 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
An experiment was conducted to find out the effects of various postharvest treatments on the biochemical and bioactive compounds of custard apple cv. Balanagar fruits. In the present experiment, three chemicals viz. oxalic acid (OA) (2.5 mM, 5.0 mM, 7.5 mM, and 10 mM; salicylic acid (SA) (0.5 mM, 1.0 mM, 1.5 mM, and 2.0 mM) and Sodium Nitro Prusside (SNP) at 0.5 mM, 1.0 mM, 1.5 mM, and 2.0 mM along with control were applied. Fruits were stored at ambient condition (Temp. 25 ± 2°C and relative humidity (RH) 44 ± 2%). Results indicated that fruits treated with SNP (2.0 mM) had significantly (p≤ 0.05) higher total soluble solids (30.80°B), titratable acidity (TA) (0.33%) and retained higher total sugar content (TS) (25.09%) at the end of the 9th day of storage. At the end of storage, the least reduction in total phenol content (TPC) (492.52 mg gallic acid equivalent (GAE)/100 g (11.62%) was found in SNP (2.0 mM) treated fruits. Likewise, SNP (2.0 mM) significantly (p≤ 0.05) retained higher ascorbic acid (AA) (43.20 mg/100 g), antioxidant activity (AOX) (0.92 µmol Trolox Equivalent (TE)/g), total flavonoids (TF) (71.49 µg CE/100 g) and tannin content (TC) (1.28 mg/g) compared to control during 9 days of storage. Thus, in the present investigation, SNP treatment was most suited to preserve the nutritional quality and bioactive compounds.
Introduction
Custard apple (Annona squamosa L.) is a delicious fruit cultivated in tropical and sub-tropical parts of the World. The custard apple can be cultivated up to 100 meter above mean sea level. It is very hardy and drought resistant crop and can be grown in undulated, marshy and problematic soils (Singh, Citation1992). The ripened fruits are consumed mainly in fresh form and some processed products such as ice creams, chips can be prepared. Fruit contains minerals, vitamins, sugars and several bioactive compounds. In the recent period, exploration of bioactive compounds such as phenols, antioxidants, vitamins and certain other flavonoids from fruits has been attempted (Chen et al., Citation2011). Researchers have isolated and characterized the bioactive compounds from custard fruits. They also have explored their commercial application from folk medicine to commercial application in pharmaceutical industries (Rojas-García et al., Citation2022). The bioactive compounds especially antioxidants can scavenge the free radicals and protect the cells from oxidation and custard apple is rich in AOX properties thus can be used as potential source in pharmaceutical industry.
Custard apple fruit is highly perishable due to its climacteric nature. It ripens within 2–3 days after harvesting at the commercial maturity stage. Due to highly climacteric and faster metabolic activities, on slight pressure fruits easily get disintegrate into segments (Jawadagi et al., Citation2013). The fruits can remain acceptable only for 2–3 days after ripening at room temperature. Fruits are highly prone to decay as they respire more and become soften rapidly. There is gap in supply chain of custard apple as it remains unattended.
Exogenous application of eco-friendly and safe chemicals becomes a major concern. Continuous use of synthetic and harmful fungicides deteriorates the quality as well as raised health issues. Thus, there is a need to explore some alternate safe chemicals. Recently, the use of OA, SA and SNP have got attention due to their wider adaptability, and safe and easy uses. OA delays the activities of pectic enzymes such as polygalacturonase (PG) and pectin methyl esterase (PME), which are responsible for cell wall degradation (Wu et al., Citation2011). Furthermore, OA inhibits the biosynthesis pathway of ethylene thus reducing the physiological activities and enhancing the shelf life. SA is an endogenous plant hormone which plays an important role in enhancing fruit quality and has positive effects on reducing respiration and ethylene biosynthesis rates, weight loss, decay and softening of the fruits (Shafiee et al., Citation2010). Recently SA gained the status of commercial importance owing to its pleiotropic functions in plant systems. SNP can release nitric oxide (NO) by reduction and decomposition. Both ethylene and NO have antagonistic effects in climacteric fruit (Liu et al., Citation2007), since NO decreases ethylene synthesis, during ripening and fruit senescence. The application of these eco-friendly generally recognize as safe (GRAS) chemicals have been explored in many fruits but remains unexplored in custard apple. Hence, there is an urgent need to develop low-cost and eco-friendly approaches. This present study aimed to elucidate the effects of OA, SA, and SNP on biochemical traits and bioactive compounds of custard apple cv. Balanagar.
Material and Methods
Fruit Sample and Treatment Application
The mature, freshly harvested, uniform size and quality fruits of custard apple cv. “Balanagar” free from insect pest and microbial infection were procured from the experimental orchard of AICRP on AZF, College of Horticulture and Forestry, Jhalawar and brought to the PHT laboratory. After preliminary sorting, the fruits were washed in tap water to remove the dirt and dust from the surface of the fruit and then fruits were dried in the air. The whole lots of fruits were randomly divided into 13 lots for each treatment and each lot containing three replications with 20 fruits per replication. The fruits were dipped In the following aqueous solution of treatments viz.: OA- (2.5 mM, 5.0 mM, 7.5 mM, and 10.0 mM) SA (0.5 mM, 1.0 mM, 1.5 mM, and 2.0 mM), SNP (0.5 mM, 1.0 mM, 1.5 mM, and 2.0 mM) for 10 minutes followed by air drying. Control fruits were dipped in distilled water for 10 minutes. These fruits were kept in aerated corrugated boxes at ambient storage conditions [Temp. 25 ± 2°C and relative humidity (RH) 44 ± 2%] for further biochemical analysis
Biochemical Analysis
A total soluble solid was measured by a hand refractometer (Model Atago, Japan). For this, one drop of fruit juice was placed on the prism of the refractometer and the reading was noted against the light and expressed in °Brix. TA of fruit pulp was estimated by taking pulp followed by grinding it with double distilled water. The filtered juice was titrated against standard 0.1 N sodium hydroxide solution using one drop of phenolphthalein as an indicator (AOAC, Citation2007). Total sugars were estimated by Lane and Eynon copper titration method as described by Ranganna (Citation1986) and expressed in percent.
The TPC in the fruit pulp was determined by the method of Singleton and Rossi (Citation1965). For this 5 g of fruit, the pulp was crushed in 10 ml of 80% ethanol and centrifuged at 15000 × g for 20 min at 4°C. After centrifugation, the supernatant was collected and 2.8 ml of distilled water was added to it followed by a 200 μl sample and 0.5 ml of 2 N Folin-Ciocalteau reagents. After 3 min, 2 ml 20% of Na2CO3 was added to it. The absorbance was measured at 750 nm using a 1 cm cuvette in a spectrophotometer. The amount of TPC was presented in mg GAE/100 g.
The antioxidant capacity of custard apple fruits was measured by CUPRAC (cupric reducing antioxidant capacity) method previously standardized (Apak et al., Citation2004). The antioxidant solution was mixed with copper (II) chloride solution, neocuproine alcoholic solution, and ammonium acetate buffer at 7.0 pH. The absorbance was taken at 450 nm against a reagent blank. The antioxidant activity was expressed as μmol Trolox Equiv (TE)/g.
AA content was estimated by using 2, 6-Dichlorophenol indophenols dye (DCPIP) as a visual titration method (Ranganna, Citation2007). Dye was standardized by taking 5 ml of each ascorbic acid solution and HPO3 against dye until a pink color appeared. The fruit pulp was crushed using 3% HPO3 followed by centrifugation. The filtered sample was taken for titration against dye. The ascorbic acid content was calculated with the formula mentioned underneath-
The total flavonoid content of custard apple fruit was determined by the colourimetric method as followed (Jia et al., Citation1999). Fruit pulp was crushed in ethanol maintaining a ratio of 1:25 and shaken at ambient conditions for a day. After filtration of the mixture, 1 ml of ethanol extract was mixed into 5% sodium nitrite solution followed by 3 ml aluminum chloride solution. The absorbance was recorded at 510 nm and expressed as µg catechin equivalent (CE)/100 g of fresh weight.
TC was estimated by the spectrophotometric method as previously described by Sasidharan and Jayadev (Citation2017). One gram sample was crushed into 50% methanol followed by centrifugation at 10000 × g. Vanillin hydrochloride reagents were added to the mixture and absorbance was recorded at 500 nm. A standard plot was curved by using 20 µg catechin and the value was presented in mg CE/g.
Statistical Analysis
The data collected from each lot and at each interval were collected in three replicates. The observed data were subjected to analysis of variance by the Completely Randomized Design (CRD) method as per the method suggested by Panse and Sukhatme (Citation1989). The data analysis was carried out with OPSTAT software and the mean value of three replicates was presented. The percent increment and decrement were also mentioned in parentheses with the Arc sign transformation.
Results and Discussion
Total Soluble Solids
Fruit TSS was estimated in all treated samples at 3 days interval. Results indicated that TSS was increased in all treatments during storage(). It was significantly (p ≤ 0.05) affected by different postharvest treatments during the entire storage period. On the 9th day of storage, maximum increment in TSS 30.80°B (38.87%) was obtained in SNP (2.0 mM) compared to the lowest 28.10°B (25.61%) in control. TSS plays a crucial role in the ripening process and is also used as an indicator of consumer acceptance (Quintero et al., Citation2013). Increased TSS in treated fruits might be due to moisture loss, conversion of reserved starch and polysaccharide to soluble forms of sugar in climacteric fruits (Singh et al., Citation1981). SNP (0.2 mM) treatment creates a strong barrier than the other treatments which might be attributed to slower respiration and catabolic processes. Nolpradubphan and Lichanporn (Citation2016) concluded that lime immersed with 5 μg/l SNP significantly delayed the increase of total soluble solids contents during storage.
Table 1. Effect of different postharvest treatments on total soluble solids (°Brix) of custard apple cv. Balanagar during ambient storage.
Titratable Acidity
A nonsignificant impact on TA in custard apple fruit was reported. According to , a slight decline in TA during storage was observed in all treatments. However, it was statistically non-significant (p ≤ 0.05) with other treatments at the end of storage. Value wise increment percent in TA content was perceived in SA (2.0 mM) (57.81%) followed by OA (5.0 mM) (57.81%). Titratable acidity is strongly correlated with the presence of citric acid and varies with the degree of ripeness and also determines the final taste and flavor (Briceño et al., Citation2005). In custard apple fruit, a small amount of organic acids were reported and it sharply decreases with the advancement of ripening. It is well-known fact that these organic acids are used as substrate in the respiration process during the storage of fruits (Jacobi et al., Citation2001). In our case, SA treatment (2.00 mM) delayed the respiration and retained the acids by protecting against respiratory substrate whereas, in control fruits, these acids might be used in the metabolic processes thus sudden decline was observed. Rasouli et al. (Citation2019) reported that orange cv. ‘Thomson Navel’ treated with SA (2 mM) significantly showed higher fruit titratable acidity.
Table 2. Effect of different postharvest treatments on titratable acidity (%) of custard apple cv. Balanagar during ambient storage.
Total Sugars
shows the changes in total sugars affected by different postharvest treatments during storage. After end of storage, a significant (p ≤ 0.05) variation was obtained treated fruits over control. Fruits treated with SNP (2.0 mM) exhibited a significantly (p ≤ 0.05) higher increment [25.09% (76.26%)] in total sugar. In other hand, control fruits showed the least increment [20.19% (26.26%)] after the 9th day of storage. It was interesting to note that OA and SA were found to be non-significant after end of storage. Total sugar is a handy parameter in custard apple fruit. Hubbard et al. (Citation1991) reported that high sugar content is linked with the activity of sucrose-phosphate synthase (Langenkamper et al., Citation1998) which can be accelerated by the ethylene and ripening process during storage. Fruits, before the ripening stage, possess a great amount of starch which is converted into sugars during ripening. Depletion of sucrose along with other respiratory substrates has a great impact on fruit quality during postharvest storage. In our study, we observed that SNP treatment significantly delayed the degradation of sugars. SNP treatments also reduced the respiration rate and other metabolic activities thus less breakdown of sugar-like compounds. Findings concerning the increment in total sugars during storage conform with the results obtained by Chen et al. (Citation2019) in apples ‘Fuji’ and Ge et al. (Citation2019) in blueberry.
Table 3. Effect of different postharvest treatments on total sugars (%) of custard apple cv. Balanagar during ambient storage.
Total Phenol Content
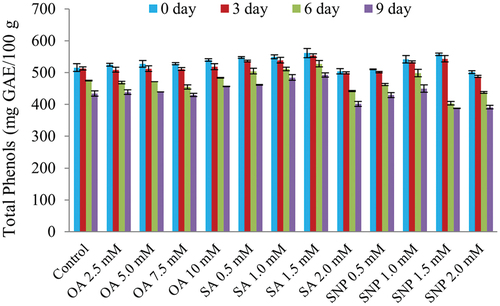
The TPC content significantly (p ≤ 0.05) reduced during the entire storage period (). It was interesting to note that fruits treated SA significantly (p ≤ 0.05) preserved the phenols over control but found non-significant with SNP (2.0 mM). The minimum reduction [492.66 mg GAE/100 g (11.62%)] in TPC was found in the SA (1.5 mM) treated fruits followed by SNP (2.0 mM) 387.52 mg GAE/100 g. Control fruits did not preserve the TPC value during storage. It is well known that phenolics are the naturally occurring substances in plants as well as in fruits which act as strong antioxidants (Ma et al., Citation2011). The reduction in phenolics during the ripening in both treatments could be due to its oxidation of phenols or any other compounds or owing to their transformation from a soluble into an insoluble form (Singh et al., Citation1981). The phenolic content is directly correlated with certain enzymes such as peroxidase (Zhanga et al., Citation2019) which might be higher in treated fruits. Findings for the decrease in TPC during storage are similar to the results obtained by Zhang et al. (Citation2016) in bananas and Ren et al. (Citation2017) in mango cv. ‘Tainong.’ It has been reported that TPC in SNP-treated fruits decreased more slowly than in the control fruits. Phenolic compounds have shown a complicated and intriguing reaction in enzymatic browning. The intensity of the brown color caused by PPO and phenolics in the presence of oxygen depends on the type of phenolic compounds involved (Lee and Jaworski, Citation1988). Thus, during the storage period, the treatment might have inhibited the activities of polyphenol oxidase (PPO) and peroxidase (POD) enzymes that catalyze the oxidation of phenolic compounds associated with high TPC in custard apple. The studies carried out by Duan et al. (Citation2007) in longan and Shahkoomahally et al. (Citation2015) in persimmon support and validate our findings.
Figure 1. Effect of different postharvest treatments on total phenol content (mg GAE/100 g) of custard apple cv. Balanagar during ambient storage *The graph shows mean value of three replicates and the vertical bars represent the standard error value of the replicates. Abbr. OA: Oxalic acid; SA: Salicylic acid; SNP: Sodium nitroprusside.
Antioxidant Activity
The AOX activity was significantly (P ≤ 0.05) affected by different treatments during storage period. As shows that AOX activity slightly declined during storage. Nevertheless, SNP (2.00 mM) treated fruit produced maximum amount of AOX (0.92 µmol TE/g) at the end of 9th day followed by SA (0.62 µmol TE/g). The maximum reduction (85.71%) in AOX was obtained in control fruits after end of storage. Antioxidant capacity is important for fruit quality, and in fruits, antioxidant capacity is relatively stable during long-term storage (Huang et al., Citation2008). Antioxidant activity of SNP-treated fruits might be due to less free radicle production thus helping in retaining the AOX value. Several pieces of evidence suggest that sodium nitroprusside protects the cell against stressful situations, and scavenges the free radicals by activating the antioxidative enzymes (Barman et al., Citation2014; Shi et al., Citation2007). The value of antioxidants reduces during the ripening and advancement of storage. In our results, a decreased value of AOX was noticed in all treatments. It is well-established fact that the antioxidant capacity of any fruit is largely governed and affected by phenolic compounds (Ma et al., Citation2011). These compounds can inhibit the process of oxidation of cells (Pennycooke et al., Citation2005). The reduction of AOX might be attributed to the development of more free radicles during the storage utilizing the AOX-like compounds and thus preventing oxidative damage. The study by Ali et al. (Citation2016) and by Hassanpour (Citation2015) on raspberry fruits endorsed our findings. Nevertheless, the antioxidant property of fruit was not entirely related to ascorbic acid (Piga et al., Citation2002). The maintenance of higher phenolics could be imparted more antioxidant value as previously reported in mango (Khaliq et al., Citation2016). Another finding related to the decrease in antioxidant activity during storage supports our results obtained by Saba and Moradi (Citation2017) in peach cultivar ‘G. H. Hill.’
Table 4. Effect of different postharvest treatments on antioxidant activity (µmol Trolox Equivalent (TE) /g) of custard apple cv. Balanagar during ambient storage.
Ascorbic Acid
The results from different treatments on AA content during storage evaluated are shown in . The AA content differed (p ≤ 0.05) among the treatments during storage. In present study, AA was found increased up to 3rd day and thereafter substantially declined in all treatments. It was noticed that fruits treated with SNP (2.0 mM) exhibited maximum (43.20 mg/100 g) and (9.92%) retention in AA content followed by SA (2.0 mM) (8.79%). In other hand, after 9 days of storage, minimum AA (36.29 mg/100 g) was recorded in control fruits which showed least reduction (0.24%). Ascorbic acid content plays important role in maintaining the nutritive value of stored fruits.The mechanism of AA content in ripened fruits is well recognized and established and it falls during the ripening under storage condition (Fennema, Citation1996; Rueda, Citation2005). The activity of dehydroascorbates might influence the AA content. The level of hormones such as SA and SNP may crosstalk with other hormones and regulate the process driven by dehydro-ascorbates. Ascorbic acid is lost in fruits stored at ambient temperatures (Sayyari et al., Citation2010). Ren et al. (Citation2017) obtained similar results in mango cv. ‘Tainong’ and Zhang et al. (Citation2016) in banana.
Table 5. Effect of different postharvest treatments on ascorbic acid (mg/100 g) of custard apple cv. Balanagar during ambient storage.
Total Flavonoids
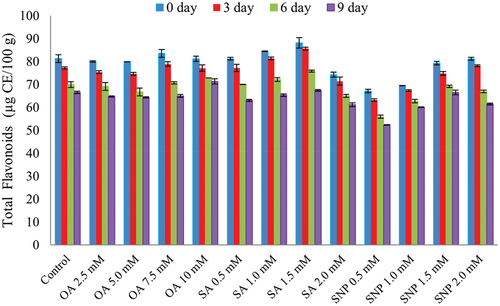
In , the changes in TF content as affected by OA, SA and SNP treatments. The TF content reported initially higher and then declined with ripening and storage days in all treatments. The minimum reduction (11.99%) in TF was obtained in SNP (2.0 mM) treated fruits. The treatments were differed at (p ≤ 0.05) with control. In other hand, control fruit exhibited maximum reduction (25.57%) on 9th day of storage. Flavonoids are also considered an antioxidant as they can scavenge free radicals and positively affect shelf life of fruit (Korkina and Afanasev, Citation1997). Several authors have reported a reduction in total flavonoids in fruits during ripening. This might be due to reduced activity of phenylalanine ammonia-lyase (PAL), a key enzyme responsible for the synthesis of flavonoids. Several contradictory reports on guava (Nair et al., Citation2018) and Hassanpour, (Citation2015) on raspberry were published where they reported an increment in total flavonoids in treated fruits respectively. In the present study, the minimum reduction in TF might be due to less oxidation of PAL and higher activity of PAL enzyme in the fruits during storage. Another study by Rahman et al. (Citation2020) reported contrasting results in the case of coated guava.
Figure 2. Effect of different postharvest treatments on total flavonoid (µg CE /100 g) of custard apple cv. Balanagar during ambient storage *The graph shows mean value of three replicates and the vertical bars represent the standard error value of the replicates. Abbr. OA: Oxalic acid; SA: Salicylic acid; SNP: Sodium nitroprusside.
Tannin Content
The TC content was significantly (p ≤ 0.05) decreased during storage at ambient. As shown in , the fruits treated with SNP (2.0 mM) maintained higher value (1.28 mg CE/g) compared to control (0.54 mg CE/g). Nevertheless, treated fruits showed least (20%) loss in TC content at the end of storage (9 days). In other hand, control fruits exhibited maximum (44.89%) loss in TC content. Tannins are naturally occurring substances found in almost every part of custard apple and they are soluble in both water and alcohol (Sasidharan and Jayadev, Citation2017). The reduction in tannin content of SNP-treated fruits might be correlated with the synthesis and action of phenyl ammonia lyases enzyme which could synthesize more flavonoids and helps in the retention of tannin compounds. Rahman et al. (Citation2020) reported similar findings with coated fruits of guava. Bibi et al. (Citation2007) reported that some astringent fruit show reduction in tannins during ripening due to a decrease in extractability/ polymerization accompanied by a loss in fluidity and a decrease in astringency.
Table 6. Effect of different postharvest treatments on tannin content (mg catechin equivalent (CE)/g) of custard apple cv. Balanagar during ambient storage.
Conclusion
In conclusion, SNP (2.0 mM) proven the best treatment compared to others that preserved the most of bioactive compounds and maintained overall quality of custard apple fruits. Fruits treated with SNP (2.0 mM) exhibited higher TSS, sugars, AA and showed minimum reduction in TPC and AOX content at the end of storage period of 9 days. Thus, SNP could be recommended as postharvest treatment for better shelf life and quality of fruits during supply chain. However, there is further scope to elucidate the mechanism and regulatory processes at the genetic level, especially in minor fruits like a custard apple.
Acknowledgments
The authors are highly grateful to the Department of Postharvest Technology, Agriculture University, Kota for providing the necessary facilities for the research.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ali, S., A.S. Khan, A.U. Malik, and M. Shahid. 2016. Effect of controlled atmosphere storage on pericarp browning, bioactive compounds and antioxidant enzymes of litchi fruits. Food Chem. 206:18–29. doi: 10.1016/j.foodchem.2016.03.021.
- AOAC. 2007. Association of Official Analytical Chemists. Official methods of analysis of the Association of Official Analytical Chemists (18th ed.). Gaithersburg.
- Apak, R., K. Güçlü, M. Özyürek, and S.E. Karademir. 2004. Novel total antioxidant capacity index for dietary polyphenols and Vitamins C and E, Using their cupric ion reducing capability in the presence of neocuproine. CUPRAC Method.J. Agric. Food Chem 52(26):7970–7981. doi: 10.1021/jf048741x.
- Barman, K., M.W. Siddiqui, V.B. Patel, and M. Prasad. 2014. Nitric oxide reduces pericarp browning and preserves bioactive antioxidants in litchi. Sci. Hortic 171(171):71–77. doi: 10.1016/j.scienta.2014.03.036.
- Bibi, N., A.B. Khattak, and Z. Mehmood. 2007. Quality improvement and shelf life extension of persimmon fruit (Diospyros kaki). J. Food Eng 79(4):1359–1363. doi: 10.1016/j.jfoodeng.2006.04.016.
- Briceño, S., J. Zambrano, W. Materano, I. Quintero, and A. Valera. 2005. Calidad de los frutos de mango bocado, maduradosen la planta y fuera de la planta cosechadosenmadurezfisiológica. Agron. Trop 55:461–473.
- Chen, J., Y. Chen, and X. Li. 2011. Beneficial aspects of custard apple (Annona squamosa L.) seeds. Nuts and Seeds in Health and Disease Prevention. 439–445.
- Chen, Y., Y. Ge, J. Zhao, M. Wei, C. Li, J. Hou, Y. Cheng, and J. Chen. 2019. Postharvest sodium nitroprusside treatment maintains storage quality of apple fruit by regulating sucrose metabolism. Postharvest Biol. Technol. 154:115–120. doi: 10.1016/j.postharvbio.2019.04.024.
- Duan, X., X. Su, Y. You, H. Qu, Y. Li, and Y. Jiang. 2007. Effect of nitric oxide on pericarp browning of harvested longan fruit in relation to phenolic metabolism. Food Chem. 104(2):571–576. doi: 10.1016/j.foodchem.2006.12.007.
- Fennema, O.R. 1996. Food Chemistry. 3rd Ed. Marcel Dekker, New York, USA.
- Ge, Y., Y. Chen, C. Li, J. Zhao, M. Wei, X. Li, S. Yang, and Y. Mi. 2019. Effect of sodium nitroprusside treatment on shikimate and phenylpropanoid pathways of apple fruit. Food Chem. 290:263–269. doi: 10.1016/j.foodchem.2019.04.010.
- Hassanpour, H. 2015. Effect of Aloe vera gel coating on antioxidant capacity, antioxidant enzyme activities and decay in raspberry fruit. LWT - Food Sci. Technol 60(1):495–501. doi: 10.1016/j.lwt.2014.07.049.
- Huang, R., R. Xia, Y. Lu, L. Hu, and Y. Xu. 2008. Effect of pre-harvest salicylic acid spray treatment on post-harvest antioxidant in the pulp and peel of ‘Cara Cara’ Navel Orange (Citrus sinensis L. Osbeck). J. Sci. Food Agric 88(2):229–236. doi: 10.1002/jsfa.3076.
- Hubbard, N.L., D.M. Pharr, and S.C. Huber. 1991. Sucrose phosphate synthase and other sucrose metabolizing enzymes in fruits of various species. Plant Physiol. 82(2):191–196. doi: 10.1111/j.1399-3054.1991.tb00080.x.
- Jacobi, K.K., E.A. MacRae, and S.E. Hetherington. 2001. Postharvest heat disinfestation treatments of Mango fruit. Sci. Hortic 89(3):171–193. doi: 10.1016/S0304-4238(00)00240-5.
- Jawadagi, R.S., D.R. Patil, D.A. Peerajade, D. Shreedhar, and R. Achari. 2013. Studies on effect of post-harvest treatments on quality and shelf life of custard apple (Annona squamosa L) cv. Balanagar. Asian J. Hort 8(2):494–497.
- Jia, Z., M. Tang, and J. Wu. 1999. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 64(4):555–559. doi: 10.1016/S0308-8146(98)00102-2.
- Khaliq, G., M.T.M. Mohamed, H.M. Ghazali, P. Ding, and A. Ali. 2016. Influence of gum Arabic coating enriched with calcium chloride on physiological, biochemical and quality responses of Mango (Mangifera indica L.) fruit stored under low temperature stress. Postharvest Biol. Technol. 111:362–369. doi: 10.1016/j.postharvbio.2015.09.029.
- Korkina, L., and I. Afanasev. 1997. Antioxidant and chelating properties of flavonoids. Adv. Pharmacol 38:151–163.
- Langenkamper, G., R. McHale, R.C. Gardner, and E. MacRae. 1998. Sucrose phosphate synthase steady-state mRNA increases in ripening kiwifruit. Plant Molecular Biol. 36(6):857–869. doi: 10.1023/A:1005964812161.
- Lee, C.Y., and A.W. Jaworski. 1988. Phenolics and browning potential of white grapes grown in New York. American J. Enol. Vitic 39:337–340.
- Liu, M.C., W.H. Song, S.H. Zhu, and J. Zhou. 2007. Effects of nitric oxide and exogenous ethylene treatments on ethylene biosynthesis in Feicheng peach. Agric. Sci. China 6(3):290–295. doi: 10.1016/S1671-2927(07)60047-9.
- Ma, X., H. Wu, L. Liu, Q. Yao, S. Wang, R. Zhan, S. Xing, and Y. Zhou. 2011. Polyphenolic compounds and antioxidant properties in Mango fruits. Sci Hortic. 129(1):102–107. doi: 10.1016/j.scienta.2011.03.015.
- Nair, M.S., A. Saxena, and C. Kaur. 2018. Effect of chitosan and alginate-based coatings enriched with pomegranate peel extract to extend the postharvest quality of guava (Psidium guajava L.). Food Chem. 240:245–252. doi: 10.1016/j.foodchem.2017.07.122.
- Nolpradubphan, A., and I. Lichanporn. 2016. Effect of nitric oxide on postharvest quality of lime fruit (Citrus aurantifolia Swingle. KKU Res. J 21(1):86–96.
- Panse, V.G., and P.V. Sukhatme. 1989. Statistical methods for agricultural workers. Indian Council of Agricultural Research, New Delhi.
- Pennycooke, J.C., S. Cox, and C. Stushnoff. 2005. Relationship of cold acclimation, total phenolic content and antioxidant capacity with chilling tolerance in petunia (Petunia × hybrida). Environ. Exp. Bot. 53(2):225–232. doi: 10.1016/j.envexpbot.2004.04.002.
- Piga, A., M. Agabbio, F. Gambella, and M.C. Nicoli. 2002. Retention of antioxidant activity in minimally processed mandarin and satsuma fruits. Food Science Technol. 35:344–347.
- Quintero, V., G. Giraldo, J. Lucas, and J. Vasco. 2013. Caracterizaciónfisicoquímica del mango común (Mangifera indica L.) durantesuproceso de maduración. Biotecnol. Sect. Agropecu. Agroind 11:8–18.
- Rahman, M.A., M.R. Asi, A. Hameed, and L.D. Bourquin. 2020. Effect of postharvest application of aloe vera gel on shelf life, activities of anti-oxidative enzymes, and quality of ‘Gola’ guava fruit. Foods (Basel, Switzerland). 9(10):E1361. doi: 10.3390/foods9101361.
- Ranganna, S. 1986. Manual of analysis of fruit and vegetable products. Tata McGraw Hill Publishing Company Ltd. New Delhi. 7–12 and 109.
- Ranganna, S. 2007. Handbook of analysis and quality control for fruit and vegetable products. 2nd. New Delhi: Tata McGraw Hill Publishing Company Ltd., 1112.
- Rasouli, M., M.K. Saba, and A. Ramezanian. 2019. Inhibitory effect of salicylic acid and Aloe vera gel edible coating on microbial load and chilling injury of Orange fruit. Sci. Hortic. 247:27–34. doi: 10.1016/j.scienta.2018.12.004.
- Ren, Y., J. He, H. Liu, G. Liu, and X. Ren. 2017. Nitric oxide alleviates deterioration and preserves antioxidant properties in ‘Tainong’ Mango fruit during ripening. Hortic. Environ. Biotechnol 58(1):27–37. doi: 10.1007/s13580-017-0001-z.
- Rojas-García, A., L. Rodríguez, M.D.L.L. Cádiz-Gurrea, A. García-Villegas, E. Fuentes, M.D.C. Villegas-Aguilar, I. Palomo, D. Arráez-Román, and A. Segura-Carretero. 2022. Determination of the bioactive effect of custard apple by-products by in vitro assays. Int. J. Mol. Sci 23(16):9238. doi: 10.3390/ijms23169238.
- Rueda, F.D.N. (2005). Guava (Psidium guajava L.) fruit phytochemicals, antioxidant properties and overall quality as influenced by postharvest treatments. M. Sc. Thesis. The Graduate School of the University of Florida, USA.
- Saba, M.K., and S. Moradi. 2017. Sodium nitroprusside (SNP) spray to maintain fruit quality and alleviate postharvest chilling injury of peach fruit. Sci. Hortic. 216:193–199. doi: 10.1016/j.scienta.2017.01.009.
- Sasidharan, S., and A. Jayadev. 2017. A comparative analysis of antioxidant properties of three varieties of Annona sp. Int. J. Applied Res 3(7):1174–1178.
- Sayyari, M., D. Valero, M. Babalar, S. Kalantari, P.J. Zapata, and M. Serrano. 2010. Pre-storage oxalic acid treatment maintained visual quality, bioactive compounds, and antioxidant potential of pomegranate after long-term storage at 2°C. J. Agric. Food Chem 58(11):6804–6808. doi: 10.1021/jf100196h.
- Shafiee, M., T.S. Taghavi, and M. Babalar. 2010. Addition of salicylic acid to nutrient solution combined with postharvest treatments (hot water, salicylic acid, and calcium dipping) improved postharvest fruit quality of strawberry. Sci. Hortic 124(1):40–45. doi: 10.1016/j.scienta.2009.12.004.
- Shahkoomahally, S., A. Ramezanian, and A. Farahnaky. 2015. Postharvest nitric oxide treatment of persimmon (Diospyros kaki L.) improves fruit quality during storage. Fruits. 70(2):63–68. doi: 10.1051/fruits/2014045.
- Shi, Q., F. Ding, X. Wang, and M. Wei. 2007. Exogenous nitric oxide protects cucumber roots against oxidative stress induced by salt stress. Plant Physiol. Biochem 45(8):542–550. doi: 10.1016/j.plaphy.2007.05.005.
- Singh, S.P. 1992. Fruit crops for wastelands. Scientific Publishers, New Pali Road, Jodhpur-342001, India, 49–64.
- Singh, B.P., S.P. Singh, and K.S. Chauhan. 1981. Certain chemical changes and rate of respiration in different cultivars of ber during ripening. Haryana Agric. Univ. J. Res 11:60–64.
- Singleton, V.L., and J.A. Rossi. 1965. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American J. Enol. Viticulture 16:144–158.
- Wu, F., D. Zhang, H. Zhang, G. Jiang, X. Su, and H. Qu. 2011. Physiological and biochemical response of harvested plum fruit to oxalic acid during ripening or shelf-life. Food Res. Int 44(5):1299–1305. doi: 10.1016/j.foodres.2010.12.027.
- Zhanga, Z., J. Xua, Y. Chenb, J. Weia, and B. Wua. 2019. Nitric oxide treatment maintains postharvest quality of table grapes by mitigation of oxidative damage. Postharvest Biol. Technol. 152:9–18. doi: 10.1016/j.postharvbio.2019.01.015.
- Zhang, Q., Q. Liu, Z. Zhang, K. Luo, and P. Chen. 2016. Effects of sodium nitroprusside on postharvest quality and physiological indexes of banana. J. Southern Agric 47(7):1198–1202.
