ABSTRACT
In Ethiopia, particularly in the East wollega zone, several underutilized wild edible fruits, such as Gardenia erubescens, Ficus mucuso, Dovyalis abyssinica, and Ziziphus spinachristi, are consumed only by local communities. However, information on nutritional composition, antinutrients, and antioxidant properties of edible parts of the fruits are limited. Given this, the study aimed to determine the nutritional composition, antinutrients, and antioxidant properties of the four selected wild edible fruits collected from the East Wollega zone following official standard analytical producers. The result on dry matter basis of the four wild edible fruits had moisture (9.83–13.10%), crude protein (3.01–5.31%), crude fat (1.40–3.31%), crude fiber (0.71–2.11%), total ash (4.84–9.23%) and utilizable carbohydrate (70.41–78.27%) and gross energy (316.05–342.63 kcal/100 g). The results also depicted high in utilizable carbohydrate and gross energy contents recorded in the Dovyalis Abyssinica. In contrast, high protein and fat contents were observed in the Ziziphus spina-chris and Ficus mucusa. The findings indicated that Ziziphus spina christi fruit was high in iron, zinc, and magnesium, while Ficus mucusa was high in calcium and phosphorus. Gardenia erubescens were low in oxalate and phytate contents. The molar ratios of the wild fruits in this study were below the standard and showed the high mineral bioavailability in all wild edible fruits. The Gardenia erubescens fruit was high total phenolic (230.76 mg/GAE/g) and flavonoid (112.85 mg CE/g) contents. The findings showed that among wild edible fruits considered in this study, Gardenia erubescens and Ziziphus spina-chris may have less anti nutrient activity that may favor mineral bioavailability.
Introduction
Regarding food security, health, and revenue sources, the underused wild edible fruit is a crucial component of human nutrition and cannot be disregarded (Bvenura and Sivakumar, Citation2017). Essential components such as dietary fiber, protein, sugar, phytochemicals, and minerals are present in wild edible fruits (Kubola et al., Citation2011). Ethiopia, like the majority of African nations, enjoys abundant access to both domesticated and wild fruits and spices throughout the year. However, it is depressing that vitamin inadequacies are widespread and cause for concern, particularly among the nation’s children and pregnant women.
According to Alissa et al (Alissa and Ferns, Citation2017), due to their high fiber and antioxidant content, wild fruits can treat various diseases, including diabetes, cardiovascular issues, inflammations, and digestive and urogenital disorders. The WHO recommends that each individual consume more than 400 g of fruit per day to stave against non-communicable illnesses associated with diet (WHO World Health Organization, Citation2003). Lack of accessibility, cost, ignorance, and neglect are the reasons for poor consumption (Miller et al., Citation2016).
Regular eating of starchy crops (cereals and roots), particularly in rural areas are common, which leads to protein-energy malnutrition and micronutrient deficiencies (Afari-Sefa et al., Citation2012). Diversifying and consuming wild edible plants is vital to overcome the problem. Even in Ethiopia, hundreds of underutilized wild edible fruits gathered from the wild contribute essential roles in the community’s nutrition, mainly to the rural population. Due to a lack of understanding about the nutritional value and health-promoting bioactive chemicals of wild fruits, ignorance and neglect may be jointly to blame for the poor intake of these fruits in some regions (Omari et al., Citation2017). The level of antinutrients in wild edible parts is the main factor in fully exploiting available resources (Keyata et al., Citation2020).
In the western part of Ethiopia, especially Eastern Wollega zone rich in endemic wild edible fruits. Among these fruit of Komsho (Dovyalis abyssinica), Harbu (Ficus mucusoWelw ex. Fialho), Qurqura (Ziziphus Spina-Christi (L) Desf.), and Gardenia erubescens are common wild edible fruits. However, there were limited reports on the nutritional composition, antinutrient, and antioxidant properties of those mentioned above wild edible fruit in the study area for further utilization and full exploitation. Therefore, the study aimed to evaluate the proximate composition, mineral contents, antinutrients, and antioxidant properties of selected underutilized wild edible fruit (Dovyalis abyssinica, Ficus mucuso, Ziziphus spina-christi, and Gardenia erubescens.
Material and Methods
Descriptions of the Sampling Site
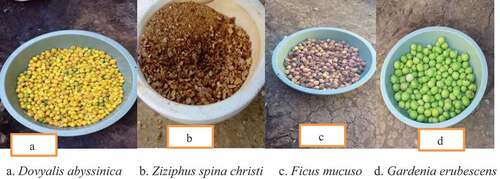
The four underutilized wild edible fruits, namely: Gardenia erubescens, Ficus mucuso, Dovyalis abyssinica, and Ziziphus spina-christi were collected from the East Wollega zone (Gute, Arjo, and Sassiga) located in Oromia regional state at a distance of 327 km west of Addis Ababa during the main fruits harvesting season (June-August) in 2021.
Sample Collection and Preparation
A 5 kg of wild edible fruits such as Gardenia erubescens, Ficus mucuso, Dovyalis abyssinica, and Ziziphus spina-christis were collected from Gute, Arjo, and Sassiga districts, Eastern Wollega zone, Oromia, Ethiopia. Representative working samples were chosen based on the category of edible wild fruits from the bulked and homogenized samples (). Each underused wild edible fruit was tagged, placed in polyethylene bags, maintained in an ice box, and shipped to the Wollega University, Ethiopia’s Food Engineering Laboratory. The selected wild edible fruit was cleaned using distilled water to remove dirt particles; then, it was cut into uniform slices using a stainless steel knife before being sorted. The sliced, under-utilized wild edible fruits were first dried in the sun and then dried again in an oven at 40°C. The dried fruits were grounded into a fine powder using a pestle and mortar and sieved (0.425 mm) mesh size. The powder was then sealed inside an airtight polyethylene plastic bag and kept in the refrigerator until used.
Determination of Nutritional Composition of Underutilized Wild Edible Fruit
Determination of Proximate Composition
The moisture content of the samples was determined by the convective oven drying method (130°C for 1 hour) by taking about 3 g of sample (dried sample powder) as described in the AOAC (Association of Official Analytical Chemists, Citation2000) method 925.10. Crude protein content was determined by the micro-Kjeldahl method by taking about 1.0 g of the sample as described in the AOAC (Association of Official Analytical Chemists, Citation2000) method, 920.87. The crude fat content was determined by taking about 1.5 g of the sample by the Soxhlet extraction method using petroleum ether as a solvent (Association of Official Analytical Chemists, Citation2000), method 920.39. The crude fiber content was determined following the AOAC (Association of Official Analytical Chemists, Citation2000). Method 962.09 after sequential digestion with 1.25% H2SO4 and 28% KOH, screening through 75 microns, drying, and ignition in a muffle furnace (Sx2-4-10, Zhejiang, China) to subtract ash from the crude fiber. The total ash content was determined gravimetrically after the carbonization of about 2.0 g of sample on a blue flame of a Bunsen burner, followed by ignition of the sample at 550°C until ashing was completed (Association of Official Analytical Chemists, Citation2000), method 923.03.The total carbohydrate content (TCC) was determined by the difference (Citation0000), and gross energy content was calculated by Atwater’s conversion factors (FAO, Citation2002).
Determination of Mineral Content
The mineral contents were determined according to the standard method of AOAC (Association of Official Analytical Chemists, Citation2000). The calcium, iron, and zinc were determined using atomic absorption spectrophotometer (AAS) (Agilent FS240 AA, USA). In contrast, sodium and potassium contents were determined using a flame photometer (Jenway, PF 7). Phosphorus was determined by the colorimetric method using ammonium molybdate (AOAC, Citation1984).
Determination of Mineral Ratios
The mole of minerals was determined by dividing the mass of minerals with its atomic weight (K = 39 g/mol, Na = 23 g/mol, P = 31 g/mol, Fe = 56 g/mol; Zn = 65 g/mol; Ca = 40 g/mol) and then the mineral ratio was calculated by dividing the first-mole mineral to the second-mole mineral (Jacob et al., Citation2015).
Determination of Anti-Nutrient Contents
UV-Visible spectrophotometer (Model: JASCO V-630, Shimadzu Corporation, Tokyo, Japan), the methods outlined by Vaintraub and Lapteva (Vaintraub and Lapteva, Citation1988) and Maxson and Rooney (Maxson and Rooney, Citation1972), respectively, were used to determine the amounts of phytate and tannin. In contrast, total oxalate was investigated by titration techniques described by Ukpabi and Ejidoh (Ukpabi and Ejidoh, Citation1989).
Determination of Molar Ratio of Anti-Nutrients to Minerals
The molar ratio of phytate to minerals (Ca, Zn and Fe) was calculated by dividing the mole of phytate (the molar mass of phytate = 660 g/mol) by the mole of minerals (the molar mass of Ca = 40 g/mol; the molar mass of Zn = 65 g/mol; the molar mass of Fe = 56 g/mol) (Norhaizan and Nor Faizadatul Ain, Citation2009). The oxalate to calcium molar ratio was calculated by dividing the mole of oxalate (88 g/mol) by that of calcium.
Antioxidant Properties
Preparation of the Methanolic Extract
Edible fruits of studied wild fruits were extracted according to Keyata et al. (). Ground sample (100 mg) were soaked in 100 mL of methanol (99.8%) to produce about 1 mg/mL of concentration using the maceration technique, soaking in the solvents for 24 h and shaking in a mechanical shaker (Hy-2(C), Shanghai, China) at room temperature and finally filtered using Whatman No. 1 filter paper. The filtered extract was used to determine the total phenolic and flavonoids contents
Total Phenolic Content
Total phenolic contents were measured using the Folin-Ciocalteu reagent described in Dewanto et al (Dewanto et al., Citation2002). Standards or aliquots of properly diluted extracts (1 mL) were mixed with 0.5 mL of distilled water and 0.125 mL of 10-fold diluted freshly made Folin – Ciocalteu reagent. The mixtures were shaken and allowed to stand for 6 min at room temperature. After that, 1.25 mL of saturated sodium carbonate (Na2CO3) (7%, v/v) was added to the mixture, and the volume of the solution was adjusted with double distilled water to a final volume of 3 mL. The mixtures were thoroughly mixed, and the contents were incubated for 2 hrs in the dark at room temperature. The absorbance was recorded using a spectrophotometer against the methanol blank at 760 nm. Total phenolic contents were calculated from the calibration curve (y = 0.0044×+0.021, R2 = 0.9926) of gallic acid with 0, 100, 200, 300, 400, 500 and 600 mg/mL and expressed as milligrams of gallic acid equivalents (GAE) per gram of the flour on dry weight (mg GAE/g dm).
Total Flavonoids
The aluminum trichloride method determined the flavonoid content using catechin as a reference standard, as described in Dewanto et al (Dewanto et al., Citation2002). This method is based on forming a complex flavonoid aluminum with a maximum absorbance at 510 nm. Standards solution of (+)-catechin or aliquots of diluted sample extracts were mixed with 75 mL of NaNO2 solution (5%, w/v) and distilled water (1 mL) and vortex mixed. After 6 min, 0.15 mL AlCl3 (10%) was added, and the incubation of the mixture was continued for another 5 min. The reactions were terminated by adding 0.5 mL of NaOH (1 M). The contents were allowed to stand for 15 min in the dark at room temperature. The final volume was adjusted to 2.5 mL with distilled water and mixed thoroughly. The absorbance was measured spectrophotometrically at 510 nm against a blank. The total flavonoid contents were derived from the calibration curve indicated in the annex (Y = 0.028× + 0.0067, R2 = 0.9903) of (+)-catechin (0 to 100 mg/mL) and expressed in mg catechin equivalents per gram of flour on dry matter basis (mg CE/g dm).
Statically Analysis
The obtained results were analyzed using SPSS version 25.0 software. Duncan’s multiple range test results were used to separate mean and standard error (SE). The difference was regarded as statistically significant if the p-value was 0.05 or below.
Results and Discussions
Proximate Composition
The moisture contents of the four under-utilized wild edible fruits ranged from 9.83 to 13.10% (). The highest moisture contents were obtained in the Ziziphus spina-christi (13.10%). In comparison, the lowest was recorded for Dovyalis abyssinica (9.83%) on a dry matter basis. The moisture contents values of the Ziziphus Spina-chris and Ficutas mucusom fruits obtained in this finding were much higher than those reported by Aynachew et al (Tafesse, Citation2018). (5.70%, 3.10%), respectively. This might be due to techniques of drying methods applied during sample preparations. The findings showed that high moisture contents in the Ziziphus spina-christi lessen the shelf life. Therefore, to overcome the problem, it needs for processing into more stable products.
Table 1. Proximate composition (%, dwb) and gross energy (kcal/100 g) of the underutilized wild edible fruit.
The under-utilized wild edible fruits considered in this study had significant (p < .05) variation in the crude protein contents (3.01–5.31%). The result also indicated that the highest protein contents were recorded for Ziziphus cdsrspina-christi (5.31%). In contrast, the lowest was obtained for Dovyalis abyssinica (3.01%) on a dry matter basis. The recorded result was somewhat lower than the crude protein contents of wild edible fruits (4.72–10.30%) reported by Mokria et al (Mokria et al., Citation2022). The variation could be due to climatic conditions, soil fertility, and genetic difference.
There Was a Significant (P < .05) Variation in the Crude Fat Contents (%) Between Dovyalis
Abyssinica and Ficus mucuso while there was non-significant (p > .05) difference between Gardenia erubescens and Ziziphus spina-chris as indicated in . The result also showed that the highest and lowest contents of crude fat content were observed in Ficus mucuso (3.31%) and Gardenia erubescens (1.40%), respectively. The obtained result in crude fat contents was higher than wild edible fruit (Balanites aegyptiaca) (0.41%) reported from Senegal (Sagna et al., Citation2014). This might be due to differences in the type of fruits, extraction solvent, and oil extraction methods.
Table 2. Mineral content (mg/100 g, dwb) of the underutilized wild edible fruits.
The crude fiber contents of the under-utilized wild edible fruits were 1.26%, 0.93%, 2.11%, and 0.71% for Gardenia erubescens, Ficus mucuso, Dovyalis abyssinica, and Ziziphus spina-chris flour respectively. The finding of the study showed that Dovyalis abyssinica consists of significantly (p < .05) the highest crude fiber content (2.11 g/100 g) when compared to other studied edible fruits. The crude fiber contents (0.71–2.11%) observed in this study were much lower than wild, semi-wild edible plants reported from Southern Ethiopia by Getachew et al (Getachew et al., Citation2013). (4.30–15.9%). However, this is comparable with the findings of Achaglinkam et al (Achaglinkame et al., Citation2019). (2.0%). The lower fiber content found in this study was highly recommended for infant and children food formulation.
The level of ash content of the fruit was significantly (p < .05) high in Ziziphus spina-christi (9.23%) and low in Dovyalis abyssinica (4.84%). The recorded results were consistent with the value reported by Mokria et al (Mokria et al., Citation2022). (4.45–22.41%). The finding showed that a good amount of total ash in wild edible fruits reflects high mineral elements.
The total carbohydrate content (TCC) (70.41–78.27%) found in the wild edible fruits in this study were comparable with dried calyces of Hibiscus sabdariffa reported by Keyata et al (Keyata et al., Citation2020). (68.7–71.0%). The results also indicated that the TCC of the underutilized wild edible fruit was significantly (p < .05) high for Dovyalis abyssinica (78.27%). In contrast, Ziziphus spinachristi (70.19%) were low in carbohydrate content. This finding revealed that four under-utilized wild edible fruits had a great potential to supply the human body with reasonable amounts of its primary energy source.
The gross energy content found in the Gardenia erubescens (341.99 kcal/100 g) and Dovyalis abyssinica fruits (342.63kcal/100 g) were significantly (p < .05) higher compared to Ficus mucuso (337.71 kcal/100 g) and Ziziphus spina-chris (316.05 kcal/100 g). The gross energy content of wild edible fruits considered in this study was similar to Spinacia oleracea leaves (334.04 kcal/100) reported by Agarwal et al (Agarwal et al., Citation2017).
Mineral Contents of the Underutilized Wild Edible Fruits
The mineral contents (calcium, sodium, iron, potassium, zinc, magnesium, and phosphorus) of the four utilized wild edible fruits used in this study are indicated in . Mineral contents of the under-utilized wild edible fruits ranged from 67.17 to 190.18, 2.09 to 29.13, and 0.62 to 8.34 for calcium, iron, and zinc, respectively.
The result obtained in this study was similar to calcium (94.37–217.77 mg/100 g), iron (11.70–37.13 mg/100 g), and zinc (0.26–1.53 mg/100 g) contents reported for wild edible plant species grown in Ethiopia (Mokria et al., Citation2022). However, potassium content (107.54–183.36 mg/100 g) in this study was more than ten times lower than the findings of the authors above (1176.54–3536.24 mg/100 g). This might be due to differences in plants, and soil.
The sodium content found in the studied wild edible fruits (4.88–10.86) was lower than Eruca sativa leaves (44.0 mg/100 g) reported by Ellahi et al (Ellahi et al., Citation2007). The findings highlighted that the low sodium content in foods is vital for better health, particularly for people susceptible to high blood pressure. The magnesium (3.44–81.15 mg/100 g) content recorded in this study was similar to the findings reported in Cameroon (0.31–59.85 mg/100 g) (Mih et al., Citation2017). However, the phosphorous content (55.76 to 168 mg/100 g) of the studied wild edible fruits was much higher than the result reported by the authors mentioned above (0.19–14.00 mg/100 g). The high phosphorus content found in edible plants is essential for the formation of body and bones structure (Onwordi et al., Citation2009).
Mineral Ratios of the Underutilized Wild Edible Fruits
The mineral ratios are often more critical than individual mineral levels because they help determine nutritional interrelationships (Hoskin and Ireland, Citation2000). The mineral ratios of the underutilized wild edible fruit are shown in .
Table 3. Mineral ratios of the underutilized wild edible fruits.
Ijarotimi et al (Ijarotimi et al., Citation2013). Reported that a Na/K ratio of less than one is recommended for healthy foods. The result showed that Na: K ratios (0.029 to 0.067) of all the wild edible fruits considered in this study are within the recommended values. Reducing the Na: K ratio below one is essential to reduce hypertension and cardiovascular diseases (Binia et al., Citation2015). Therefore, consumption of wild edible fruits considered in this study reduces hypertension and might lower blood pressure in hypertensive patients.
The recommended values of the Ca/P should be greater than (>0.5) for healthy foods to contribute to calcium absorption in the small intestine (Jacob et al., Citation2015). All edible parts had a high Ca:P ratio (1.132–1.249), which helps calcium absorption for growing children who require a high intake of calcium and phosphorus for bone and teeth formation (Ijarotimi et al., Citation2013).
Watts (Watts, Citation2010) reported that the ideal ratio of Ca/K is 4:1 with an acceptable ideal range of 2.2 to 6.2. In line with these findings, the Ca: K ratios of wild edible fruits (0.451–1.148) were found within the recommended rate of healthy foods, which may help thyroid activity (Oghbaei et al., Citation2016).
The Fe/Zn ratio of the underutilized fruit ranged from 2.414 to 4.796. Peres et al (Pérès et al., Citation2001). Indicated that iron did not impair zinc absorption up to an iron: zinc ratio of 2:1. A dose-dependent effect was observed up to ratios from 5:1 to 10:1. Therefore, the iron present in the wild edible fruit did not impair zinc absorption which is a remedial solution to overcome the problem of poor growth and cognitive retardation (Dangoggo et al., Citation2011).
Anti-Nutritional Analysis
The antinutrients considered in this study are presented in . Ugwu and Oranye, Citation2006. [35) reported that the anti-nutritional factors reduce the maximum utilization of nutrients, especially proteins, vitamins, and minerals.
Table 4. Anti-nutritional factors (mg/100 g, dwb) of the underutilized wild edible fruits.
The phytate contents of the studied underutilized wild edible fruits ranged from 0.27 to 3.17 mg/100 g. The results showed that phytate contents in the samples are below the maximum tolerable level (200 mg/100 g) (Hurrell and , Citation2004), which may not impair the bioavailability of zinc, calcium, iron, and protein digestibility (Oghbaei et al., Citation2016). A similar result was reported by Aynachew et al (Tafesse, Citation2018).
The total oxalate contents of the underutilized wild edible fruits were 1.06, 2.86, 0.86, and 0.81 mg/100 g for Gardenia erubescens, Ficus mucuso, Dovyalis abyssinica, and Ziziphus spina-chris flour respectively. The total oxalate contents in this study were similar to the value reported from Ghana (Achaglinkame et al., Citation2019). The total oxalate content in this study is safe for human consumption because toxic levels of oxalate are between 3–5 g for men (Ekop et al., Citation2008).
There was a significant (p < .05) variation in the condensed tannin (4.11–9.33 mg/100 g) among wild edible fruit. The highest tannin contents (9.33 mg/100 g) were observed in the Ziziphus spina-christi fruit. At the same time, the lowest was recorded in the Ficus mucuso. The tannin contents of wild edible fruits considered in this study were below the toxicity level of daily intake (560 mg) reported by Fekadu et al.(Citation2013)
Molar Ratios and Bioavailability of Minerals
The molar ratios for calcium, zinc, iron, oxalate, and phytate were calculated to evaluate the effect of oxalate and phytate on the bioavailability of dietary minerals (Šimić et al., Citation2009). The calculated Ca: Phy, Ox: Ca, Phy: Zn, Phy: Fe, and [Ca] [Phy]/[Zn] molar ratios of the underutilized wild edible fruits are shown in .
Table 5. Calculated molar ratios of underutilized wild edible fruits.
Phytic acids markedly decrease Ca bioavailability. Woldegiorgis et al (Woldegiorgis et al., Citation2015). Reported that the critical molar ratio of [phy]: [Ca] of <0.24 indicating good calcium bioavailability. The result obtained in this study was also showed that the molar ratios of Phy: Ca of the fruits ranged from 0.0002 to 0.0018. The Phy: Ca molar ratios of the fruits were lower than the critical molar ratio of phytate to calcium. The findings indicated that absorption of calcium in all wild edible fruits is not adversely affected by phytate.
The Phy: Fe molar ratio lower than 0.4 is highly preferred for good iron absorption (Hurrell and , Citation2004). In line with this, the result found in all the wild edible fruits (0.0015 to 0.1283) was lower than the critical value. Thus, all the studied fruit samples may favor good iron bio-available to the human body.
Foods with a Phytate:Zn molar ratio of less than ten are sufficient zinc availability. However, problem is encountered when a value is greater than 15 (Gemede, Citation2020). Based on this context, the result obtained in this study (0.0043–0.5065) was within standard and high in zinc bioavailability. Similar results were reported in the wild edible plants grown in the Benishangul Gumuz region by Hailu and Addis (Hailu and Addis, Citation2016).
The Ox: Ca ratios of the fruits ranged from 0.0033 to 0.0069. Frontera et al (WHO World Health Organization, Citation2003). Showed that dietary calcium availability is limited when oxalate: Ca is higher than one. The oxalate: Ca molar ratios of all edible parts of the studied fruits were below the critical level of one. The finding implies that oxalate may not adversely affect the bioavailability of dietary calcium in the fruits of Gardenia erubescens, Ficus mucuso, Dovyalis abyssinica, and Ziziphus spina-chris. A molar ratio of Phy·Ca: Zn higher than 200 might negatively affect zinc bioavailability (Castro‐alba et al., Citation2019). The value obtained in this study is much lower (0.0106–1.5186) than the standard indicated above and suitable for zinc bioavailability.
Phytate Phosphorus and Non Phytate Phosphorous
The percentages of the phytate phosphorus to total phosphorus are very important; hence the phytate phosphorus cannot utilize by the human body, as reported by Umeta et al. (Citation2005). The fruits’ phytate phosphorous and nonphytate phosphorus contents are indicated in .
Table 6. Phytate phosphorus and non phytate phosphorous contents (mg/100 g, dwb) of under-utilized wild edible fruits.
The phytate phosphorus contents of the fruits ranged from 15.713 mg/100 g (Ziziphus spina-christi) to 47.342 mg/100 g (Ficus mucuso). The phytate phosphorous of the fruits was highest in Ficus mucuso (47.342 mg/100 g) and lowest in Ziziphus spina-christi (15.713 mg/100 g), but no significant (p > .05) difference among them. The nonphytate phosphorus content of the fruit ranged from 40.047 to 120.658 mg/100 g in . The nonphytate phosphorus content of the Ficus mucuso (120.658 mg/100 g) was significantly (p < .05) high, while Ziziphus spina-christi (40.047 mg/100 g) was the lowest.
According to Umeta et al (Umeta et al., Citation2005), diets with a proportion of phosphorus as phytate (percent) 50% in foods are regarded as adequate in bioavailable phosphate. This is because phytate’s impact on phosphorous absorption at high levels in the presence of high phytate intake has led to the suggestion that the proportion of phosphorus as phytate may be a good index of phosphorus bioavailability. The percentage of phosphorus in the underused wild edible fruits in this study was lower than the crucial proportion of phosphorus as phytate (50%), indicating the high bioavailability of phosphorus in the fruits. Therefore, eating neglected wild edible fruits may improve the mineral status and alleviate widespread mineral deficits brought on by their low bioavailability.
Antioxidant Properties of Underutilized Wild Edible Fruits
The total phenolic and flavonoid content of the underutilized wild edible fruits are shown in . The total phenolic content (TPC) of the under-utilized wild edible fruits were 230.76, 191.61, 191.36, and 108.32 mg GAE/100 g for Gardenia erubescens, Ficus mucuso, Dovyalis abyssinica, and Ziziphus spina-christi flour respectively. The findings highlighted there was a significant (p < .05) difference in the TPC among Gardenia erubescens, Dovyalis abyssinica, and Ficus mucuso. The TPC contents found in the Ziziphus spina-christi were lower than those reported in Cameroon (169:12 mg/100 g dry fruit) (Koubala et al., Citation2021). The findings are also lower than Ziziphus mauritiana Lam wild fruit grown in Burkina Faso (190.58 mg/100 g) (Meda et al., Citation2008). The variations in the TPC could be because of factors such as climatic conditions, ripeness at harvest time, genetic factors, and sunlight exposure. The TPC found in the Ficus mucuso is similar to the results reported for Fig varieties (81.77–212.36 mg GAE/100 g) reported from Turkey (Meda et al., Citation2008). Wild fruits considered in this study, particularly Gardenia erubescens rich in the TPC, can potentially develop functional foods to prevent several chronic diseases (Li et al., Citation2016).
Table 7. Antioxidant compounds [total phenolics (mg GAE/100 g) and flavonoids contents (mg CE/100 g)] of the underutilized wild edible fruits.
The total flavonoid contents (TFC) of the under-utilized wild edible fruits investigated in this study ranged from 79.70 to 112.85 mg CE/100 g. The highest TFC was recorded in the Gardenia erubescens, while the lowest was observed in the Ziziphus spinachristi. The found TFC in this study within the range reported edible indigenous wild fruits grown in Tanzania (45.24–178.46 (mg RE/100 g) (Mapunda and Mligo, Citation2019). The TFC of Ziziphus spina-christi observed in this study was lower than the result reported by Koubala et al (Koubala et al., Citation2021). (98:65 mg/100 g). The TFC of Ficus mucuso in this study was more than three times higher than Ficus carica fruits grown in Nigeria (21.63 mgQE/100 g) (Sirajo, Citation2018). This might be due to genetic variations and the type of standard chemical used for the calibration curve. A high TFC is essential to possess a robust anti-inflammatory effect to prevent the body cells’ free radical damage (Tiwari and Husain, Citation2017).
Conclusion
In this study, nutritional composition, anti-nutrient content, in-vitro mineral bioavailability, and antioxidant properties of underutilized wild edible fruits (Gardenia erubescens, Ficus mucuso, Dovyalis abyssinica, and Ziziphus spinachristi) grown in East Wollega, Ethiopia were investigated. The findings showed that all of the fruits appear to fall into a category of providing minerals and antioxidants although both gardenia and ziziphus may have less anti nutrient activity that may favor mineral bioavailability and have the potential to be used in various food formulations to treat micro and micronutrient deficiencies. The results also revealed that, compared to comparable fruits in other areas of the world, the underused edible fruits considered in this study more or less have the same nutritional makeup. Using these underused wild edible fruits can diversify the nation’s cereal-based eating patterns and improve nutritional value. To combat the nation’s difficulties with food and nutrition security, stakeholders at all levels should recognize the potential of these plants for expanded production and consumption.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Achaglinkame, M.A., R.O. Aderibigbe, O. Hensel, B. Sturm, and J.K. Korese. 2019. Nutritional characteristics of four underutilized edible wild fruits of dietary interest in Ghana. Foods 8(3):104. doi: 10.3390/foods8030104.
- Afari-Sefa, V., A. Tenkouano, C.O. Ojiewo, J.D. Keatinge, and J. d’A Hughes. 2012. Vegetable breeding in Africa: Constraints, complexity and contributions toward achieving food and nutritional security. Food Secur. 4(1):115–127. doi: 10.1007/s12571-011-0158-8.
- Agarwal, A., N. Raj, and N. Chaturvedi. 2017. A comparative study on proximate and antioxidant activity of Brassica oleracea (kale) and Spinacea oleracea (spinach) leaves. Int. J. Adv. Res. Biol. Sci. (IJARBS) 4(4):22–29. doi: 10.22192/ijarbs.2017.04.04.004.
- Alissa, E.M., and G.A. Ferns. 2017. Dietary fruits and vegetables and cardiovascular diseases risk. Crit. Rev. Food Sci. Nutr. 57(9):1950–1962. doi: 10.1080/10408398.2015.1040487.
- AOAC. 1984. Official methods of analysis association of official analytical chemists. 4th ed. AOAC, Washington, DC.
- AOAC,2000. Official methods of Analysis, 17th Ed. Methods 925.10, 965.17, 974.24, 992.16. The Association of Official Analytical Chemists, Gaithersburg, MD, USA.
- Binia, A., J. Jaeger, Y. Hu, A. Singh, and D. Zimmermann. 2015. Daily potassium intake and sodium-to-potassium ratio in the reduction of blood pressure: A meta-analysis of randomized controlled trials. J. Hypertens. 33(8):1509–1520. doi: 10.1097/HJH.0000000000000611.
- Bvenura, C., and D. Sivakumar. 2017. The role of wild fruits and vegetables in delivering a balanced and healthy diet. Food Res. Int. 99:15–30. doi: 10.1016/j.foodres.2017.06.046.
- Castro‐alba, V., C.E. Lazarte, B. Bergenståhl, and Y. Granfeldt. 2019. Phytate, iron, zinc, and calcium content of common Bolivian foods and their estimated mineral bioavailability. Food Sci. Nut. 7(9):2854–2865. doi: 10.1002/fsn3.1127.
- Dangoggo, S.M., A. Muhammad, A.I. Tsafe, A.A. Aliero, and A.U. Itodo. 2011. Proximate, mineral and anti-nutrient composition of Gardenia aqualla seeds. Arch. Appl. Sci. Res. 3(4):485–492.
- Dewanto, V., X. Wu, K.K. Adom, and R.H. Liu. 2002. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 50(10):3010–3014. doi: 10.1021/jf0115589.
- Ekop, A.S., I.B. Obot, and E.N. Ikpatt. 2008. Anti-nutritional factors and potassium bromate content in bread and flour samples in Uyo Metropolis, Nigeria. E-J. Chem. 5(4):736–741. doi: 10.1155/2008/530596.
- Ellahi, B., A. Salman, and S. Sheikh. 2007. Estimation of nutritional value and trace elements content of Carthamus oxyacantha, Eruca sativa and Plantago ovate. Pak. J. Bot. 39(4):1181–1187.
- FAO. Carbohydrates in human nutrition. Report of a Joint FAO/WHO Expert Consultation. FAO Food and Nutrition Paper No.66. Rome, Italy.1998
- FAO. Food energy-methods of analysis and conversion factors. Report of a technical workshop. FAO Food and Nutrition Paper No. 77. Rome, Italy.2002.
- Fekadu, H., F. Beyene, and G. Desse. 2013. Effect of traditional processing methods on nutritional composition and anti-nutritional factors of anchote (Coccinia Abyssinica (lam.) Cogn) tubers grown in Western Ethiopia. Journal of Food Processing & Technology 4(7):249–251.
- Gemede, H.F. 2020. Nutritional and antinutritional evaluation of complementary foods formulated from maize, pea, and anchote flours. Food Sci. Nut. 8(4):2156–2164. doi: 10.1002/fsn3.1516.
- Getachew, G.A., Z. Asfaw, V. Singh, Z. Woldu, J.J. Baidu-Forson, and S. Bhattacharya. 2013. Dietary values of wild and semi-wild edible plants in Southern Ethiopia. Afr. J. Food Agric.Nutr. Dev. 13(57):7486–7503. doi: 10.18697/ajfand.57.11125.
- Hailu, A.A., and G. Addis. 2016. The content and bioavailability of mineral nutrients of selected wild and traditional edible plants as affected by household preparation methods practiced by local community in Benishangul Gumuz Regional State, Ethiopia. Int. J. Food Sci. 2016:1–7. doi: 10.1155/2016/7615853.
- Hoskin, P.W., and T.R. Ireland. 2000. Rare earth element chemistry of zircon and its use as a provenance indicator. Geology 28(7):627–630. doi: 10.1130/0091-7613(2000)28<627:REECOZ>2.0.CO;2.
- Hurrell. 2004. Phytic acid degradation as a means of improving iron absorption. Int. J. Vitamin and Nutr. Res. 74(6):445–452. doi: 10.1024/0300-9831.74.6.445.
- Ijarotimi, O.S., O.A. Adeoti, and O. Ariyo. 2013. Comparative study on nutrient composition, phytochemical, and functional characteristics of raw, germinated, and fermented Moringa oleifera seed flour. Food Sci. Nut. 1(6):452–463. doi: 10.1002/fsn3.70.
- Jacob, A.G., D.I. Etong, and A. Tijjani. 2015. Proximate, mineral and anti-nutritional compositions of melon (Citrullus lanatus) seeds. Br. J. Res. 2(5):142–151.
- Keyata, E.O., Y.B. Tola, G. Bultosa, and S.F. Forsido. 2020. Proximate, mineral, and anti-nutrient compositions of underutilized plants of Ethiopia: Figl (Raphanus sativus L.), Girgir (Eruca sativa L) and Karkade (Hibiscus sabdariffa): Implications for in-vitro mineral bioavailability. Food Res. Int. 137:109724. doi: 10.1016/j.foodres.2020.109724.
- Koubala, B.B., J.P. Bayang, H. Wangso, M.C. Kolla, A. Laya, and M. MakniVariation of phenolics (bound and free), minerals, and antioxidant activity of twenty-eight wild edible fruits of twenty-three species from far north region of CameroonBiomed. Res. Int.2021Jul1220211–1410.1155/2021/4154381
- Kubola, J., S. Siriamornpun, and N. Meeso. 2011. Phytochemicals, vitamin C and sugar content of Thai wild fruits. Food Chem. 126(3): p.972–981. doi: 10.1016/j.foodchem.2010.11.104.
- Li, Y., J.J. Zhang, D.P. Xu, T. Zhou, Y. Zhou, S. Li, and H.B. LiBioactivities and health benefits of wild fruits. 2016 Aug 4 Int. J. Mol. Sci. 17(8): p.1258. doi:10.3390/ijms17081258
- Mapunda, E.P., and C. MligoNutritional content and antioxidant properties of edible indigenous wild fruits from miombo woodlands in TanzaniaInt. J. Biol. Chem. Sci.2019 Aug 28132849–86010.4314/ijbcs.v13i2.22
- Maxson, E.D., and L.W. Rooney. 1972. Evaluation of methods for tannin analysis in sorghum grain. Cereal Chem. 49(6):719–728.
- Meda, A.L., C.E. Lamien, M.M. Compaoré, R.N. Meda, M. Kiendrebeogo, B. Zeba, J.F. Millogo, and O.G. NacoulmaPolyphenol content and antioxidant activity of fourteen wild edible fruits from Burkina FasoMolecules2008 Mar 6133581–59410.3390/molecules13030581
- Mih, A.M., A.M. Ngone, and L.M. Ndam. 2017. Assessment of nutritional composition of wild vegetables consumed by the people of Lebialem Highlands, South Western Cameroon. Food Nutr. Sci. 8(6):647–657. doi: 10.4236/fns.2017.86046.
- Miller, V., S. Yusuf, C.K. Chow, M. Dehghan, D.J. Corsi, K. Lock, B. Popkin, S. Rangarajan, R. Khatib, S.A. Lear, et al. 2016. Availability, affordability, and consumption of fruits and vegetables in 18 countries across income levels: Findings from the Prospective Urban Rural Epidemiology (PURE) study. The Lancet Global Health 4(10):e695–703. doi: 10.1016/S2214-109X(16)30186-3.
- Mokria, M., Y. Gebretsadik, E. Birhane, S. McMullin, E. Ngethe, K.M. Hadgu, N. Hagazi, and S. Tewolde-Berhan. 2022. Nutritional and ecoclimatic importance of indigenous and naturalized wild edible plant species in Ethiopia. Food Chem. 4:100084. doi: 10.1016/j.fochms.2022.100084.
- Norhaizan, M.E., and A.W. Nor Faizadatul Ain. 2009. Determination of phytate, iron, zinc, calcium contents and their molar ratios in commonly consumed raw and prepared food in Malaysia. Malays. J. Nutr. 15(2):213–222.
- Oghbaei, M., J. Prakash, and F. Yildiz. 2016. Effect of primary processing of cereals and legumes on its nutritional quality: A comprehensive review. Cogent Food Agr. 2(1):1136015. doi: 10.1080/23311932.2015.1136015.
- Omari, R., K.E. Quorantsen, and P.K. Omari. 2017. Nutrition knowledge and food consumption practices and barriers in rural Ghana: The case of foods for preventing vitamin a and iron deficiencies. Afr. J. Food Agr. Nutr. Dev. 17(1):11639–11656. doi: 10.18697/ajfand.77.15815.
- Onwordi, C.T., A.M. Ogungbade, and A.D. Wusu. 2009. The proximate and mineral composition of three leafy vegetables commonly consumed in Lagos, Nigeria. Afr. J. Pure Appl. Chem. 3(6):102–107.
- Pérès, J.M., F. Bureau, D. Neuville, P. Arhan, and D. Bouglé. 2001. Inhibition of zinc absorption by iron depends on their ratio. J. Trace Elem. Med. Biol. 15(4):237–241. doi: 10.1016/S0946-672X(01)80039-0.
- Sagna, M.B., A. Diallo, P.S. Sarr, O. Ndiaye, D. Goffner, and A. Guisse. 2014. Biochemical composition and nutritional value of Balanites aegyptiaca (L.) Del fruit pulps from Northern Ferlo in Senegal. Afr. J. Biotechnol. 13(2):336–342. doi: 10.5897/AJB2013.12395.
- Šimić, D., R. Sudar, T. Ledenčan, A. Jambrović, Z. Zdunić, I. Brkić, and V. Kovačević. 2009. Genetic variation of bioavailable iron and zinc in grain of a maize population. J. Cereal Sci. 50(3):392–397. doi: 10.1016/j.jcs.2009.06.014.
- Sirajo, M.Ascorbic acid, total polyphenols and antioxidant activity of Ficus carica fruits. J. Food Chem. Nutr. 2018 Jul 15 6(1): p.21–2710.33687/jfcn.006.01.2497
- Tafesse, A.2018. Nutritional quality of underutilized wild edible fruits grown in Ethiopia. Addis Ababa.
- Tiwari, S.C., and N.I. Husain. 2017. Biological activities and role of flavonoids in human health–a. Indian J. Sci. Res. 12(2):193–196.
- Ugwu, F.M., and N.A. Oranye. 2006. Effects of some processing methods on the toxic components of African breadfruit (Treculia africana). Afr. J. Biotechnol. 5(22):2329–2333.
- Ukpabi, U.J., J.I. Ejidoh Effect of deep oil frying on the oxalate content and the degree of itching of cocoyams (Xanthosoma and Colocasia spp). InTechnical paper presented at the 5th Annuel Conference of the Agricultural Society of Nigeria, Federal University of Technology Owerri, Nigeria 1989: 3–6.
- Umeta, M., C.E. West, and H. Fufa. 2005. Content of zinc, iron, calcium and their absorption inhibitors in foods commonly consumed in Ethiopia. J. Food Compos. Anal. 18(8):803–817. doi: 10.1016/j.jfca.2004.09.008.
- Vaintraub, I.A., and N.A. Lapteva. 1988. Colorimetric determination of phytate in unpurified extracts of seeds and the products of their processing. Anal. Biochem. 175(1):227–230. doi: 10.1016/0003-2697(88)90382-X.
- Watts, D.L. 2010. HTMA mineral ratios. A brief discussion of their clinical importance. Trace Elem. Newsletter. 21:1–3.
- WHO (World Health Organization). 2003. Diet, nutrition, and the prevention of chronic diseases: Report of a joint WHO/FAO expert consultation. World Health Organ.
- Woldegiorgis, A.Z., D. Abate, G.D. Haki, and G.R. Ziegler. 2015. Major, minor and toxic minerals and anti-nutrients composition in edible mushrooms collected from Ethiopia. J. Food Process. Technol. 6(3):1.