ABSTRACT
The aim of this study was to isolate and purify flavonoids from Jujube (Zizipus jujuba Mill.) fruit and further evaluate antibacterial activities. Semi-preparative high-performance liquid chromatography (HPLC) was used to isolate and purify the flavonoids, while nuclear magnetic resonance spectroscopy (NMR) was employed for structural identification. Five flavonoids, including epicatechin, quercetin, rutin, isoquercitrin, and hyperin were identified. Antibacterial activity assays showed quercetin had the greatest inhibitory effect on Escherichia coli, Shigella, and Pseudomonas aeruginosa, and the minimum inhibitory concentration (MIC) against Escherichia coli or Pseudomonas aeruginosa was 250 μg/mL and against Shigella was 125 μg/mL. Rutin had the greatest inhibitory effect on Staphylococcus aureus, the MIC of which was 62.5 μg/mL. Hyperin had the greatest inhibitory effect on Bacillus subtilis, the MIC of which against Bacillus subtilis was 250 μg/mL, whereas that against Staphylococcus aureus was 62.5 μg/mL. We found that the antibacterial effects of the flavonoids were strong at a pH less than 6 and that they dropped sharply with increasing pH. Furthermore, the five metal ions Na+, Ca2+, K+, Fe2+, and Mg2+ generally increased the antibacterial activity of quercetin against Escherichia coli, Shigella, and Pseudomonas aeruginosa, and that of rutin against Staphylococcus aureus. For hyperin, Ca2+ and K+ had the strongest antibacterial effects. The present study suggests that the flavonoids extracted from Jujube (Zizipus jujuba Mill.) fruit were considered a promising candidate for natural and healthy antibacterial agents for the pharmaceutical and food industries.
Introduction
Antimicrobial resistance has become an increasingly important and pressing global problem (Hodaei et al., Citation2021). Antibiotics resistance is important one of antimicrobial resistance, which was play an important role in getting rid of bad bacteria, while they are unavoidable to lead to the resistance due to innate and acquired resistance mechanisms There is an urgent need of searching for new type antibacterial agents against drug-resistant bacteria. Because of their nontoxicity to the human body, natural products have been particularly a rich source of antimicrobial agents. Over the past decade, studies on natural products with antimicrobial activity have been conducted and their results have been encouraging (Milanezi et al., Citation2019). Natural antibacterial molecules found in plants is another valuable alternative to antibiotics. For example, spice plants, Chinese herbal medicines, traditional Mongolian and Tibetan medicines, and many other plants have shown significant antibacterial effects (Al-Maharik et al., Citation2022; Salami et al., Citation2016).
Flavonoids are a type of polyphenols with a C6-C3-C6 skeleton. As one of the largest classes of plant secondary metabolite, flavonoids can be widely found in various parts of plants (Kumar and Pandey, Citation2013). The antibacterial activities of flavonoids have been increasingly paid attention to, which can enhance the sensitivity of bacteria to antibiotics and then even reverse antimicrobial resistance (Parhi et al., Citation2020). Recently, flavonoids have attracted much interest because of the potential to be substitutes for antibiotics. The antibacterial activity of flavonoids is being increasingly documented.
Jujube (Ziziphusjujuba Mill.) is the most important member of the large and diverse Rhamnaceae family and is widely distributed in southeastern Europe and Asian countries, including China (Gao et al., Citation2013; Liu et al., Citation2019). China is the largest producer of jujube, where there are more than 700 cultivars of jujube and about 450,000 t produced annually. Jujube has been cultivated for over 1000 years. Jujube fruit has been used as healthy food for thousands of years in traditional Chinese medicine. Modern pharmacological studies demonstrated that jujube fruit possessed wide-ranging beneficial health-promoting properties as well. As one of the most important functional components,flavonoids play a key role in bacteriostasis, antioxidation, antitumor, and anti-inflammatory effects (Sobhani et al., Citation2019).
In our previous study, the total flavonoid content in Jujube reached as high as 6.70 mg/g under an optimum extraction process. In this study, Semi-preparative high-performance liquid chromatography (HPLC) was used to isolate and purify flavonoids from Jujube extracts. Then, structural identification of the flavonoids was performed. Further studies of their antibacterial activities have provided enhanced theoretical references for the identification and application of natural flavonoids as antibacterial agents.
Materials and Methods
Materials and Reagents
Jujube (Zizipus jujuba Mill.) fruits were provided by the Pomology Institute of the Academy of Agricultural Sciences, Shanxi (1000 m a.s.l.). The fruits were first cleaned, pitted, vacuum-frozen, and dried to a constant weight. Then, they were pulverized, passed through a screen (60 meshes), and sealed for storage. Escherichia coli ATCC 25,922, Staphylococcus aureus CICC 20,235, Salmonella CICC 21,482, Shigella CICC 21,534, Pseudomonas aeruginosa CICC 21,636, and Bacillus subtilis were purchased from the Center of Industrial Culture Collection (CICC), China. XCharge C18 (20 × 250 mm, 5 μm) preparative column was purchased from ACCHROM Corporation (Beijing, China).
Dimethyl sulfoxide (DMSO), methanol, and formic acid were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Commercial and pure flavonoid monomers, including epicatechin, quercetin, rutin, isoquercitrin, and hyperin were purchased from Shanghai Yi En Chemical Technology Co., Ltd. (Shanghai, China). Nutrient agar (NA) was purchased from Beijing Land Bridge Technology Co., Ltd. (Beijing, China). All other chemicals and solvents were of analytical grade.
Extraction, Isolation and Purification of the Flavonoids from Jujube Fruit
Jujube fruit powder was extracted three times with 65% ethanol in a weight ratio of 1:50 at 83°C. The crude sample was further extracted with ethyl acetate and sequentially separated with silica gel column and Sephadex LH-20 column. The purified sample was further purified with an ODS reversed-phase column and eluted with a gradient of 20%-100% methanol. A thin-layer chromatography (TLC) test was performed to determine the concentration of each fraction, which was then used for further purification.
Further separation of the target fraction was performed on the XCharge C18 preparative column (20 × 250 mm,10 μm) for Semi-preparative liquid chromatography (LC3000; Beijing Tong Heng Innovation Technology Co., Ltd.), and two mobile phases, A (0.4% phosphorus aqueous solution) and B (methanol) (A:B = 40:60), were employed. The flow rate was 2.0 mL/min, and the monitoring wavelength was 280/360 nm. The injection volume was 20 μL and the equilibration time was 10 min.
Structural Identification of Flavonoid Monomers
To obtain the UV absorption spectra for each flavonoid monomer, the monomers were dissolved in methanol and scanned using a UV-visible spectrophotometer with a scanning wavelength of 200–800 nm.
The ZORBAX Eclipse Plus C18 column was applied to high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS). The liquid phase of HPLC included a mobile phase A, which was water (containing 0.4% methanol), and a mobile phase B, which was methanol, in a ratio of 40:60. The other HPLC conditions were flow velocity of 0.4 mL/min, sample size of 2 μL, and column temperature of 40°C. Mass spectrometry (MS) was performed using a triple quadrupole mass spectrometer system, electrospray ionization (ESI) source, negative ion acquisition mode, and MS scanning acquisition range of 200–800 m/z. The conditions of MS included a drying gas temperature of 350°C, capillary voltage of 3.5 kV, atomizer pressure of 40 psi, drying gas flow rate of 10 L/min, fragmentation voltage of 135 V, and an acceleration voltage of 4 V.
For nuclear magnetic resonance spectroscopy (NMR), the flavonoid monomers were dissolved in deuterium methyl sulfoxide (DMSO-d6) and deuterium chloroform (CDCl3) respectively, and transferred to sample tubes for NMR spectrum determination.
Evaluation of the Antibacterial Activities of the Flavonoid Monomers
Bacterial strains were inoculated in a nutrient solution and cultured at 37°C for 1–2 days. A hemocytometer was used to control the concentration of the bacterial solution at 106-107 cfu/mL. The flavonoid monomers were then dissolved in DMSO to prepare a solution with a concentration of 1.0 mg/mL. Amoxicillin at a concentration of 1.0 mg/mL in saline was used as the control.
The antibacterial activities of the flavonoid monomers were detected by the cylinder-plate method (Tang et al., Citation2020). Briefly, 1 mL of a bacteria solution and 15–20 mL of nutrient agar with a temperature of 46°C were added to a sterile petri dish. After solidification, a sterilized Oxford cup was gently placed on the agar surface. After 2 min, 100 μL of the flavonoid monomer solution was added to the Oxford cups in triplicate. The Petri dishes were incubated at 36 ± 1°C for 24 h, and the diameter of the clear band was measured.
The minimum inhibitory concentration of the five flavonoid monomers was estimated using a two-fold dilution method (Rajaei et al., Citation2020). A total of eight concentrations, including 1000 μg/mL, 500 μg/mL, 250 μg/mL, 125 μg/mL, 62.5 μg/mL, 31.2 μg/mL, 15.6 μg/mL and 0 μg/mL were investigated, respectively. The minimum concentration (MIC) was the lowest flavonoid concentration with no bacterial growth.
To explore the effect of temperature on the antibacterial activities of the flavonoid monomers, 1.0 mg/mL of the flavonoid solution was treated at 20, 40, 60, 80, 100 and 121°C for 20 min and then tested on bacterial strains by the cylinder-plate method as described previously. Furthermore, to determine the effect of UV treatment on the antibacterial activities of the flavonoids, 1.0 mg/mL of the flavonoid monomer solution was treated under UV for 0, 5, 10, 15, 20, 25, and 30 min, and the antibacterial activities were tested as described above. To explore the effect of pH on the antibacterial activity, the pH values of the flavonoid solutions were adjusted to 1, 3, 5, 7, 9, and 11. The cylinder-plate method was then used as described above. The effect of metal ions on the antibacterial activities of the flavonoids were also measured. Briefly, KCl, CaCl2, NaCl, FeSO4, and FeCl3 were added to the flavonoid solution until the final concentration was 0.05 mol/L. The solution was then incubated at room temperature for 3 h. The antibacterial activities were tested by the cylinder-plate method as previously described. Each test was repeated thrice.
Statistical Analysis
All data obtained from triplicate determinations were expressed as means ± SD and a comparison between multiple groups was performed by one-way analysis of variance (ANOVA). The differences between the two groups were corrected using the Bonferroni method. SPSS version 22.0 was used for statistical analysis and the statistical significance of all tests was defined as P<0.05.
Results
Identification of the Structure of the Monomer Flavonoids
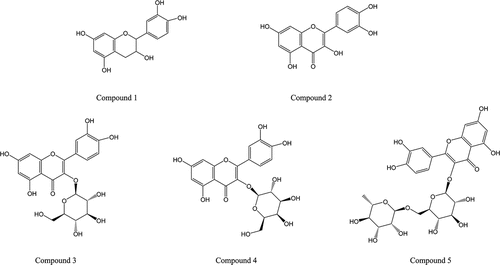
Five flavonoid monomers were identified by UV optical spectroscopy, HPLC-MS, and NMR. The monomer properties, including molecular weight, molecular formula, and molecular structure, of the five flavonoids were confirmed. Their molecular structures are shown in .
Compound 1 was a white powder with a melting point of 239°C. Dark spots were observed under UV light at 254 nm. The maximum absorption peak was observed at 280 nm. Its molecular formula was C15H14O6, and its molecular weight was 290.1 (m/z = 290.1 [M+H]+). 13C NMR (DMSO) data showed a 15 carbon skeleton signal. By integrating the 1 H NMR (DMSO) data, phenolic hydroxy H signals were detected in the low-field region. Based on this information and a previous study (Yang et al., Citation2017) the compound was confirmed to be epicatechin.
Compound 2 was a white powder with a melting point of 313°C. Dark spots were observed under UV light at 254 nm. The maximum absorption peak was observed at 360 nm. Its molecular formula was C15H10O7, and its molecular weight was 302 (m/z = 302 [M+H]+). 13C NMR (DMSO) data indicated 15 carbon skeleton signals, with a carbon carbonyl signal at δ 176.30. By integrating the 1 H NMR (DMSO) data, phenolic hydroxy H signals were detected in the low-field region. Based on this information and previous studies (Yang et al., Citation2017), the compound was confirmed to be quercetin.
Compound 3 was a yellow needlelike crystal with a melting point of 225–227°C. Dark spots were observed under UV light at 254 nm. Its maximum absorption peak was observed at 360 nm, and its molecular formula was C21H20O12. Its molecular weight was 464.1 (m/z=[M+H]+). 13C NMR (DMSO) data showed a 21 carbon skeleton signal. By integrating the 1H NMR (DMSO) data, phenolic hydroxy H signals were detected in the low-field region. Based on the above information and a previous study (Yang et al., Citation2017) the compounds were confirmed to be quercetin-3-O-glucoside and isoquercitrin.
Compound 4 was a pale-yellow powder with a melting point of 225–226°C. Dark spots were observed under UV light at 254 nm. Its maximum absorption peak was at 360 nm, and its molecular formula was C21H20O12. It had a molecular weight of 464.1 (m/z = 464.1[M+H]+). 13C NMR (DMSO) data showed a 21 carbon skeleton signal. By integrating the 1H NMR (DMSO) data, phenolic hydroxy H signals were detected in the low-field region. Based on the above information and a previous study (Zhang et al., Citation2019), the compounds were confirmed to be quercetin-3-O-β-D-galactopyranoside and hyperin.
Compound 5 was a pale-yellow powder with a melting point of 225–226°C. Dark spots were observed under UV light at 254 nm, and the maximum absorption peak occurred at 360 nm. Its molecular formula was C27H30O16, and its molecular weight was 610.2 (m/z = 610.2[M+H]+). 13C NMR (DMSO) data showed a 27 carbon skeleton signal. By integrating the 1H NMR (DMSO) data, phenolic hydroxy H signals were identified in the low-field area. Based on the above information and a previous study (Wei et al., Citation2013), the compounds were confirmed to contain quercetin-3-o-rutinose and rutin.
Analysis of the Antibacterial Activities of the Flavonoids
The intestinal bacterial strains Escherichia coli, Shigella, and Staphylococcus aureus, the microbial pollutant Pseudomonas aeruginosa, and a gram-positive bacillus, Bacillus subtilis, were chosen for antibacterial activity analysis.
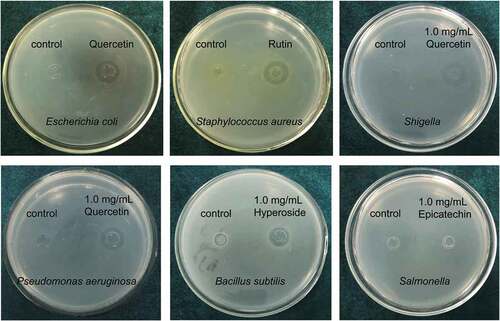
As shown in and , epicatechin, quercetin, and rutin exhibited significant inhibitory effects on Escherichia coli, Staphylococcus aureus, Shigella, and Pseudomonas aeruginosa. Isoquercitrin exhibited an inhibitory effect on Staphylococcus aureus and Bacillus subtilis. Hyperin had an inhibitory effect only on Bacillus subtilis. In addition, quercetin exhibited relatively greater inhibitory effect on Escherichia coli, Shigella, and Pseudomonas aeruginosa than the other flavonoid monomers; rutin exhibited a relatively greater inhibitory effect on Staphylococcus aureus than the other flavonoids; and hyperin exhibited relatively greater inhibitory effects on Bacillus subtilis than the other flavonoids. However, none of the five flavonoids had an inhibitory effect on Salmonella.
Table 1. Antibacterial activity test of different flavonoid compounds (Diameters of inhibition zones: mm).
Determination of the Minimum Inhibitory Concentrations (MICs) of Five Flavonoids
The MICs of quercetin, rutin, and hyperin were investigated. The results are presented in . Briefly, the MICs of quentin against Escherichia coli, Shigella, and Pseudomonas aeruginosa were 250, 125, and 250 μg/mL, respectively. Moreover, the MICs of rutin against Staphylococcus aureus and hyperin against Bacillus subtilis were 62.5 and 250 μg/mL, respectively.
Table 2. The antibacterial activities of quercetin, rutin and hyperin.
Analysis of the antibacterial stabilities of the flavonoids
Effect of temperature on the antibacterial activities of the flavonoids
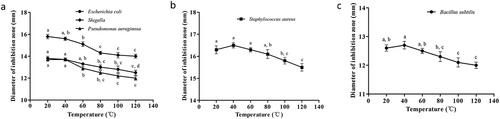
The influence of temperature on the antibacterial activity of the flavonoids is illustrated in . The results revealed that the antibacterial activity of quercetin against Escherichia coli, Shigella, and Pseudomonas aeruginosa decreased gradually with an increase in temperature (). In particular, the antibacterial activity of quercetin against Shigella at 60°C, 80°C,100°C, and 120°C was significantly lower than that at 20°C (p < .05). Similarly, compared to that at 20°C, the antibacterial activity of quercetin against Pseudomonas aeruginosa at 60°C, 80°C, 100°C, and 120°C was significantly lower (p < .05). The activity of quercetin against Escherichia coli at 80°C, 100°C, and 120°C was higher than that at 20°C and 40°C (p < .05). As shown in , the antibacterial activity of rutin against Staphylococcus aureus decreased gradually with increasing temperature from 40°C to 120°C, and the diameters of the inhibition zones at 100°C and 120°C were higher than those at 20°C and 40°C (p < .05). Additionally, the antibacterial activity of hyperin against Bacillus subtilis decreased when the temperature exceeded 60°C (). Compared with the diameters of the inhibition zones at 40°C and 60°C, the diameters of the inhibition zones at 100°C and 120°C were smaller (p < .05).
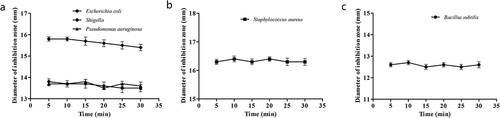
Effect of UV Treatment on the Antibacterial Activities of the Flavonoids
As shown in , the effects of UV irradiation on the antibacterial activity of quercetin against Escherichia coli, Shigella, and Pseudomonas aeruginosa,rutin against Staphylococcus aureus, and hyperin against Bacillus subtilis were not significant.
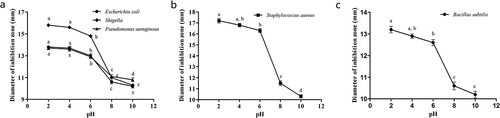
Effect of pH on the Antibacterial Activities of the Flavonoids
To further explore the antibacterial stabilities of the flavonoids, their pH values were adjusted to 1, 3, 5, 7, 9 and 11. As shown in , quercetin had a stronger antibacterial effect on Escherichia coli, Shigella, and Pseudomonas aeruginosa at low pH, and when the pH was higher than 6, the antibacterial effect decreased sharply, and no antibacterial effect was found at a pH over 8. Rutin had a stronger antibacterial effect against Staphylococcus aureus when the pH was less than 6 (). The antibacterial effect decreased significantly with an increase in pH from 6 to 10 (p < .05). When the pH was between 8 and 10, no antibacterial activity was found. Similarly, the antibacterial activity of hyperin against Bacillus subtilis decreased with increasing pH (). When the pH was between 8 and 10, the antibacterial activity against Bacillus subtilis was not detected, indicating that an acidic environment is crucial to the antibacterial activity of hyperin.
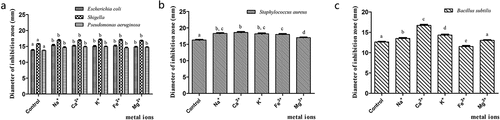
Effect of Metal Ions on the Antibacterial Activity of the Flavonoids
As shown in , the five metal ions Na+, Ca2+, K+, Fe2+, and Mg2+ increased the antibacterial activity of quercetin against Escherichia coli, Shigella, and Pseudomonas aeruginosa (p < .05). Furthermore, rutin was tested for its metal ion stability against Staphylococcus aureus. The results showed that all five metal ions, significantly enhanced the antibacterial effect of rutin against Staphylococcus aureus (p < .05; ). The order of antibacterial activity was Ca2+ > Na+ > K+. An analysis of the effect of the metal ions on the antibacterial activity of hyperin against Bacillus subtilis revealed that four metal ions, i.e., Na+, Ca2+, Mg2+, and K+, could enhance the antibacterial activity of hyperin (P < .05, ). The order of antibacterial activity was Ca2+, K+, Na+, and Mg2+. In contrast, Fe2+ decreased the antibacterial activity of hyperin against Bacillus subtilis.
Discussion
Plant flavonoids have shown anti-ulcer, anti-depression, antibacterial, antiviral, antibacterial, anti-diabetes, anti-inflammatory, anti-angiogenesis, anti-proliferation, and anti-tumor activities in vitro (Rana and Gulliya, Citation2019). Although flavonoids are not listed as nutrients, their intake is considered to be important for human health. In the present study, the flavonoid monomers were purified from the crude extracts of Jujube (Zizipus jujuba Mill.) fruit, and five flavonoids, epicatechin, quercetin, rutin, isoquercitrin, and hyperin, were identified. Epicatechin, quercetin, and rutin are flavonol derivatives, and isoquercitrin and hyperin are hyperoside and flavonol glycosides, respectively. Flavonoids have been found to perform antibacterial actions through a series of mechanisms, such as bacterial toxin production, inhibition of nucleic acid synthesis, inhibition of cytoplasmic membrane function, inhibition of energy metabolism, inhibition of the attachment and biofilm formation, inhibition of the porin on the cell membrane, alteration of the membrane permeability, and attenuation of the pathogenicity (Takó et al., Citation2020; Xie et al., Citation2015). It has been proved that flavonoids-rich Ziziphus jujuba Mill. extract could inhibit biofilm formation, improve the bacterial pH environment, and eliminate the hydrophobic effect of reactive oxygen species and flavonoids in a previous study (Miao et al., Citation2020). The analysis of antibacterial activities in this study suggested that quercetin had the greatest inhibitory effect on Escherichia coli, Shigella, and Pseudomonas aeruginosa; rutin had the greatest inhibitory effect on Staphylococcus aureus; and hyperin exhibited the greatest inhibitory effect on Bacillus subtilis.
As a flavonoid, quercetin is present in various common fruits, vegetables, and beverages and is mostly found in the form of quercetin glycosides. Because of its antioxidant and anti-inflammatory activities, quercetin is also used as an active ingredient in cosmetic preparations as a plant extract (Andres et al., Citation2018). A previous study reported quercetin as a precursor for the synthesis of novel nanoscale Cu (II) complexes, which are catalysts for alcohol oxidation with high antibacterial activity against Staphylococcus aureus and Escherichia coli (Moodi et al., Citation2021). Rutin belongs to the flavonoid family and is abundant in all plants and fruits (Ganeshpurkar and Saluja, Citation2017). It has been shown that rutin synergistically increases the antibacterial activity of alternative flavonoids against Salmonella enteritidis and Bacillus cereus. Hyperin, also known as quercetin-3-O-β-D-galactoside, is widely found in many plants, including Tengxanthaceae, Leguminosae, Azaleaceae, and Celastraceae (Wang et al., Citation2018). However, studies on quercetin, rutin, and hyperin in the Jujube (Zizipus jujuba Mill.) are limited.
The antibacterial activities of flavonoids are closely related to their structural characteristics. For example, chalcone has a C3-chain open-ring structure, and the hydroxylation and lipophilicity of ring A of chalcone play important roles in its antibacterial activity. Hydroxylation of ring A at 2“, 4,” and other positions can enhance the antibacterial activity of chalcone (Avila et al., Citation2008; Liu et al., Citation2008). The lipophilicity of ring A also significantly enhanced the antibacterial activity of chalcone (Batovska et al., Citation2009). Piperidinyl, C6-chain alkyl, or hydroxyl substitutions at the 4th position of ring B and trifluoromethyl or tribromomethyl substitution at the 3th or 5th position of ring B can also increase the antibacterial activity of chalcone. However, the lipophilicity of ring B is not as important as that of ring A.
In this study, the antibacterial activities of the flavonoids were stable at a pH of 6. When the pH was 8–10, all five flavonoids lost their antibacterial activities. This is likely because the structures of flavonoids are destroyed in an alkaline environment. In addition, studies have reported that the coordination of a flavonoid with metal ions can increase its antioxidant activity and ultra-oxygen anion elimination, and the strong ability of flavonoids to chelate with different metal ions (Moodi et al., Citation2021). In this study, the five metal ions Na+, Ca2+, K+, Fe2+, and Mg2+ generally increased the antibacterial activity of quercetin against Escherichia coli, Shigella, and Pseudomonas aeruginosa, as well as that of rutin against Staphylococcus aureus. For hyperin, Ca2+ and K+ had the strongest antibacterial effects, followed by Na+ and Mg2+.
Antibacterial flavonoids may have multiple cellular targets, rather than one specific site of action. One of their molecular actions is to form complexes with proteins through nonspecific forces such as hydrogen bonding and hydrophobic effects, as well as covalent bond formation (Kumar and Pandey, Citation2013). Thus, their mode of antimicrobial action may be related to their ability to inactivate microbial adhesins, enzymes, and cell-envelope transport proteins. Lipophilic flavonoids may also disrupt microbial membranes (Mishra et al., Citation2009).
Conclusion
Flavonoid monomers were purified from crude extracts of Jujube (Zizipus jujuba Mill.) fruit using silica gel column chromatography, Sephadex column chromatography, ODS reversed-phase column chromatography, and semi-preparative liquid chromatography. UV spectroscopy, liquid phase mass chromatography, and NMR were used to identify and confirm the structures of the flavonoids. Finally, a total of five flavonoids were identified, among which epicatechin, quercetin, and rutin are flavonol derivatives, but isoquercitrin and hyperin are hyperoside and flavonol glycosides, respectively. Quercetin had a greater inhibitory effect on Escherichia coli, Shigella, and Pseudomonas aeruginosa, whereas rutin had a greater inhibitory effect on Staphylococcus aureus than the other flavonoids. Hyperin had the greatest inhibitory effect on Bacillus subtilis. The MIC of quercetin against Escherichia coli or Pseudomonas aeruginosa was 250 μg/mL, and the MIC for Shigella was 125 μg/mL. The MIC of hyperin against Bacillus subtilis was 250 μg/mL, whereas that against Staphylococcus aureus was 62.5 μg/mL. The thermal and UV treatment of Jujube flavonoids did not have a significant effect on their antibacterial activities. However, the pH had a significant effect on the activity of Jujube flavonoids. Briefly, when the pH was less than 6, the flavonoids had a strong antibacterial effect. When the pH increased, the antibacterial effect dropped sharply. When the pH was greater than 8, no antibacterial effects were detected. Furthermore, the five metal ions Na+, Ca2+, K+, Fe2+, and Mg2+ mostly increased the antibacterial activity of quercetin against Escherichia coli, Shigella, and Pseudomonas aeruginosa, as well as that of rutin against Staphylococcus aureus. For hyperin, Ca2+ and K+ had the strongest antibacterial effects, followed by Na+ and Mg2+. This study could provide certain directions for the antibacterial agent purposes development of jujube flavonoids. This study may provide a direction toward the use of jujube flavonoids as antibacterial agents.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Al-Maharik, N., N. Jaradat, N. Bassalat, M. Hawash, and H. Zaid. 2022. Isolation, identification and pharmacological effects of Mandragora autumnalis fruit flavonoids fraction. Molecules 27(3):1046. doi: 10.3390/molecules27031046.
- Andres, S., S. Pevny, R. Ziegenhagen, N. Bakhiya, B. Schäfer, K.I. Hirsch-Ernst, and A. Lampen. 2018. Safety aspects of the use of quercetin as a dietary supplement. Molecular Nutrition & Food Research 62(1):1700447. doi: 10.1002/mnfr.201700447.
- Avila, H.P., F. Smânia Ede, F.D. Monache, and A. Smânia Jr. 2008. Structure-activity relationship of antibacterial chalcones. Bioorganic & Medicinal Chemistry 16(22):9790–9794. doi: 10.1016/j.bmc.2008.09.064.
- Batovska, D., S. Parushev, B. Stamboliyska, I. Tsvetkova, M. Ninova, and H. Najdenski. 2009. Examination of growth inhibitory properties of synthetic chalcones for which antibacterial activity was predicted. Eur. J Med. Chem 44(5):2211–2218. doi: 10.1016/j.ejmech.2008.05.010.
- Ganeshpurkar, A., &. and A. Saluja. 2017. The pharmacological potential of rutin. Saudi Pharmaceutical Journal 25(2):149–164. doi: 10.1016/j.jsps.2016.04.025.
- Gao, Q., C. Wu, and M. Wang. 2013. The jujube (Ziziphus jujuba Mill.) fruit: A review of current knowledge of fruit composition and health benefits. Journal of Agriculture and Food Chemistry 61(14):3351–3363. doi: 10.1021/jf4007032.
- Hodaei, M. M. Rahimmalek, and A. Arzani. 2021. Variation in bioactive compounds, antioxidant and antibacterial activity of Iranian Chrysanthemum morifolium cultivars and determination of major polyphenolic compounds based on HPLC analysis. J Food Sci. Technol 58(4):1538–1548. doi: 10.1007/s13197-020-04666-1.
- Kumar, S., and A.K. Pandey. 2013. Chemistry and biological activities of flavonoids: An overview. The Scientific World Journal 2013:162750. doi: 10.1155/2013/162750.
- Liu, X., H. Liu, Y. Yan, L. Fan, J. Yang, X. Wang, and G. Qin. 2019. Structural characterization and antioxidant activity of polysaccharides extracted from jujube using subcritical water. Lebensmittel-Wissenschaft und-Technologie 117(2):108645. doi: 10.1016/j.lwt.2019.108645.
- Liu, X., Y. Xu, and M. Go. 2008. Functionalized chalcones with basic functionalities have antibacterial activity against drug sensitive Staphylococcus aureus. Eur. J. Med. Chem 43(8):1681–1687. doi: 10.1016/j.ejmech.2007.10.007.
- Miao, W., L. Sheng, T. Yang, G. Wu, M. Zhang, J. Sun, and A. Ainiwaer. 2020. The impact of flavonoids-rich Ziziphus jujuba Mill. Extract on Staphylococcus aureus biofilm formation. BMC Complement Med. Ther 20(1):187. doi: 10.1186/s12906-020-2833-9.
- Milanezi, F.G., L.M. Meireles, M.M. de Christo Scherer, J.P. de Oliveira, A.R. da Silva, M.L. de Araujo, D.C. Endringer, M. Fronza, M.C.C. Guimarães, and R. Scherer. 2019. Antioxidant, antimicrobial and cytotoxic activities of gold nanoparticles capped with quercetin. Saudi Pharmaceutical Journal 27(7):968–974. doi: 10.1016/j.jsps.2019.07.005.
- Mishra, A.K., A. Mishra, H.K. Kehri, B. Sharma, and A.K. Pandey. 2009. Inhibitory activity of Indian spice plant Cinnamomum zeylanicum extracts against Alternaria solani and Curvularia lunata, the pathogenic dematiaceous moulds. Ann. Gen. Psychiatry 8(1):9. doi: 10.1186/1476-0711-8-9.
- Moodi, Z., G. Bagherzade, J. Peters, and I. Sovago. 2021. Quercetin as a precursor for the synthesis of novel nanoscale Cu (II) complex as a catalyst for alcohol oxidation with high antibacterial activity. Bioinorg. Chem. Appl 2021:8818452. doi: 10.1155/2021/8818452.
- Parhi, B., D. Bharatiya, and S.K. Swain. 2020. Application of quercetin flavonoid based hybrid nanocomposites: A review. Saudi Pharmaceutical Journal 28(12):1719–1732. doi: 10.1016/j.jsps.2020.10.017.
- Rajaei, A., D. Salarbashi, N. Asrari, B. Fazly Bazzaz, S. Aboutorabzade, and R. Shaddel. 2020. Antioxidant, antimicrobial, and cytotoxic activities of extracts from the seed and pulp of Jujube (Ziziphus jujuba) grown in Iran. Food Science and Nutrition 9(2):682–691. doi: 10.1002/fsn3.2031.
- Rana, A., and B. Gulliya. 2019. Chemistry and pharmacology of flavonoids-a review. Indian Journal of Pharmaceutical Education and Research 53(1):8–20. doi: 10.5530/ijper.53.1.3.
- Salami, M., M. Rahimmalek, and M.H. Ehtemam. 2016. Inhibitory effect of different fennel (Foeniculum vulgare) samples and their phenolic compounds on formation of advanced glycation products and comparison of antimicrobial and antioxidant activities. Food Chem. 213:196–205. doi: 10.1016/j.foodchem.2016.06.070.
- Sobhani, Z., S. Nikoofal-Sahlabadi, M.S. Amiri, M. Ramezani, S.A. Emami, and A. Sahebkar. 2019. Therapeutic effects of Ziziphus jujuba Mill. fruit in traditional and modern medicine: A review. Med Chem. (Los Angeles) 16(8):1069–1088. doi: 10.2174/1573406415666191031143553.
- Takó, M., E.B. Kerekes, C. Zambrano, A. Kotogán, T. Papp, J. Krisch, and C. Vágvölgyi. 2020. Plant phenolics and phenolic-enriched extracts as antimicrobial agents against food-contaminating microorganisms. Antioxidants 9(2):165. doi: 10.3390/antiox9020165.
- Tang, Z., Y. Wang, J. Yang, Y. Xiao, Y. Cai, Y. Wan, H. Chen, H. Yao, Z. Shan, C. Li, et al. 2020. Isolation and identification of flavonoid-producing endophytic fungi from medicinal plant Conyza blinii H.Lév that exhibit higher antioxidant and antibacterial activities. PeerJ 8:e8978. doi: 10.7717/peerj.8978.
- Wang, L., M. Zhang, Q. Zhu, C. Lu, and X. Bai. 2018. Hyperin enhances the sensitivity of HCT8/VCR colon cancer cell line to vincristine by down-regulating p-glycoprotein. Clin. Lab. 64(3):269–275. doi: 10.7754/Clin.Lab.2017.170923.
- Wei, X., S. Yang, N. Liang, D. Hu, L. Jin, W. Xue, and S. Yang. 2013. Chemical constituents of Caesalpinia decapetala (Roth) Alston. Molecules 18(1):1325–1336. doi: 10.3390/molecules18011325.
- Xie, Y., W. Yang, F. Tang, X. Chen, and L. Ren. 2015. Antibacterial activities of flavonoids: Structure-activity relationship and mechanism. Curr. Med. Chem. 22(1):132–149. doi: 10.2174/0929867321666140916113443.
- Yang, Y., M. An, Y. Jin, and H. Chen. 2017. Chemical constituents from the rhizome of and their antifungal activity. J. Asian Nat. Prod. Res. 19(1):47–52. doi: 10.1080/10286020.2016.1196672.
- Zhang, X., D. An, L. Guo, N. Yang, and H. Zhang. 2019. Identification and screening of active components from Ziziphora clinopodioides Lam. in regulating autophagy. Nat. Prod. Res. 33(17):2549–2553. doi: 10.1080/14786419.2018.1452002.