in total phenolic compounds in the Kasturi lime juice. Aadil et al. (Citation2013) found increased total phenolic contents in grapefruit juice after application of ultrasound (using water bath) for 90 min. The increase in total phenolic contents might be from the addition of hydroxyl radical produced by application of sonication treatment. Ultrasound treatment process may cause liberation of phenolic compounds due to breakdown of cell walls of plant cells. The polyphenolic compounds in apple juice were significantly (p ≤ .05) higher after ultrasound treatment at 20°C, which might be due to sudden change in pressure or shear force exerted by bubble implosion that causes cavitation because of cell breakdown. This phenomenon of ultrasound treated juice samples was significantly higher than control samples (Abid et al., Citation2013; Nadeem et al., Citation2018).
Table 3. Effect of ultrasound on the total phenolic contents (gallic acid equivalent µg/mL) and total flavonoid contents (µg/mL catechin equivalent) of citrus pulp.
Citrus fruits usually contain flavonoids such as flavanones, polymethoxyflavones, and aglycones (taxifolin, acacetin) (Rice-Evans et al., Citation1997). The total flavonoids in control juice sample were more in ‘Pamelo,’ followed by ‘Red Blood’ and ‘Shamber,’ and after ultrasound treatment, the values of total flavonoids were the highest in ‘Shamber,’ followed by ‘Pamelo’ and ‘Red Blood.’ Aadil et al. (Citation2013) studied the effect of ultrasound treatment on 28 days storage of grapefruit juice. They observed an increase in flavonoid contents of grapefruit juice after ultrasonic treatment. This increase in bioactive compounds might be beneficial for the health and commercial point of view.
Effect of Ultrasound on DPPH Radical Scavenging Activity and the Total Antioxidant Activity of Citrus Juices
The results of DPPH radical scavenging activity are shown in . The DPPH-RSA (%) values were also higher in all cultivars when measured in µmol/mL trolox equivalent. The DPPH values of all juice samples ranged from 791.5 to 1251.9 µmol/mL trolox equivalent. After ultrasound treatment, these values of juice samples increased significantly (p ≤ .05) from 1001.5 to 1336.8 µmol/mL trolox equivalent. The juices from all cultivars showed an increasing trend of DPPH activity except the juice from ‘Shamber’ cultivar, which showed a small decrease in DPPH activity. Aadil et al. (Citation2013) also observed the same increasing trend in DPPH free radical scavenging activity while studying the grapefruit juices after ultrasound treatment for a storage period of 28 days. This increase may be due to the increase in total phenolic contents of juices. Vitamin C and phenolic compounds in citrus are responsible for antioxidant capacity and DPPH free radical scavenging activity. Phenols have redox property due to which these act as reducing agent, hydrogen donators, and metal chelation properties (Rice-Evans et al., Citation1997). Our results are also concomitant with previous findings that showed an increasing trend in DPPH free radical scavenging activity of grapefruit juice treated with thermosonication (Aadil et al., Citation2013). Observations made with Kasturi lime juice also showed an increase in radical scavenging activity of ultrasound treated lime juice (Bhat et al., Citation2011). In our research findings, all citrus fruit cultivars showed an increasing trend in radical scavenging activity that might be due to the release of phenolic compounds as a result of cavitation process of ultrasound treatment. The same reason might be for the increase in antioxidant capacity of grapefruit juices. This capacity is used to evaluate the antioxidant potential of any cell or tissue.
Figure 1. Effect of ultrasound on DPPH values in primary x-axis (blue bars showing ultrasonic-treated juice and red bars showing untreated juice) and radical scavenging activity (RSA %) in secondary x-axis (green lines showing untreated juice and purple lines showing ultrasonic-treated juice) of citrus fruits.
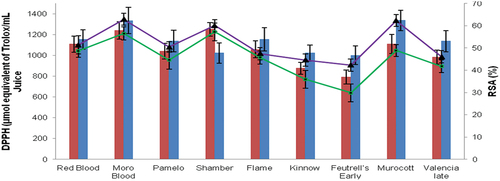
The results regarding the percent inhibition of DPPH radical scavenging activity (RSA %) are presented in . The results indicated that the radical scavenging activity in control fruit samples ranged from 29.8% (‘Feutrell’s Early’) to 57.2% (‘Shamber’), and in ultrasound treated samples, it ranged from 42.3% (‘Feutrell’s Early’) to 62.9% (‘Moro Blood’). It was found that the DPPH radical scavenging activity of all ultrasound treated juice samples was significantly higher than control juice samples. The radical scavenging activity in control juice sample was more in ‘Shamber’ (57.2%), ‘Moro Blood’ (56.5%) and ‘Murocott’ (48.8%) and after ultrasound treatment the radical scavenging activity was 62.9% in ‘Moro Blood,’ 62.3% in Morocott and 60.1% in ‘Shamber.’
The results of total antioxidant activity are shown in . The total antioxidant activity of citrus fruits is usually due to the presence of phenolic compounds, fflavonoids,and ascorbic acids (Gorinstein et al., Citation2001). In this study, it was observed that all juice samples of different cultivars possess excellent antioxidant activity and ranged from 100.5 (‘Murocott’) to 203.1 mg/100 mL ascorbic acid equivalent (‘Moro Blood’). The antioxidant activity of juice samples increased from 127.3 to 288.6 mg/100 mL ascorbic acid equivalent treated with ultrasound treatment. In ‘Kinnow’ juice the total antioxidant activity was 190.8 mg/100 mL ascorbic acid equivalent that increased to 288.5 mg/100 mL ascorbic acid equivalent after ultrasound treatment. In fruits and vegetables, a co-relation between total phenolic and antioxidant activity was observed by many researchers. Antioxidant activity increased significantly with high concentration of phenolic compounds (Jingfei and Vasantha, Citation2012).
Figure 2. Effect of ultrasound on the antioxidant activity (red bars showing untreated juice and blue bars showing ultrasonic-treated juice) and reducing power (green lines showing untreated juice and purple lines showing ultrasonic-treated juice) of citrus fruit. Values are mean ± SD (n = 6); statistical difference is noted as p ≤ .05.
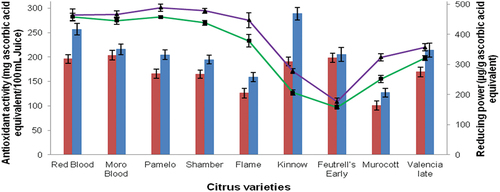
The Effect of Ultrasound on the Reducing Power of Citrus Juices
The results of reducing power of citrus fruit juices are given in . The results indicated that the reducing power in control fruit samples ranged from 157.1 (‘Feutrell’s Early’) to 458.9 µg/g ascorbic acid equivalent (‘Pamelo’) and in ultrasound treated samples it was ranged from 176.2 (‘Feutrell’s Early’) to 489.2 µg/g ascorbic acid (‘Pamelo’). It was found that the reducing power of all ultrasound treated juice samples was significantly higher than the control juice samples. The reducing power in control juice sample was more in ‘Pamelo’ (458.9 µg/g ascorbic acid equivalent), ‘Shamber’ (457.6 µg/g ascorbic acid equivalent) and ‘Moro Blood’ (445.5 µg/g ascorbic acid equivalent) and after ultrasound treatment, the reducing power of juice samples was 489.2 µg/g ascorbic acid equivalent in ‘Pamelo,’ 478.7 µg/g ascorbic acid equivalent in ‘Shamber’ and 466.6 µg/g ascorbic acid equivalent in ‘Red Blood.’ The increased reducing power after ultrasonic treatment might be due to more liberation of phenolics and flavonoids from cell walls after ultrasonic treatment.
Conclusions
The results of the study of varietal analysis are a type of endeavor to proceed on the development side by diversifying the citrus-based production both for direct consumption and value addition. The results revealed that sonication treatment could produce high-quality citrus fruit juices with higher amount of total phenols, total flavonoids, total antioxidant capacity, DPPH radical scavenging activity, and reducing power. On the basis of the results of the present study, it is suggested that sonication is highly feasible for practical use in the fruit and vegetables processing industry for the production of citrus fruit juices with improved quality in terms of nutrients.
Conflict of intrest
No conflict of interest exists in this manuscript.
Acknowledgments
The authors acknowledge the financial help of the Higher Education Commission, the United States Agency for International Development, and the University of Sargodha for execution of this research work.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Aadil, R.M., X.A. Zeng, Z. Han, and D.W. Sun. 2013. Effects of ultrasound treatments on quality of grapefruit juice. Food Chem. 141(3):3201–3206. doi: 10.1016/j.foodchem.2013.06.008.
- Abid, M., S. Jabbar, T. Wu, M.M. Hashim, B. Hu, S. Lei, X. Zeng, and X. Zeng. 2013. Effect of ultrasound on different quality parameters of apple juice. Ultrason. Sonochem 20(5):1182–1187. doi: 10.1016/j.ultsonch.2013.02.010.
- Aliakbarian, B., A.A. Casazza, E.J.O. Montoya, and A. Convert. 2010. Valorisation of olive oil solid wastes: valuable compounds recovery using high pressure-high temperature. J. Biotechnol. 150:332. doi: 10.1016/j.jbiotec.2010.09.341.
- Anagnostopoulou, M.A., P. Kefalas, V.P. Papageorgiou, A.N. Assimopoulou, and D. Boskou. 2006. Radical scavenging activity of various extracts and fractions of sweet orange peel (Citrus sinensis). Food Chem. 94(1):19–25. doi: 10.1016/j.foodchem.2004.09.047.
- AOAC. 2016. Official methods of analysis of AOAC International. 20 ed. AOAC International, Rockville, MD.
- Asjad, H.M.M., M.S. Akhtar, S. Bashir, B. Din, F. Gulzar, R. Khalid, and M. Asad. 2013. Phenol, flavonoid contents and antioxidant activity of six common citrus plants in Pakistan. J. Pharm. Cos. Sci 1(1):1–5.
- Batiston, W.P., S.A. Maruyama, S.T.M. Gomes, J.V. Visentainer, N.E. de Souza, and M. Matsushita. 2013. Total phenolic content and antioxidant capacity of methanolic extracts of ten fruits. Acta Scien. Technol 35(3):581–585.
- Bhat, R., N.S.B.C. Kamaruddin, L. Min-Tze, and A. Karim. 2011. Sonication improves kasturi lime (Citrus microcarpa) juice quality. Ultrason. Sonochem 18(6):1295–1300. doi: 10.1016/j.ultsonch.2011.04.002.
- Caminiti, I.M., I. Palgan, F. Noci, A. Muñoz, P. Whyte, D.A. Cronin, J.G. Lyng, and J.G. Lyng. 2011. The effect of pulsed electric fields (PEF) in combination with high intensity light pulses (HILP) on Escherichia coli inactivation and quality attributes in apple juice. Innov. Food Sci. Emerg. Technol 12(2):118–123. doi: 10.1016/j.ifset.2011.01.003.
- Cheng, L., C. Soh, S. Liew, and F. Teh. 2007. Effects of sonication and carbonation on guava juice quality. Food Chem. 104(4):1396–1401. doi: 10.1016/j.foodchem.2007.02.001.
- Din, A., M. Asghar, S. Parveen, and M.A. Azhar. 2012. Evaluation of ‘kinnow’ mandarin as influenced by pre-harvest management practices. J. Agric. Res 50(3):381–392.
- Fucik, J., and J. Norwine. 1979. Climatological parameters and grapefruit size relationships in the Rio Grande Valley of Texas. J. Rio Grande Vall. Hort. Soc 33:83–89.
- Ghafar, M., K.N. Prasad, K.K. Weng, and A. Ismail. 2010. Flavonoid, hesperidine, total phenolic contents and antioxidant activities from Citrus species. Afr. J. Biotechnol 9(3):326–330.
- Gorinstein, S., O. Martı́n-Belloso, Y.S. Park, R. Haruenkit, A. Lojek, M. Ĉı́ž, S. Trakhtenberg, I. Libman, and S. Trakhtenberg. 2001. Comparison of some biochemical characteristics of different citrus fruits. Food Chem. 74(3):309–315. doi: 10.1016/S0308-8146(01)00157-1.
- Gull, J., B. Sultana, F. Anwar, R. Naseer, M. Ashraf, and M. Ashrafuzzaman. 2012. Variation in antioxidant attributes at three ripening stages of guava (Psidium guajava L.) fruit from different geographical regions of Pakistan. Molecules 17(3):3165–3180. doi: 10.3390/molecules17033165.
- Hegazy, A., and M. Ibrahium. 2012. Antioxidant activities of orange peel extracts. World Appl. Sci. J 18(5):684–688.
- Herken, E.N., and S. Guzel. 2010. Total antioxidant capacity and total phenol contents of selected commercial fruit juices in Turkey. Int. J. Food Prop 13(6):1373–1379. doi: 10.1080/10942912.2010.499039.
- Ishfaq, M., S. Ahmad, M.Z. Awan, and M.A. Nasir. 2007. Performance of grape fruit cultivars under agro-climatic conditions of Chakwal. Pak. J. Agricul. Sci 44(3):472–474.
- Jingfei, G., and H.P.R. Vasantha. 2012. Nutritional, physicochemical and microbial quality of ultrasound-treated apple-carrot juice blends. Food Nutr. Sci 3(2):212–218.
- Khan, A.S., M. Naseer, A.U. Malik, S.M. Basra, M.S. Khalid, S. Khalid, M. Amin, B.A. Saleem, I.A. Rajwana, and M.U. Din. 2011. Location, soil and tree nutrient status influence the quality of Kinnow. Mandarin. Int. J. Agric. Biol 13(4):498–504.
- Kim, D.O., S.W. Jeong, and C.Y. Lee. 2003. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 81(3):321–326. doi: 10.1016/S0308-8146(02)00423-5.
- Knorr, D., M. Zenker, V. Heinz, and D.U. Lee. 2004. Applications and potential of ultrasonics in food processing. Trends Food Sci. Technol 15(5):261–266. doi: 10.1016/j.tifs.2003.12.001.
- Kumar, R., S. Vijay, and N. Khan. 2013. Comparative nutritional analysis and antioxidant activity of fruit juices of some citrus spp. Octa J. Biosci 1(1):44–53.
- Lacey, K., N. Hancock, and H. Ramsey. 2009. Measuring internal maturity of citrus.
- Latocha, P., B. Łata, and A. Stasiak. 2015. Phenolics, ascorbate and the antioxidant potential of kiwiberry vs. common kiwifruit: the effect of cultivar and tissue type. J. Funct. Food. 19:155–163. doi: 10.1016/j.jff.2015.09.024.
- Latoui, M., B. Aliakbarian, A.A. Casazza, M. Seffen, A. Converti, and P. Perego. 2012. Extraction of phenolic compounds from Vitex agnus-castus L. Food Bioprod. Process 90(4):748–754. doi: 10.1016/j.fbp.2012.01.003.
- Lee, S.K., and A.A. Kader. 2000. Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biol. Technol 20(3):207–220. doi: 10.1016/S0925-5214(00)00133-2.
- Liu, Y., E. Heying, and S.A. Tanumihardjo. 2012. History, global distribution, and nutritional importance of citrus fruits. Compr. Rev. Food Sci. Food Saf 11(6):530–545. doi: 10.1111/j.1541-4337.2012.00201.x.
- Marzouk, B. 2013. Characterization of bioactive compounds in tunisian bitter orange (Citrus aurantium L.) peel and juice and determination of their antioxidant activities. Biomed Res. Int 2013:1–12.
- Mathew, B.B., S.K. Jatawa, and A. Tiwari. 2012. Phytochemical analysis of Citrus limonum pulp and peel. Int. J. Pharm. Pharm 4(2):369–371.
- Mehmood, A., M. Ishaq, L. Zhao, S. Yaqoob, B. Safdar, M. Nadeem, M. Munir, and C. Wang. 2019. Impact of ultrasound and conventional extraction techniques on bioactive compounds and biological activities of blue butterfly pea flower (Clitoria ternatea L.). Ultrason. Sonochem. 51:12–19. doi: 10.1016/j.ultsonch.2018.10.013.
- Milind, P., and C. Dev. 2012. Review article orange: Range of benefits. J. Pharm 3(7):3–7.
- Mohamed, S.A., A.L. Al-Malki, and T.A. Kumosani. 2009. Characterization of a polygalacturonase from Trichoderma harzianum grown on citrus peel with application for apple juice. Aust. J. Basic Appl. Sci 3(3):2770–2777.
- Mohamed, S.A., M.A. Awad, E.R.F. El-Dengawy, H.M. Abdel-Mageed, M.O. El-Badry, H.A. Salah, A.M. Abdel-Aty, and A.S. Fahmy. 2016. Total phenolic and flavonoid contents and antioxidant activities of sixteen commercial date cultivars grown in Saudi Arabia. RSC Adv 6(50):44814–44819. doi: 10.1039/C6RA02831D.
- Monica, S., S. Leonardo, B. Adalgisa, and G. Giacomo. 2011. Preliminary study on bioactive compounds of citrus× myrtifolia rafinesque (Chinotto) to its potential application in food industry. Food Nutr. Sci 2:685–691.
- Moshonas, M.G., P.E. Shaw, and R.D. Carter. 1991. Ambersweet Orange hybrid: Compositional evidence for variety classification. J. Agric. Food Chem 39(8):1416–1421. doi: 10.1021/jf00008a012.
- Nadeem, M., N. Ubaid, T.M. Qureshi, M. Munir, and A. Mehmood. 2018. Effect of ultrasound and chemical treatment on total phenol, flavonoids and antioxidant properties on carrot-grape juice blend during storage. Ultrason. Sonochem. 45:1–6. doi: 10.1016/j.ultsonch.2018.02.034.
- Nadeem, M., A. Ghaffar, M.M. Hashim, M.A. Murtaza, M.M.A.N. Ranjha, A. Mehmood, and M.N. Riaz. 2021a. Sonication and microwave processing of phalsa drink: A synergistic approach. Int. J. Fruit Sci 21(1):993–1007. doi: 10.1080/15538362.2021.1965942.
- Nadeem, H.R., S. Akhtar, T. Ismail, P. Sestili, J.M. Lorenzo, M.M.A.N. Ranjha, L. Jooste, C. Hano, and R.M. Aadil. 2021b. Heterocyclic aromatic amines in meat: formation, isolation, risk assessment, and inhibitory effect of plant extracts. Foods 10(7):1466. doi: 10.3390/foods10071466.
- Nayik, G.A., Y.D. Jagdale, S.A. Gaikwad, A.N. Devkatte, A.H. Dar, D.S. Dezmirean, O. Bobis, M.M.A.N. Ranjha, M.J. Ansari, H.A. Hemeg, et al. 2021. Recent insights into processing approaches and potential health benefits of goat milk and its products: A review. Front. Nutr. 8:789117. doi: 10.3389/fnut.2021.789117.
- Prieto, P., M. Pineda, and M. Aguilar. 1999. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Analyt. Biochem 269(2):337–341. doi: 10.1006/abio.1999.4019.
- Ranjha, M.M.A.N., B. Shafique, L. Wang, S. Irfan, M.N. Safdar, M.A. Murtaza, M. Nadeem, S. Mahmood, G. Mueen-ud-Din, and H.R. Nadeem. 2021a. A comprehensive review on phytochemistry, bioactivity and medicinal value of bioactive compounds of pomegranate (Punica granatum). In: Advances in traditional medicine. Singapore: Springer Science and Business Media LLC. doi:10.1007/s13596-021-00566-7.
- Ranjha, M.M.A.N., S. Irfan, J.M. Lorenzo, B. Shafique, R. Kanwal, M. Pateiro, R.N. Arshad, L. Wang, G.A. Nayik, U. Roobab, et al. 2021b. Sonication: A potential technique for extraction of phytoconstituents: A systematic review. Processes 9(8):1406. doi: 10.3390/pr9081406.
- Ranjha, M.M.A.N., R. Kanwal, B. Shafique, R.N. Arshad, S. Irfan, M. Kieliszek, P.Ł. Kowalczewski, M. Irfan, M.Z. Khalid, U. Roobab, et al. 2021c. A critical review on pulsed electric field: A novel technology for the extraction of phytoconstituents. Molecules 26(16):4893. doi: 10.3390/molecules26164893.
- Ranjha, M.M.A.N., S. Irfan, M. Nadeem, and S. Mahmood. 2022. A comprehensive review on nutritional value, medicinal uses, and processing of banana. Food Rev. Int 38(2):199–225. doi: 10.1080/87559129.2020.1725890.
- Rekha, C., G. Poornima, M. Manasa, V. Abhipsa, J.P. Devi, H.T.V. Kumar, and T.R.P. Kekuda. 2012. Ascorbic acid, total phenol content and antioxidant activity of fresh juices of four ripe and unripe citrus fruits. Chem. Sci. Trans 1(2):303–310. doi: 10.7598/cst2012.182.
- Rice-Evans, C., N. Miller, and G. Paganga. 1997. Antioxidant properties of phenolic compounds. Trends Plant Sci. 2(4):152–159. doi: 10.1016/S1360-1385(97)01018-2.
- Roobab, U., R. Afzal, M.M.A.N. Ranjha, X. Zeng, Z. Ahmed, and R.M. Aadil. 2022. High pressure‐based hurdle interventions for raw and processed meat: A clean‐label prospective. Int. J. Food Sci. Technol 57(2):816–826. doi: 10.1111/ijfs.15499.
- Sabir, H.M., M.B. Khan, and Z. Hussain. 2010. Marketing margin of mandarin: a case study of sargodha region, Pakistan. Pak. J. Soc. Sci 30(2):275–291.
- Siddiq, S., A. Raza, K. Altaf-ur-Rehman, and M. Avais. 2011. An insight into prominent soil characters of Sargodha areas for the establishment of healthy citrus orchards. J. Agric. Res 49(1):27–37.
- Sikora, E., E. Cieślik, and K. Topolska. 2008. The sources of natural antioxidants. Acta Sci. Pol. Technol. Aliment 7(1):5–17.
- Simona, B., F. Alexandrina, Ţ.D. Mirela, and S. Ildikó. 2011. Studies on citrus species fruits ascorbic acid content using kinetic, spectrophotometric and iodometric methods. Analele Univ. din Oradea, Fasc. Biol 16:212–217.
- Singleton, V.L., R. Orthofer, and R.M. Lamuela-Raventós. 1999. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In: N. Jura and J.M. Murphy (eds.), Methods in enzymology (Vol. 299, pp. 152–178). Cambridge, Massachusetts, United States: Elsevier.
- Skrede, G., V.B. Larsen, K. Aaby, A.S. Jørgensen, and S.E. Birkeland. 2004. Antioxidative properties of commercial fruit preparations and stability of bilberry and black currant extracts in milk products. J. Food Sci 69(9):S351–S356. doi: 10.1111/j.1365-2621.2004.tb09948.x.
- Tsai, H.L., S.K. Chang, and S.J. Chang. 2007. Antioxidant content and free radical scavenging ability of fresh red pummelo [Citrus grandis (L.) Osbeck] juice and freeze-dried products. J. Agric. Food Chem 55(8):2867–2872. doi: 10.1021/jf0633847.
- Valero, M., N. Recrosio, D. Saura, N. Muñoz, N. Martí, and V. Lizama. 2007. Effects of ultrasonic treatments in orange juice processing. J. Food Eng 80(2):509–516. doi: 10.1016/j.jfoodeng.2006.06.009.
- Walkling-Ribeiro, M., F. Noci, J. Riener, D. Cronin, J. Lyng, and D. Morgan. 2009. The impact of thermosonication and pulsed electric fields on staphylococcus aureus inactivation and selected quality parameters in orange juice. Food Bioproc. Tech 2(4):422–430. doi: 10.1007/s11947-007-0045-7.
- Wu, J., T. Gamage, K. Vilkhu, L. Simons, and R. Mawson. 2008. Effect of thermosonication on quality improvement of tomato juice. Innov. Food Sci. Emerg. Technol 9(2):186–195. doi: 10.1016/j.ifset.2007.07.007.
- Yi, Z., Y. Yu, Y. Liang, and B. Zeng. 2008. In vitro antioxidant and antimicrobial activities of the extract of pericarpium citri reticulatae of a new citrus cultivar and its main flavonoids. LWT-Food Sci. Tech 41(4):597–603. doi: 10.1016/j.lwt.2007.04.008.
- Zhang, B., X.A. Zeng, W.T. Lin, D.W. Sun, and J.L. Cai. 2013. Effects of electric field treatments on phenol compounds of brandy aging in oak barrels. Innov. Food Sci. Emerg. Technol. 20:106–114. doi: 10.1016/j.ifset.2013.07.003.
- Zulkifli, K.S., N. Abdullah, A. Abdullah, N. Aziman, and W. Kamarudin. 2012. Bioactive phenolic compounds and antioxidant activity of selected fruit peels. paper presented at the international conference on environment. Chem. Biol. 49(14):66–70.
