ABSTRACT
Apomixis is a mode of asexual reproduction through seed. Apomixis can be classified into two types – gametophytic and sporophytic apomixis. The phenomenon of apomixis in higher plants is limited; however, it seems to play a critical role in the genetic diversity of Garcinia species. Many species in Garcinia undergo apomixis and this review highlights reproductive pathways of various Garcinia species documented over the years. Most of the Garcinia species are endemic. Garcinia species are known for their edible fruits. For the conservation of species and maintenance of genetic diversity, it is necessary to understand the processes by which Garcinia species reproduce.
Introduction
Apomixis is a mode of asexual reproduction through seed. Sexual seed development is initiated by the process of double fertilization, which involves the fusion of reduced female and male gametes that lead to the development of the embryo and the endosperm () (Hojsgaard and Hörandl, Citation2019). However, apomixis is a type of asexual reproduction occurring without fertilization and maternal haploid gamete leads to the formation of the embryo and asexual seeds (Koltunow, Citation1993). The word apomixis is derived from the Greek words apo and mixis – apo means “away from” and mixis is the “act of mixing” (Hojsgaard and Pullaiah, Citation2022). Apomixis can be classified into two types – gametophytic and sporophytic apomixis. Each of the apomictic mechanisms differs in the time at which it is initiated.
Figure 1. Various types of apomictic pathway in flowering plants: normal sexual pathway, gametophytic apomixis (a) Diplospory, (b) Apospory and (c) Adventitious apomixis.
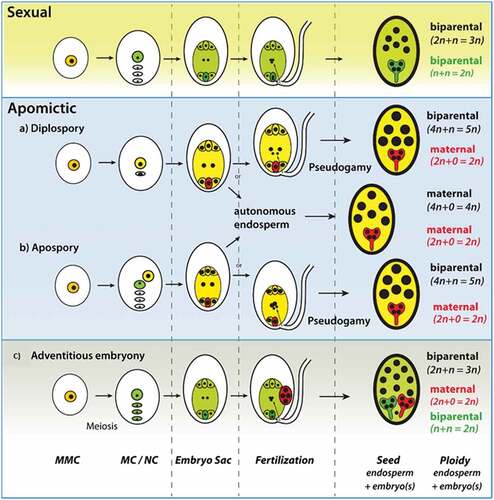
Gametophytic apomixis (Stebbins, Citation1950) is a common term for all types of apomixis where the embryo develops from the egg cell of a well-developed embryo sac, without fertilization (Savidan, Citation2001). Gametophytic apomixis is represented by either diplospory or apospory, based on the origin of embryo-sacs (ES) in the ovule (Asker and Jerling, Citation1992; Nogler, Citation1984). In Diplospory, the embryo sac originates from the megaspore mother cell either directly by mitosis and/or after interrupting meiosis (). Apospory occurs when a somatic, unreduced cell of the nucellus develops into an embryo sac ().
Sporophytic apomixis, also known as adventitious embryony, is widespread throughout the plant kingdom and is frequent in Citrus and members of Orchidaceae (Schmidt, Citation2020). In sporophytic apomixis, one or more adventitious embryos derive from sporophytic cells, surrounding the sexually formed gametophyte (). They may be derived from nucellar cells or from the cells of the integument. Both the sexually derived embryo and its asexual siblings compete for their nutritional resources. Unlike sexual reproduction, which usually leads to the formation of a single embryo per seed, adventitious embryony is frequently marked by polyembryony (Hojsgaard and Hörandl, Citation2019).
The basic mechanism leading to seed clonality relies on bypassing the two phases of variability generation, meiotic recombination, and fertilization, during seed formation, eventually resulting in seeds with copied maternal genotype. Meiosis is avoided (or eliminated/modified) via the formation of apomeiotic embryo sacs (ES), while fertilization is bypassed via parthenogenetic development of the egg cell (Kaushal et al., Citation2019).
Apomixis in Garcinia Species
The phenomenon of apomixis in higher plants is limited; however, it seems to play a critical role in the genetic diversity of Garcinia (Sharma et al., Citation2013). Most Garcinia species are apomictic (Corner, Citation1988) and fruit and seed development in these species is through agamospermy i.e., production of seed without the fusion of gametes (Murthy et al., Citation2018; Thomas, Citation1997). Various teams around the world have worked on the apomictic behavior of various species in Garcinia.
Obligate Apomixis in Absence of Male Members
Lan (Citation1984) made a detailed study of embryogenesis in Garcinia mangostana L. Male trees are hardly seen in their natural populations although their taxonomic evidence has been reported (Idris and Rukayah, Citation1987). In the female flower of G. mangostana, the base of the ovary was surrounded by 14 to 16 staminodes. Anthers showed degeneration at various stages of microsporogenesis, and few microspores produced are non-viable (Nuanjunkong et al., Citation2011; Sutthinon et al., Citation2018). One of the hypodermal, nucellar cells enlarged to form the archesporial cell and functioned directly as the megaspore mother cell. Two or three archesporial cells were even formed in rare cases. After completion of meiosis, only the chalazal megaspore enlarged further to form the female gametophyte, while the other three megaspores degenerated. The nucleus of the functional megaspore underwent three successive divisions to form an eight-nucleate embryo sac, which further degenerated. The degeneration of the egg apparatus was followed by antipodals and the fusion of the polar nuclei led to the formation of the endosperm. Preliminary bagging experiments confirmed that the seeds were formed without fertilization. The division in the endosperm was free nuclear and remained scattered within the enlarging embryo sac. The degeneration of the nucellar tissue was followed by the inner integument. Cells of the outer integument divided by periclinal and anticlinal divisions produced buds, which projected into the embryo sac but only a few of these attained maturity and button-shaped “proembryos” were eventually seen. The development of the adventive integumentary embryos is irregular, and sometimes within the same embryo sac several embryos may be found. The degraded ovules remained; only outer integuments developed into the seed coat. Thus, G. mangostana reproduces by sporophytic apomixis. The details regarding the apomixis in G. mangostana has been discussed in many reviews such as Sprecher (Citation1919), Horn (Citation1940) and Lan (Citation1984).
Garcinia parviflora Benth. is dioecious and known to possess two types of plants: males and fruit-bearing hermaphrodites in forests of Malaysia and Singapore. But Ha et al. (Citation1988) studied the reproductive pattern in the Pasoh Forest Reserve of Malaysia, where the population consisted of only hermaphrodite trees; male trees were absent. The staminodes of the structurally hermaphrodite flowers contain aborted, sterile pollen. Bagging experiments carried out on two trees revealed, about 19% of the fruit sets occurred in absence of an external pollen source. Of the aborted buds, 70% contained either a mature embryo sac or an eight-celled proembryo. This indicates that the embryos are derived apomictically in the absence of pollen grains. In the ovule, archesporial cell develops that directly functions as a megaspore mother cell to form a linear tetrad. The functional megaspore produces eight-nucleate embryo sacs. Before the division of the egg cell, the two polar nuclei fuse to form the secondary nucleus and subsequent endosperm formation occurs. The egg cell in G. parvifolia does not degenerate as observed by Lan (Citation1984) in G. mangostana. Instead, it divides first horizontally and then vertically to give rise to a four-celled embryo, which undergoes further divisions to form a spherical multicellular, rather than a well-differentiated embryo. Thus, in G. parvifolia, the embryo is non-zygotic and develops without fertilization and in the absence of pollen stimulus. As the embryo sac develops, the nucellus degenerated and the layers of the inner integument become meristematic and form few-celled proembryos. But unlike G. mangostana, these proembryos do not completely develop to form adventive embryos. This conforms to gametophytic agamospermy but whether parthenogenesis was haploid or diploid in nature remains to be established (Ha et al., Citation1988).
Garcinia scortechinii King. was assumed to be facultatively apomictic, but in a population of entirely of pistillate individuals with no male trees recorded within a 25-ha area, it was documented that most flowering individuals also fruited (27 of 40, or 68%). Structure comparable to the mature embryo of G. parvifolia was found in some ovules of G. scortechinii. In the mature seed, the embryo may differentiate further to give rise to one or more embryos. Dissected fruits from these individuals exhibited apparently normal seed development and developed into seedlings. The seed germination of both species conforms to the Garcinia Type (Vogel, Citation1980) where the shoot emerges at one pole of the seed, the first root usually at the opposite pole. This root is then followed by a second one formed immediately below the shoot (Ha et al., Citation1988). Hence, G. scortechinii undergoes obligate apomixis due to the absence of males (Thomas, Citation1997).
Facultative Apomixis when both Male and Female Plants Exist
Richards (Citation1990a) made a systematic study of agamospermy in Garcinia hombroniana Pierre and compared it with G. mangostana. G. hombroniana is not obligatory apomictic like G. mangostana. The study was carried out at two locations where male plants were available within the population. Reproduction was studied in both artificially pollinated and non-pollinated plants. The specialty of G. hombroniana was non-pollinated flowers that showed the presence of proembryos in the ovules even before anthesis. The proportion of ovules containing proembryos reaches a maximum of 62.3% at 4 days after anthesis. Most ovules with proembryos survived, and those lacking proembryos were aborted. In the absence of males, the mean number of seeds for mature fruits was 2.58. The development of sexual embryos in artificially pollinated flowers was studied in detail. An archesporium was detected in floral buds, and meiosis occurred leading to the formation of a linear tetrad. In several cases, it was presumed that the meiosis is regular and reductional. Mature embryo-sacs were formed about 48 h before anthesis. From this stage to the anthesis, embryo-sacs with synergids, egg-cells, and primary endosperm nuclei (PEN) of a “normal” appearance are regularly encountered. The absence of antipodals – which is a common feature of Garcinia was also observed here. The development of sexual embryos and endosperms was studied at 24 h, 72 h, and 96 h after controlled crosses. Sexual embryos are invariably located at the point of entry of the micropyle into the embryo sac. The PEN or young endosperm is invariably located close to the embryo, to the chalazal side. In pollinated flowers, the development of proembryos was also observed before anthesis, but their number was reduced as sexual embryos advanced. Thus, G. hombroniana is the best example of facultative apomixis where sexual embryos are formed when flowers are pollinated, and plants develop proembryos in the absence of pollination. Sexuality was probably limited by a combination of poor pollination, synchronicity of male and female flowering, and competition with asexual embryos (Richards, Citation1990b) (Richards, Citation1990a) noted that another close relative of G. mangostana, i.e., Garcinia mangostana var. malaccensis Hook. f. was a facultative agamospermous diploid species.
Recently, facultative apomixis is reported in many dioecious species of Garcinia – G. indica, Garcinia imbertii Bourd. and Garcinia prainiana King. Both these species produce male and female plants on separate plants and the plants undergo both reproductive systems sexual and facultative. G. imberti is a critically endangered tree species found in the Agasthyamalai Hills of the Western Ghats of Kerala (Kandhasamy et al., Citation2015). The fruit set was recorded in various pollination regimens. It was observed that in natural pollination fruit set was 36.67%, in artificial pollination it improved to 53.33%. The fruit set (30.00%) was also observed in bagged flowers without pollination. They suggested that a lack of pollinators transferring pollen from female flowers might be a reason for fewer fruit set in naturally pollinated plants (Kandhasamy et al., Citation2015).
A recent book on Apomixis in Angiosperms enlists eight species of Garcinia and their respective modes of apomixis in a tabular format - Garcinia atroviridis Griff. ex T. Anderson, Garcinia cowa Roxb. ex Choisy, G. hombroniana, Garcinia livingstonei T. Anderson, G. mangostana, G. parvifolia, G. scortechinii, Garcinia treubii Pierri (Hojsgaard and Pullaiah, Citation2022). Although the formation and development of the embryo sac is described in G. livingstonei by Puri (Citation1939), it does not throw any light on the mode of apomixis occurring in this species. Apomixis in other Garcinia species is discussed below.
Facultative Apomixis in Gynodioecious Populations
Pangsuban et al. (Citation2007) reported that G. atroviridis had a gynodioecious system with females (trees producing pistillate flowers) and hermaphrodites (trees producing perfect flowers) co-occurring. In a comprehensive study of their reproductive biology, different pollination treatments namely – no pollination, manual pollination, and natural pollination were carried out. The fruit set was observed irrespective of the treatment. This indicated the possibility of apomixis, which was later confirmed with RAPD analysis. On average, 58% of the offspring had a genetic constitution identical to that of the maternal parents (ranging from 36% to 87%) confirming apomixis. However, the remainder showed polymorphism, demonstrating the occurrence of sexual reproduction. Hence, G. atroviridis shows facultative mode of reproduction. This behavior is advantageous for female trees, as it ensures reproduction and allows them to escape extinction even in the absence of hermaphrodite trees (Pangsuban et al., Citation2009).
Garcinia celebica Linn. is another example that exhibits gynodioecious flowers as in G. atroviridis. Field observations have revealed that G. celebica bears perfect flowers, with numerous stamens surrounding the pistillode. Female flowers are borne on separate trees (Sutthinon et al., Citation2018). According to the histological observations, a microspore mother cell undergoes the general development in fertile anthers with pollen production at the anthesis. Female flowers on separate trees have been observed to undergo both sexual and asexual reproductions, as they may produce seeds without fertilization (apomixis), i.e., they show facultative agamospermy (Sutthinon et al., Citation2018, Citation2019).
Sexually Reproducing Garcinia Species
Garcinia brasiliensis Mart. is a neotropical species unlike other pantropical species, which are female biased, the population in Rio de Janeiro, Brazil, is cryptic dioecious with a sex ratio of 4.5:1:male:female. It comprises male individuals with staminate flowers and female individuals with morphologically perfect flowers that have no pollen grains. Leal et al. (Citation2013) made a detailed study of reproduction in the same. The reproductive system was studied through hand-pollination experiments. The different experiments were conducted in female plants: cross-pollination, autonomous apomixis, and pollination under natural conditions – control. The production of mature fruits was quantified. In the four female individuals tested for apomixis, ovaries in most of the bagged flowers underwent early abscission. Non-bagged flowers had an early ovary development and fusion of a male gamete with the polar nuclei, giving rise to the endosperm. Some ovules contained a globular embryo. Within naturally pollinated pistillate flowers (control), 80.86% initiated fruits, of which only 12.17% matured. Manually pollinated flowers had the greatest success for both initiated fruits (85%) and matured fruits (30%). Thus, the buds and flowers showed no signs of alteration or suppression of the sexual events during development, and all indications of sexual reproduction were recorded, including differentiation of the megaspore dyad and tetrad from the nucellus, megagametogenesis, and maturation of the embryo sac (Leal et al., Citation2012, Citation2013).
Garcinia prainiana King. is also a dioecious plant where male plants produce pollens with high viability and germination ability. Meanwhile, the female plant showed a well-developed ovule. The ovule was bitegmic and anatropous. The micropyle was formed from both integuments and produced a path with a zig-zag pattern. The nucellus is tenuinucellate where only a single layer of nucellus cells was present and degenerates before the embryo sac reached maturity. At a later stage, a globular embryo was observed near the micropylar end. The location of the embryo shows that the embryo could have formed from sexual reproduction. Furthermore, there was no embryo structure on the integument (adventive embryo) of the ovule observed (Rohani et al., Citation2021). A cross-section of a mature seed shows the presence of a procambium ring. Bagging female flowers to assess the possibility of apomixis was unsuccessful due to flower clusters and environmental conditions. The RAPD analysis produced a total of 53 bands, of which 45 (84.9%) were polymorphic. Both well-functioning male and female reproductive organs along with polymorphic progeny bands indicate a high occurrence of sexual reproduction (Rohani et al., Citation2021).
Garcinia Species in which Pollen Stimulus is Needed Even for Apomictic Fruit Development
About 30 species of Garcinia are distributed in India, including Garcinia cambogia Desr., Garcinia xanthochymus Hook. f., G. cowa, Garcinia indica (Thouars) Choisy. Garcinia gummi-gutta (L.) N. Robson is a dioecious plant found in southern India producing male and female flowers on separate plants. It does not reproduce via apomixis as none of the emasculated and bagged flowers set fruit. Further studies were undertaken to rule out the possibility of facultative apomixis. Artificially pollinated flowers resulted in a 96% fruit set as compared to 20% in naturally pollinated flowers. According to Jansen (Citation1992), G. gummi-gutta germinated through seed, which is represented by hypocotyls enclosing a linear embryo devoid of cotyledons (Aswathi et al., Citation2018).
Apomixis in G. indica
G. indica is also unique where male plants develop viable pollen grains, which reach the stigmatic surface and successfully germinate. The embryo sac appears to develop before anthesis and even the embryo development does occur, the embryo might be derived by gametophytic apomixis, where the megaspore mother cell fails to undergo meiosis; instead, it undergoes mitotic division to produce an embryo sac. A well-developed globular embryo was observed in flowers collected at 8 h after the anthesis. Further development of an embryo was not observed. From Day 2, i.e., 48 h after the anthesis, cellularization progressed from the chalazal end and filled most of the embryo sac. The embryo is inconspicuous and is situated in an extreme upper portion of the seed in a protuberant radicle (Baskaware and Deodhar, Citation2022). As the ovule is transformed into a seed, the embryo appears as a long hypocotyl-radicle axis with vestigial cotyledons, which traverse from the micropylar end to the chalazal end. It was referred to as “embryonic axis” by Teo in G. mangostana (Teo, Citation1992). G. indica also shows a typical Garcinia type seed germination, plumule, and radicle emerging from both radicular and germicular ends of the embryonic axis. The nature of seeds (Malik et al., Citation2005) also show polyembryony. Regeneration of multiple seedlings from whole seed and seed halves or segments further indicated the apomictic (agamospermous) effect.
Earlier breeding experiments conducted by Dike et al. (Citation2020), also suggests the possibility of apomixis in G. indica. To confirm or rule out the possibility of apomixis or sexual reproduction, male and female plants with polymorphic banding pattern were selected. Although G. indica shows only 4–10% polymorphism (Thatte and Deodhar, Citation2012), out of 60 plants screened with 11 ISSR markers – only two females F159 and F244 and a male M214 showed different banding patterns for female and male plants. In this experiment, the flowers of F159 and F244 female plants were divided into three sets.
The first set of flowers was bagged with butter paper bags. In the above study, to check the possibility of sexual reproduction or apomixis in G. indica, paper bags without pollination to study the possibility of apomixis. The second set of flowers was artificially pollinated with pollen grains from the known male plant M214. Furthermore, to avoid natural pollination, these flowers were also protected with butter paper bags. In the third set, the flowers were left open to pollinate naturally. In the case of the first set, i.e., flowers bagged without any pollination, none of the 100 flowers could set fruit and dropped down after 5–6 days.
This observation suggests that the plant is not obligate apomictic. Fertilization and further fruit sets were observed in artificially and naturally pollinated flowers. From a total of 100 artificially pollinated flowers of plant F159, 35% could set fruit, while in naturally pollinated flowers of the same plant showed 55% fruit set. In the case of plant F244, 25% fruit set was observed for artificially pollinated flowers, whereas for naturally pollinated flowers the fruit set percentage was 40%.
Fruits formed by artificial and natural pollination were collected and their seeds were germinated. The plantlets obtained after germination were used for further molecular analysis. If the F1 progeny is derived by apomixis, it should show exactly similar banding pattern to that of the female parent, whereas if it is derived via sexual reproduction, its banding pattern will be different from that of the female parent. Female plant F159, when artificially pollinated with M214, out of seven randomly selected seed raised plants of F1 progeny--all plants H1 to H7 showed molecular banding patterns similar to the maternal parent, suggesting that 100% progeny was derived from apomixis. When F159 was allowed to pollinate naturally one of the seven plants showed polymorphic patterns indicating the presence of sexual reproduction in only 14.28% of the plants. Thus, results indicate the possibility of facultative apomixis but the results need to be confirmed in more plants. Thus, in G. indica in the plants that are surrounded by a number of male plants also the percentage of the sexually derived plant is as less than 15%. The percentage will still be reduced at the location where the male plants are purposefully removed as they do not produce any fruit.
The utility of DNA-based molecular markers has been demonstrated in genetic, cytogenetic, and molecular analysis of apomixis. One such class of molecular markers, the inter-simple sequence repeats (ISSR), are being utilized extensively for population genetics, species discrimination, cultivar identification, and genetic diversity. These markers can work on small quantities of DNA without requiring any prior genomic DNA sequence information. To improve reproducibility, ISSR are converted to sequence characterized amplified region (SCAR) markers. Utility of SCAR markers in apomixis research has also been earlier demonstrated in Buffelgrass, Cenchrus ciliaris L., which reproduces predominantly through gametophytic apomixis wherein unreduced embryo sac is formed from a maternal nucellar cell (apospory) (Dwivedi et al., Citation2007, Citation2015; Yadav et al., Citation2012). Hence, ISSR markers were selected for differentiating apomixis in G. indica.
There are species of Garcinia where the variation of floral types was reported. Leal et al. (Citation2013) while studying reproductive biology of G. brasilienses, found two more types of plants along with normal pistillate and staminate plants. G9 individual had more staminate flowers than pistillate flowers. The pistillate flowers were perfectly producing viable pollen grains. The individual also set fruits. They described this plant as an andromonoecious plant. Another individual G11 - was predominantly female, whereas it produced some staminate flowers bearing fertile pollen grains. At the center, there was a globular nectary. The female flowers were bearing staminodes as usual. Leal et al. (Citation2013) further concluded that andromonoecious individuals found in dioecious populations represent another viable reproductive alternative.
Similar observations were made by Manikandan in the thesis on reproductive biology of two Indian, endemic endangered species of Garcinia viz G. imberti and Garcinia travancorica Bedd. (Manikandan, Citation2017). They reported that both species are dioecious producing male and female flowers on two different trees. But at the peak of the flowering period, female plants also bear bisexual flowers. They produced viable pollen grains. In G. travancorica, female flowers reproduced apomictically producing 14% apomictic progeny, whereas in bisexual flowers the percentage of apomictic fruit set was only 2%. In a breeding experiment when female and bisexual flowers were pollinated by genitogamy, female flowers did not set any fruits, but in bisexual plants the fruit set was 31% this means that bisexual flowers were self-compatible. This indicates that bisexual flowers favor sexual reproduction and female flowers favor apomixis. Bisexual flowers observed in andromonoecious and hermaphrodite trees of G. indica occasionally set fruits (Baskaware and Deodhar, Citation2022). Joseph and Murthy (Citation2015) noted that gynomonoecious plants in a few locations in Karnataka such plants were not detected in Dr. Balasaheb Sawant Konkan Krishi Vidyapeeth, Dapoli campus. Furthermore, it is essential to study whether these bisexual flowers are self-compatible and undertake a breeding program followed by molecular fingerprinting to confirm whether these flowers produce sexual or apomictic progeny. Sexual progeny in species is essential as a conservation effort for this endangered endemic species. World-wide thousands of plant species are being included under endangered species and facing a risk of extinction. The study of the reproductive biology of these threatened, endangered plants may give clues that will help in the conservation of species.
Conclusion
It can be said that all pathways of reproduction, namely, sexual, obligate, or facultative agamospermy can be seen in the genus Garcinia. In populations where males are absent: G. mangostana and G. scortechinii or functionally absent as in the case of G. parvifolia, reproduction occurs in obligate apomixis. G. parvifolia shows gametophytic apomixis, whereas G. mangostana reproduces via sporophytic apomixis. G. hombroniana, G. atroviridis, G. celebica, and G. indica reproduce by facultative apomixis as pollen stimulus from male counterparts is required. G. imberti and G. gummi-gutta indicate reproduction via apomixis, although detailed studies are needed in their case when lack of natural pollinators is observed. G. brasiliensis and G. prainiana reproduce sexually. It has been established that G. indica has both apomictic and sexual progenies, i.e., is facultatively apomictic.
Acknowledgement
We are thankful to K.E. T’s V. G. Vaze College (Autonomous) of Arts, Science, and Commerce, Mulund, Mumbai for providing the infrastructure.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Asker, S.E., and L. Jerling. 1992. Apomixis in plants. CRC Press, Boca Raton.
- Aswathi, P., K. Aswani, and M. Sabu. 2018. reproductive biology of Malabar tamarind (Garcinia gummi-gutta (L.) Rob.: An endemic, medicinal and spice plant from Western Ghats. The Int. J. Plant Reprod. Biol. 10(1):65–68. doi: 10.14787/ijprb.2018.
- Baskaware, S.V., and M.A. Deodhar. 2022. Study of various floral types on different plants of garcinia indica (thouars) Choisy and correlation of its functionality in sexual reproduction. Int. J. Fruit Sci. 22(1):383–401. doi: 10.1080/15538362.2022.2046527.
- Corner, E.J.H. 1988. Wayside Trees of Malaya. Vol. 1. Malaysian Nature Society, Kuala Lumpur, Malaysia.
- Dike, M.S., S.K. Malik, S.V. Sawardekar, and M.A. Deodhar. 2020. Study of the mode of reproduction and fruit development ingarcinia Indica. Int. J. Fruit Sci. 20(1):20–38. doi: 10.1080/15538362.2018.1563022.
- Dwivedi, K.K., B.V. Bhat, M.G. Gupta, S.R. Bhat, and V. Bhat. 2007. Identification of a SCAR marker linked to apomixis in buffel grass (Cenchrus ciliaris L.). Plant Sci. 172(4):788–795. doi: 10.1016/j.plantsci.2006.12.006.
- Dwivedi, K.K., A. Radhakrishna, S. Kumar, M.K. Srivastava, M.G. Gupta, and P. Kaushal. 2015. Development of an ISSR-derived SCAR marker linked to apospory in buffel grass (Cenchrus ciliaris L.). Indian J. Genet75(2):271–273. https://www.researchgate.net/publication/279232037_Development_of_an_ISSR-derived_SCAR_marker_linked_to_apospory_in_buffel_grass_Cenchrus_ciliaris_L.
- Ha, C.O., V.E. Sands, E. Soepadmo, and K. Jong. 1988. Reproductive patterns of selected understorey trees in the Malaysian rain forest: The sexual species. Bot. J. Linn. Soc. 97(3):295–316. doi: 10.1111/j.1095-8339.1988.tb01585.x.
- Hojsgaard, D., and E. Hörandl. 2019. The rise of apomixis in natural plant populations. Front Plant. Sci. 10:358. doi: 10.3389/fpls.2019.00358.
- Hojsgaard, D., and T. Pullaiah. 2022. Apomixis in angiosperms: mechanisms. Occurrences, and Biotechnology. 1st. CRC Press. 10.1201/9781003088561.
- Horn, C.L. 1940. Existence of only one variety of cultivated mangosteen explained by asexually formed “seed”. Science 92(2385):237–238. doi: 10.1126/science.92.2385.237.
- Idris, S., and A. Rukayah. 1987. Description of the male mangosteen (Garcinia mangostana L.) discovered in Peninsular Malaysia. MARDI Res .Bull. 15(1):63–66.
- Jansen, P.C.M. 1992. Garcinia L. Edible fruits and nuts, In: p. 176. In: V. EWM and R. Coronel (eds.). Plant Resources of Southeast Asia. Prosea Foundation, Bogor, Indonesia.
- Joseph, K.S., and H.N. Murthy. 2015. Sexual system of Garcinia indica Choisy: Geographic variation in trioecy and sexual dimorphism in floral traits. Plant syst. Evol. 301(3):1065–1071. doi: 10.1007/s00606-014-1120-y.
- Kandhasamy, D., K. Puttaramaiah, S. Ramnath, and S. Venkataramegowda. 2015. Floral biology and breeding system garcinia imberti bourd.-a critically endangered tree species of Western Ghats, Kerala, India. Int. J. Curr. Res. 7(4):14855–14863.
- Kaushal, P., K.K. Dwivedi, A. Radhakrishna, M.K. Srivastava, V. Kumar, A.K. Roy, and D.R. Malaviya. 2019. Partitioning apomixis components to understand and utilize gametophytic apomixis. Front Plant. Sci. 10:256. doi: 10.3389/fpls.2019.00256.
- Koltunow, A.M. 1993. Apomixis: Embryo sacs and embryos formed without meiosis or fertilization in ovules. Plant Cell 5(10):1425–1437. doi: https://doi.org/10.2307/3869793.
- Lan, L.A. 1984. The embryology of Garcinia mangostana L.(Clusiaceae). Gard. Bull. Singap. 37:97–103.
- Leal, D.O., C.R. Benevides, R.C.P. Silva, L.D.R. Santiago-Fernandes, B. Sá-Haiad, and H.A. Lima. 2013. Garcinia brasiliensis: Insights into reproductive phenology and sexual system in a Neotropical environment. Plant syst. Evol. 299(8):1577–1585. doi: 10.1007/s00606-013-0833-7.
- Leal, D.O., C. Malafaia, R. Cesar, R.R. Pimentel, L.D.R. Santiago-Fernandes, H.A. Lima, and B. Sá-Haiad. 2012. Floral structure ofgarcinia brasiliensis in relation to flower biology and evolution. Int. J. Plant Sci. 173(2):172–183. doi: 10.1086/663163.
- Malik, S.K., R. Chaudhury, and Z. Abraham. 2005. Seed morphology and germination characteristics in threegarcinia species. Seed Sci. Technol. 33(3):595–604. doi: 10.15258/sst.2005.33.3.07.
- Manikandan, G. (2017) Reproductive biology of Garcinia imberti bourd. And G. travancorica bedd.: endemic and endangered tree species from Agasthyamalai biosphere reserve. PhD Thesis, Gandhigram Rural Institute-Deemed University
- Murthy, H.N., V.S. Dandin, D. Dalawai, S.Y. Park, and K.Y. Paek 2018. Breeding of Garcinia spp, In: J. Al-Khayri, S. Jain, and D. Johnson eds.. Advances in Plant Breeding Strategies: Fruits. Springer, Cham. doi: 10.1007/978-3-319-91944-7_19
- Nogler, G. 1984. Gometophytic apomixis, In: pp. 475–518. In: M. Johri (ed.). 8. Springer-Verlog, Berlin.
- Nuanjunkong, N., C. Purintavaragul, and U. Meesawat (2011) Abortive pollen development in relation to early tapetal degradation in male sterile mangosteen. In International Symposium on Tropical and Subtropical Fruits, 1024, 217–221. 10.17660/ActaHortic.2014.1024.27
- Pangsuban, S., N. Bamroongrugsa, K. Kanchanapoom, and C. Nualsri. 2007. An evaluation of the sexual system of Garcinia atroviridis L.(Clusiaceae), based on reproductive features. Songklanakarin J. Sci. Technol. 29(6):1457–1468.
- Pangsuban, S., N. Bamroongrugsa, K. Kanchanapoom, and C. Nualsri. 2009. Facultative apomixis in Garcinia atroviridis (Clusiaceae) and effects of different pollination regimes on reproductive success. Trop. Life Sci. Res. 20(2):89–108.
- Puri, V. 1939. Studies in the order Parietales. I. A contribution to the morphology of Tamarix chinensis Lour. Beih. bot. Zentralbl. 9(2):74–86. doi: 10.1007/BF03050539.
- Richards, A.J. 1990a. Studies in Garcinia, dioecious tropical forest trees: The origin of the mangosteen (G. mangostana L.). Bot. J. Linn. Soc. 103(4):301–308. doi: 10.1111/j.1095-8339.1990.tb00191.x.
- Richards, A.J. 1990b. Studies in Garcinia, dioecious tropical forest trees: The phenology, pollination biology and fertilization of G. hombroniana Pierre. Botany Journal of Linnean Society 103:251–261.
- Rohani, E.R., A.F. Norfadzilah, M.M. Clyde, and N.M. Noor. 2021. Insights into the mode of reproduction of Garcinia prainiana. FRUITS 76(1):22–29. doi: 10.17660/th2021/76.1.3.
- Savidan, Y. 2001. Gametophytic apomixis, pp. 419–433. In: S.S. Bhojwani, W.Y. Soh (eds.). Current trends in the embryology of angiosperms. Springer, Dordrecht.
- Schmidt, A. 2020. Controlling apomixis: Shared features and distinct characteristics of gene regulation. Genes 11(3):329. doi: 10.3390/genes11030329.
- Sharma, P.H.B., P.J. Handique, and H.S. Devi. 2013. A Historical and Taxonomic Overview of Garcinia L. and its reproductive ecology. Folia malaysiana 14(1):63–76.
- Sprecher, M.A. 1919. Étude sur la semence et la germination du Garcinia mangostana L. Rev. Gén. Bot. 31:513–531.
- Stebbins, G.L. 1950. Megasporogenesi e sviluppo del gemetofito femminile, Ann. di Bot. (Rome), pp. 481–504. In: Variation and Evolution in PlantsVol. 26, Columbia University Press, New York. doi:10.7312/steb94536.
- Sutthinon, P., L. Samuels, and U. Meesawat. 2018. Male functionality in Garcinia celebica L., a candidate ancestor species of mangosteen (G. mangostana L.). Botany 96(10):685–693. doi: 10.1139/cjb-2018-0079.
- Sutthinon, P., L. Samuels, and U. Meesawat. 2019. Pollen development in male sterile mangosteen (Garcinia mangostana L.) and male fertile seashore mangosteen (Garcinia celebica L.). Protoplasma 256(6):1545–1556. doi: 10.1007/s00709-019-01397-9.
- Teo, C.K.H. 1992. In vitro culture of the mangosteen seed. Acta Hortic. 292(292):81–85. doi: 10.17660/ActaHortic.1992.292.10.
- Thatte, K.S., and M.A. Deodhar. 2012. Study of flowering behavior and sex determination in Garcinia indica (Thomas-Du Pettite) Choisy by means of molecular markers. Biotechnology 11(4):232–237. doi: 10.3923/biotech.2012.232.237.
- Thomas, S.C. 1997. Geographic parthenogenesis in a tropical forest tree. Am. J. Bot. 84(7):1012–1015. doi: 10.2307/2446292.
- Vogel, E.F.D.E. 1980. Seedlings of Dicotyledons. Prudoc, Wageningen.
- Yadav, C., K.S. Anuj, M.G. Gupta, and V. Bhat. 2012. Genetic linkage maps of the chromosomal regions associated with apomictic and sexual modes of reproduction in Cenchrus ciliaris. Mol. Breed. 30(1):239–250. doi: 10.1007/s11032-011-9614-6.
