?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Mahonia jaunsarensis Ahrendt is a narrow endemic wild edible fruit-bearing species found in Uttarakhand Himalaya and has the potential of breeding compatibility with economically important Mahonia and Berberis species. Berry fruits of the species were explored for nutritional, phytochemical and antioxidant potential among three sampling locations. Fresh berries appeared a good source of diverse nutrients (carbohydrate 1.07–1.25 g/100 g, protein 0.97–1.13 g/100 g, non-reducing sugar 3.38–3.92 mg/g, and total sugar 9.86–12.87 mg/g); minerals (e.g. sodium 0.56–0.65 mg/100 g and potassium 0.99–1.14 mg/g); and vitamins (ascorbic acid 3.87–4.49 mg/g, thiamine 9.97–11.57 µg/g and carotenoids 1.67–1.94 mg/g). Similarly, phytochemicals (total anthocyanin 18.93–22.12 mg/g, phenolics 1.56–1.80 mg GAE/g, flavonoids 1.49–1.73 mg QE/g, flavonols 4.88–5.66 mg CE/g, and tannins 6.53–7.58 mg TAE/g) and in vitro antioxidant capacity (measured by ABTS assay, DPPH assay, OH· radical scavenging activity and FRAP assays) varied significantly (p < .05) among localities. Various phenolic compounds (particularly, chlorogenic acid 9.93–13.77 mg/g; caffeic acid 0.32–0.65 mg/g; syringic acid 0.30–0.47 mg/g fw) present in the methanolic extract also varied significantly among the localities. Thus, this phytonutrient- and antioxidant-rich genetic resource can be utilized for health-promoting functional foods. Also, the results of the present study indicated that the variation in nutritional, phytochemicals, and antioxidant activity among the locations can be utilized for elite selection, quality control, and breeding programs in the species.
Introduction
The genus Mahonia (family Berberidaceae) is widely distributed in tropical to temperate regions (1000–2400 m above mean sea level) across the globe. The genus has about 75 species worldwide, of these 13 species have been reported from the Indian Himalayan region and 4 from Uttarakhand (Li et al., Citation2000; Tiwari et al., Citation2012). Berry fruits of the genus Mahonia are consumed by local communities (Suyal et al., Citation2020). In addition, other parts of the plant such as roots, leaves and bark are traditionally used in the indigenous medicinal system for curing psoriasis, dermatitis, fever, inflammation, jaundice dysentery, diarrhea, constipation, hepatitis, cold, cough, conjunctivitis, tuberculosis, eczema and wounds (He and Mu, Citation2015). Rich species diversity, high nutritive value, and hybridization potential found among the species of Berberis and Mahonia signified the importance of these wild genetic resources in breeding for improved hybrids of their commercially important relative species (Rounsaville and Ranney, Citation2010). As such, berries are generally consumed as a rich source of vitamins, minerals, natural antioxidants, and other bioactive compounds and thus, considered to have high nutraceutical potential (Bhutia et al., Citation2021; Coklar and Akbulut, Citation2017; Kakar et al., Citation2019). These nutritive phytochemicals play an important role in preventing free radical-mediated non-communicable diseases, such as cardiovascular diseases, cancer, neurodegenerative diseases, diabetes, arthritis, cataracts, and atherosclerosis due to their antioxidant activities (Bhatt et al., Citation2012). Consumption of small berry fruits has been associated with the reduction of such diseases in many epidemiological studies, through balancing the level of reactive oxygen species in oxidative stress conditions, which may damage proteins, DNA, and lipids of the body (Hjartåker et al., Citation2015; Rawat et al., Citation2011).
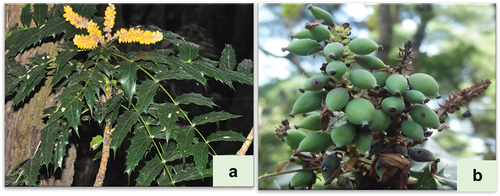
Mahonia jaunsarensis, locally known as Khoru, is endemic species to Uttarakhand (Rao et al., Citation1998). The species occupied the limited and specialized habitat of Chakrata-Jaunsar region of Uttarakhand State within an altitudinal range of 1800–2200 m asl (Tiwari et al., Citation2012). Berry fruits of M. jaunsarensis are generally considered as a rich source of phenolics and antioxidants (Suyal et al., Citation2020). Earlier, berries of other Mahonia species, such as M. aquifolium, M. bealei and M. leschenaultii have been reported as a rich source of polyphenolics, alkaloids, vitamins, minerals, and other secondary metabolites (Coklar and Akbulut, Citation2017; Kakar et al., Citation2019). Similarly, the fruit of M. aquifolium has been well-recognized for being a rich source of phenolic compounds, vitamin C, anthocyanins, sugars, and various minerals (Coklar and Akbulut, Citation2017, Citation2019; Kakar et al., Citation2019). In addition, the medicinal uses of different Mahonia species in traditional Indian and Chinese medicine are well documented ().
Table 1. Traditional utilization of Mahonia genus species.
However, a detailed investigation of the berries of Mahonia jaunsarensis has not been conducted. The quantitative assessment of morphological, nutritional, and nutraceutical parameters of the berries collected from different locations may provide the opportunity to determine the variability of these traits, which may be further helpful in the identification of elite germplasm for domestication and cultivation (Badhani et al., Citation2015). In the above context, the present study was focused on the investigation of morphology, nutrients, bioactive constituents, and antioxidant activity of the fruits of M. jaunsarensis collected from three locations from its only existing natural population of Chakrata, Uttarakhand, covering an elevation range of 1900 to 2300 m above mean sea level.
Materials and Methods
Sample Collection
Fully ripened fresh berry fruits of Mahonia jaunsarensis were collected in three localities at different altitudes (N 30°45′05;” E77°52′10;” localities presented as Mj-1 collected from 1900 m asl; Mj-2 collected from 2100 m asl; Mj-3 collected from 2300 m asl) in Banj-Oak Forest in Chakrata, Uttarakhand (). A total of 6–8 plants at each locality were targeted for the collection of fresh berries. All the infected and damaged berries were removed after collection. All the berry fruits collected from various plants within a locality were pooled, washed, and used for further morphological, nutritional, phytochemical, and antioxidant activity studies.
Morphological and physico-chemical characterization
Among the morphological parameters, fruit height and fruit width of berry fruits (10 from each localities) were measured with the help of a vernier calliper (Model LR44, India) and fruit weight of berries (10 fruits from each location) was measured by using an electronic weighing balance (Model CY510, Citizen, India). Berry fruits (50 g in three replicates) were squeezed by hand for obtaining the juice and recovered through passed from a cheese cloth. The pH of obtained fruit juice was measured using a digital pH meter. A total of 10 g fruits (in 3 replicates) were kept in a hot air oven at 65 ºC for drying till constant weight and the moisture content was calculated based on dry weight obtained. All the nutritional, phytochemical and antioxidant activity was performed in extracts prepared after the removal of seeds.
Mineral Analysis
For the quantification of minerals (e.g., sodium and potassium) standard method of Andola et al. (Citation2011) was followed. For the digestion of samples, 0.5 g of fresh fruits were mixed with 10 ml digestion mixture containing 20% nitric acid (v/v) and kept in the digestion unit at 360°C. The process was allowed to continue till the mixture turns colorless (~5 to 6 h). After cooling, the desired volume of the digested mixture was maintained by adding distilled water. The solution was filtered and mixed thoroughly till all sediments get dissolved. Subsequently, mineral elements (sodium and potassium) were determined by using Flame Photometer (SYSTRONIC-128).
Quantification of Total Carbohydrate and Protein
Total carbohydrates in fresh fruits were determined following the Anthrone reagent method described by Jugran et al. (Citation2016). Fresh fruits (1.0 g) were homogenized by a mortar and pestle, followed by the addition of 2.5 N HCl (10 ml), and placed in a water bath for 3 hrs. The mixture was cooled at room temperature and neutralized with 7% sodium carbonate until effervescence ceases. The final volume was made up of 100 ml and filtered using filter paper. Filtrate (1 ml) was mixed with 4 ml of Anthrone reagent (made in 95% H2SO4) and placed in a water bath at 90°C for 8 min. After cooling, absorbance was recorded at 630 nm using a UV-vis spectrophotometer (Hitachi U-2001, Tokyo, Japan). A calibration curve of glucose was prepared for quantification and results were expressed in g/100 g of fresh fruits.
Total protein was determined by Lowry method using the Folin’s reagent (Waterborg, Citation2009). Homogenised material of fresh fruit (1 g) was added to 10 ml water. Furthermore, copper sulfate (0.5 ml) and Folin’s phenols reagent (0.5 ml) were added to the mixture and incubated for 30 minutes at room temperature (20–22°C). The absorbance of the resulting blue-green mixture solution was measured at 660 nm using a UV-vis spectrophotometer (Hitachi U-2001, Tokyo, Japan). A calibration curve of different concentrations of Bovine Serum Albumin (01–10 mg/l) was prepared for quantification and results were represented in g/100 g of fresh fruits.
Quantification of Total Sugar and Reducing Sugar Content
The total sugar was estimated using Anthrone reagent method (Andola et al., Citation2011). Fresh fruit samples (1 g) were homogenized in a mortar and pestle, followed by the addition of 50 ml 2.5 N HCl and placed in the water bath at 90°C. In 1 ml of alcoholic extracts was taken in a test tube and chilled at −4°C. After 5 minutes, 4 ml of Anthrone reagent was carefully added through the walls of the test tube. The test tubes were thereafter immersed in ice water. The tubes were brought to ambient temperature and boiled in the water bath for 10 minutes. After proper cooling, the absorbance was measured at 625 nm using a UV-vis spectrophotometer (Hitachi U-2001, Tokyo, Japan). A calibration curve of glucose at different concentrations (01–10 mg/l) was used for quantification and results were expressed in mg/100 g fresh weight of fruit samples.
The reducing sugar was estimated using di-nitro-salicylic acid (DNS) reagent (Nisha and Radhamany, Citation2020). DNS reagent (3 ml) was added to 3 ml samples in a tightly capped test tube. The mixture was heated at 90°C for 5–15 minutes to attain a red-brown color. Thereafter, 1 ml of Rochelle’s salt solution was added to stabilize the color. After cooling to room temperature in the cold-water bath, absorbance was recorded at 575 nm using a UV-vis spectrophotometer (Hitachi U-2001, Tokyo, Japan). A calibration curve of glucose at different concentrations was used for quantification and results were expressed in mg/100 g fresh weight of fruit samples.
Quantification of Ascorbic Acid
Ascorbic acid was determined following the method described by Jugran et al. (Citation2016). Fruit samples (0.5 g) were homogenized using mortar and pestle in the presence of 4% (w/v) oxalic acid (50 ml). After filtration, the supernatant aliquots (2 ml) were taken out followed by the addition of 0.5 ml bromine water. Further, 1.0 ml 2% solution of 2,4-dinitro-phenylhydrazine reagent (w/v) was added to the mixture, followed by the addition of 0.5 ml thiourea and incubated at 37°C for 2 hrs. Subsequently, 7 ml of H2SO4 (80%) was added for dissolving osazone crystals and the absorbance was recorded at 540 nm using a UV-vis spectrophotometer (Hitachi U-2001, Tokyo, Japan). A standard curve of different concentrations (10–100 μg) of ascorbic acid was prepared for quantification, and results were expressed in mg/g fresh weight of the fruit sample.
Quantification of Thiamine Content
For quantification of thiamine content, fresh berries (10 g) were homogenized in 50 ml of ethanolic sodium hydroxide (5 N) and filtered into a flask. In 10 ml of filtered solution, 10 ml of potassium dichromate (0.5 N) was added for color development. The absorbance of the resulting colored mixture was recorded at 360 nm using a UV-vis spectrophotometer (Hitachi U-2001, Tokyo, Japan). The quantification of thiamine content was performed with the comparison to a standard curve of thiamine content prepared in 80% (v/v) ethanol (Bhutia et al., Citation2021). Results were expressed in mg/g fresh weight.
Quantification of Total Carotenoid Content
To determine the total carotenoid content, 10 g of fresh fruits were homogenized in an ice bath in the presence of 5 ml acetone in a cold mortar and pestle. Furthermore, 1.0 g anhydrous sodium sulfate (Na2SO4) was added to the homogenization and was elutriated using a paper filter. Filtrated solution was made up to a volume of 10 ml with acetone and was centrifuged for 10 minutes at 5000 rpm. The upper phase was collected and the absorbance of the obtained solution was measured at 662, 645, and 470 nm wave lengths using a UV-vis spectrophotometer (Hitachi U-2001, Tokyo, Japan) (Bhutia et al., Citation2021). Acetone was used as a control, and the carotenoids of each extract were calculated using the formulas as follows.
Extraction and Quantification of Total Anthocyanins
For extraction of anthocyanins, 5 g of fresh berries samples were homogenized with 50 ml of 80% (v/v) acidified ethanol (95% ethanol: 1.5N HCl). Samples were allowed to stand for overnight incubation at room temperature followed by filtering the extracts. Filtrates were stored at 4°C until analysis within a week. The total anthocyanins content was quantified by the pH differential method (Badhani et al., Citation2015). A UV-vis spectrophotometer (Hitachi U-2001, Tokyo, Japan) was used to measure the absorbance at 510 and 700 nm. The absorbance difference (A) between the pH-1.0 and pH-4.5 samples was calculated using the following formula:
The monomeric anthocyanin pigment concentration was calculated using the following equation:
Monomeric anthocyanin pigment (mg/l) = (A x MW x DF x 1000)/(ε x L);
(Where, MW = 449.2 and ε = 26,000, respectively, are molecular weight and molar absorptive of cyanidin-3-glucoside, which was used as a standard; DF is the dilution factor; L is the path length. The total monomeric anthocyanins were presented in mg/g fresh weight).
Extract Preparation for Phenolics and Antioxidant Activity
Fresh 20 g berries were carefully crushed using a grinder after removal of the seeds and kept in a flask with (80%) of 200 ml of aqueous methanol (80% v/v). Thereafter, extractions were sonicated (Ultrasonicator Toshiba-India) for 5 min, placed in the water bath for 1 h (30ºC), an orbital shaker for 14 hrs at 22ºC), respectively. The supernatant was removed and filtered, and further used for the analysis of total phenolic, flavonoids, flavonols, tannin contents, phenolic composition and antioxidant activity.
Determination of Total Phenolic, Flavonoid, Flavonols, Proanthocyanidin and Tannin Contents
The total phenolic content in fruit extracts was determined by Folin-Ciocalteu’s colorimetric method (Bahukhandi et al., Citation2020). Different concentrations of gallic acid were used for preparation of calibration curve and results were expressed in mg gallic acid equivalent (GAE)/g fresh weight of fruits. Total flavonoid content in the extracts of fruit was determined by the aluminum chloride colorimetric method (Badhani et al., Citation2015). Quantification of total flavonoid was done with the support of a standard curve of quercetin prepared in 80% (v/v) methanol and results were expressed in mg quercetin equivalent (QE)/g fresh weight (fw) of fruits. Total flavonol content was estimated using the method of Dhyani et al. (Citation2018), and quantification was carried out based on a standard curve of quercetin prepared in 80% (v/v) methanol, and results were expressed in mg catechin equivalent (CE)/g fresh weight (fw) of fruits. Proanthocyanidin content was determined following Bahukhandi et al. (Citation2020) and the absorbance of final reaction mixture was measured at 500 nm using UV-vis spectrophotometer (U-2001, Hitachi, Tokyo, Japan). Data were expressed in mg catechin equivalent (CE)/g fresh weight. However, total tannin content was estimated using Folin-Denis Reagent method (Bhatt et al., Citation2017). The absorbance of obtained blue mixture was recorded at 700 nm using a UV-vis spectrophotometer (Hitachi U-2001, Tokyo, Japan), and quantification was performed based on a standard curve of tannic acid prepared in 80% (v/v) methanol. Results were expressed in mg tannic acid equivalent (TAE)/g fresh weight of fruits.
Quantification of Phenolic Composition
Phenolic composition of the sample was analyzed using a reversed-phase chromatography system (Alliance Waters 2695, Waters, Milford, USA) coupled with a photodiode array detector (PDA, Waters 2998) and Empower 3 software (Waters). An isocratic mode system was used for the separation of individual phenolic compounds on SPHERISORB C18 column (5-µm particle size, 4.6 х 250 mm i.d.) at 30°C. The mobile phase consists of methanol (Merck HPLC grade) and 0.1% ortho-phosphoric acid (v/v) at a 40:60 ratio with a flow rate of 0.8 ml/minute for total run time of 40 min and the injection volume was 25 µl. The PDA was set at 190–400 nm, and the chromatograms of compounds (total three numbers) were recorded at 254 nm (caffeic acid and syringic acid) and 320 nm (chlorogenic acid). The identification of phenolic compounds was done based on the retention time of the corresponding external standard. UV-VIS spectra of the pure standard were used for plotting the standard calibration curve at different concentrations (Bhatt et al., Citation2017). The repeatability of quantitative analysis was less than 3.0%. The mean value of content was calculated with ± standard deviation (SD). The results were expressed as mg/g of fresh weight of fruits.
Antioxidant Activity
Free Radical – Scavenging Ability by Using ABTS Radical Cation (ABTS Assay)
Total antioxidant activity was measured following the improved ABTS method described by Bhatt et al. (Citation2017). ABTS salt (7.0 μM) and potassium per sulfate (2.45 μM) was added for the production of ABTS cation (ABTS˙+) and kept in dark for 16 hours at 23ºC. ABTS˙+ solution was diluted with distilled water till an absorbance of 0.700 ± 0.005 at 734 nm was obtained. Diluted ABTS˙+ solution (3.90 ml) was added in 0.10 ml of methanolic extract and allowed to stand for six minutes in dark at room temperature (~20ºC), and absorbance recorded at 734 nm using UV-VIS spectrophotometer corresponding to a blank prepared with 80% (v/v) methanol. A standard curve of various concentrations of ascorbic acid was prepared in 80% v/v methanol for the equivalent quantification of antioxidant potential. Results were expressed in millimole (mM) ascorbic acid equivalent (AAE) per 100 g fresh weight (fw) of fruits.
Free Radical – Scavenging Ability by the Use of DPPH Cation (DPPH Assay)
Traditional DPPH assay described by Coklar and Akbulut (Citation2017) was used for this study with few modifications. A cation solution of 0.1 mM DPPH prepared in 80% methanol (2.7 ml) was mixed with 0.9 ml sample extract and kept in dark at room temperature (~20 ºC) for 20 minutes. A reduction in the absorbance at 520 nm was recorded. Results were expressed in millimole (mM) ascorbic acid equivalent (AAE) per 100 g fresh weight (fw) of fruits.
Ferric Reducing Antioxidant Power (FRAP) Activity
Ferric reducing antioxidant power (FRAP) assay was performed following Jugran et al. (Citation2016) with some modifications. FRAP reagent was prepared by adding 10 volumes of 300 mM acetate buffer (i.e., 3.1 gram of sodium acetate and 16 ml glacial acetic acid/l), 1 volume of 10 mM 2,4,6-tri-2-pyridyl-1,3,5-triazine (TPTZ) in 40 mM HCl and 1 volume of 20 mM ferric chloride. The mixture was pre-warmed at 37°C and 3.0 ml of the mixture was added to 0.10 ml methanolic extract and kept at 37°C for 8 minutes. Absorbance was taken at 593 nm by using a UV-VIS spectrophotometer. A blank was prepared by ascorbic acid, and results were expressed in millimole (mM) of ascorbic acid equivalent (AAE) per 100 g fresh weight (fw) of fruits.
Statistical Analysis
All the analyzed parameters (viz. physico-chemical, nutritional, phenolics, and antioxidants) were determined in triplicates. The value for each sample was calculated as the mean of all replicates with ± standard error. The significant variations between mean values were tested using SPSS version 17.0. In addition, correlation analysis and principal component analysis (PCA) were performed for the identification of suitable habitat condition for accumulation of higher nutritive constituents in Past software (ver. 4.0) (Hammer et al., Citation2001).
Results and Discussion
Morphological and Physicochemical Characterization
In the present study, M. jaunsarensis showed significant (p < .05) variability in morphological parameters such as fruit length (9.34–9.74 mm), fruit diameter (4.46–4.69 mm), and fruit weight (0.170–0.176 g) among samples collected from different localities (). Similarly, physicochemical parameters like moisture content (80.03–83.66%) and pH (3.40–3.43) also varied significantly (p < .05) among the samples collected from different localities. Among these, higher fruit weight (0.176 g) and fruit length (9.74 mm) were found at the higher elevation (Mj-3, 2300 m). However, moisture content decreased with the higher elevation. Larger fruit size has been recorded at higher altitudes in passion fruit, Andean blackberry, Actinidia chinensis, Citrus latifolia, Fragaria ananassa, Rubus glaucus, and many other species (Fischer et al., Citation2022). It might be due to the development of genetic machinery for the adaptability of certain species to lower temperature at higher altitudes. At higher elevation, cooler night reduces the maintenance cost of respiration favored carbon balance and enhanced biomass in stage organs (Gariglio et al., Citation2007).
Table 2. Morphological and physicochemical properties of Mahonia jaunsarensis fresh fruits.
Fruits of M. jaunsarensis exhibited higher fruit weight and length as compared to M. aquifolium, which is a cultivated species of North America (Gunduz, Citation2013). Few studies on other Himalayan wild berry fruits (i.e., M. aquifolium and B. aristata) showed similar variations among populations in fruit morphology (Ahmed et al., Citation2013), which might be due to variable genetic makeup, habitats, elevation, climatic factors and other micro-climatic conditions of different locations (Gunduz, Citation2013). The variation in morphological parameters among the locations can be used for the identification of elite genotypes and suitable growing habitats (Qi et al., Citation2020). Also, the heritability and variation of such biomass-related quality traits might be an important aspect for introducing a species into the cultivation and successful breeding programs.
Nutritional and Mineral Components
The fresh berries of M. jaunsarensis showed significant variation (p < .05) in nutritional contents among the different localities. Total carbohydrates recorded between 1.07 and 1.25 g/100 g), proteins between 0.97 and 1.13 g/100 g, non-reducing sugar between 3.05 and 3.92 mg/g, and total sugar between 9.55 and 12.87 mg/g among the various studied localities. Interestingly, higher amounts of nutrients (i.e., protein content, carbohydrates, non-reducing sugars, and total sugars) were observed in fresh berries collected from the locality of higher elevation. The flavor of fruit defined as a sensory character is determined by water-soluble free sugars and organic acids. Among others, the sweetness of fruits is an essential component of fruit edible quality, which is determined by the types and totality of sugars (Cirilli et al., Citation2016). Different types of reducing and non-reducing sugars must be standardized for the acceptance and popularity of this species during domestication and harvesting. Protein is one of the most important nutrients required by body for the proper development and functioning (Gopalan et al., Citation2004). In the Himalayan region, the share of protein content is 4–5 times lower than the required share of 29% of the total diet for the proper functioning of the human body (Dutta et al., Citation2010). Thus, consumption of such local available resources might be useful for ensuring food and nutritional security of the region. Thus, such fruits might be useful for the improvement of the nutritional status of the poor undernourished population of the Himalaya (Sood et al., Citation2010).
Among minerals, sodium recorded between 0.56 and 0.65 mg/100 g and potassium between 0.97 and 1.13 mg/g among the localities (). Sodium and potassium take part in ionic balance and regulate the blood pressure of the human body (Haddy et al., Citation2006). Coklar and Akbulut (Citation2019) found that seeds of M. aquifolium are a rich natural source of essential nutrients and minerals such as protein (23.30 g/100 dw), fatty acids (14.20 g/100 g dw), potassium (628 mg/100 g), sodium (1.608 mg/100 g dw), and many others. Similarly, few other Himalayan berries, like Berberis asiatica, B. aristata, B. lycium, B. jaeschkeana, B. pseudumbellata, B. angulosa, B. napaulensis, Rubus ellipticus, R. acuminatus, Fragaria indica, Myrica esculenta, and Pyracantha crenulata, have been reported as good sources of nutritional contents (Ahmed et al., Citation2013; Andola et al., Citation2011; Badhani et al., Citation2015; Bhatt et al., Citation2017; Bhutia et al., Citation2021; Rana et al., Citation2018; Sood et al., Citation2010). These small wild berry fruits rich in diverse nutritional constituents are attracting the interest of nutritionists due to their health beneficial effects. Thus, diversity among genotypes or species of closely related taxa can be harnessed for variety development and improving quality-related traits in cultivated berry species through hybridization. In the present study, the nutritional content in the berries was recorded as comparatively higher than previously reported values of nutrients (protein content, carbohydrates, and reducing sugar) in Mahonia and Berberis species (Coklar and Akbulut, Citation2017; Sood et al., Citation2010). Thus, these berries of the species can be considered a rich source of nutritional compounds.
Table 3. Nutritional contents in fresh fruits of Mahonia jaunsarensis along the altitudinal gradients in different localities.
Vitamins and Other Metabolites
Total carotenoid (precursor of vitamin-A as β-carotene) content ranged between 1.67 and 1.94 mg/g, ascorbic acid content ranged between 3.87 to 4.49 mg/g and thiamine content ranged between 9.97 and 11.57 µg/g among different localities of M. jaunsarensis. These vitamins are considered the essential nutrients required by the body for maintaining proper cellular function (Bilgi Boyaci et al., Citation2012). Mahonia fruits are generally considered a good source of vitamins such as, carotenes and tocopherols. In a previous study, M. aquifolium fruit oil was found a rich source of α-tocopherol (74.06 mg/kg), β-tocopherol (6.03 mg/kg), γ-tocopherol (26.55 mg/kg), σ-tocopherol (4.94 mg/kg), α-tocotrienol (85.06 mg/kg), β-tocotrienol (3.98 mg/kg), γ-tocotrienol (308.30 mg/kg), total tocols (508.92 mg/kg), β-carotene (58.26 μg/100 g), α-carotene (15.21 μg/100 g), and total carotene (73.47 μg/100 g) (Andreicut et al., Citation2018). Among other berry fruits, β-carotene concentration has been recorded as (135.23 μg/100 g) in blueberry, (8.22 μg/100 g) in red raspberry, 44.27 μg/100 g in Marion berry, and (240.52 μg/100 g) oil in boysenberry seed oils (Parry et al., Citation2005). Thus, the presence of a significant amount of essential nutrients justified its potential application as a nutraceutical option for rural Himalayan people.
Similarly, anthocyanin content ranged between 18.93 and 22.12 mg/g among different studied localities. These blue-colored Mahonia fruits are a good source of anthocyanins and it have been reported as (380.99 mg/100 g fw) in ethanol extract of domesticated M. aquifolium berries (Coklar and Akbulut, Citation2017). Similarly, total monomeric anthocyanins have been recorded as (8.58 mg/100 g fw) in Mahonia leschenaultii fruits (Karuppusamy et al., Citation2011). Berry anthocyanins are generally strong antioxidants and thus have potential application as a functional food to protect against diseases related to heart, cardiovascular, blood pressure, and diabetes (Khoo et al., Citation2017).
Total Phenolic, Flavonoid, Flavonol, Proanthocyanidin, and Tannin Contents
A significant (p < .05) variation was recorded in different phenolic constituents among studied localities (). The content of total phenolics ranged between 1.56 and 1.80 mg GAE/g, total flavonoids between 1.49 and 1.73 mg QE/g, total flavonols between 4.85 and 5.66 mg CE/g, proanthocyanidin between 4.66 and 5.41 mg CE/g and total tannins between 6.53 and 7.58 mg TAE/g fresh weight. The sample collected from the higher elevation (Mj-3, 2300 m asl) exhibited higher content of phenolic constituents than other localities. Previously, total phenolic content has been reported in fruits of cultivated M. aquifolium as 17.24 mg GAE/g dry weight (Coklar and Akbulut, Citation2021). Similarly, in another study, total phenolic contents in M. aquifolium berries ranged from 1.30 (chloroform extract) to 1049.40 mg (methanol extract) (GAE/100gfw) (Coklar and Akbulut, Citation2017). Also, in berries of M. leschenaultii found in Western Ghats of India, total phenolic content has been reported as 65.8 mg/100 g GAE and total flavonoids as 95.5 mg/100 g QE (Karuppusamy et al.,Citation2011). Thus, species can be used as a rich source of phenolic constituents.
Table 4. Polyphenolics in Mahonia jaunsarensis along the altitudinal gradients in different localities.
Phenolic Composition
A total of three phenolics (caffeic acid, chlorogenic acid, and syringic acid) were quantified in fresh berries of M. jaunsarensis (; ) through a reverse-phase HPLC. A significant variation (p < .05) was found among the localities and chlorogenic acid ranged between 8.98 to 14.81 mg/g, caffeic acid between 0.32 to 0.76 mg/g, and syringic acid between 0.27 to 0.47 mg/g. The higher amounts of chlorogenic acid (14.81 mg/g) and caffeic acid (0.76 mg/g) were exhibited at higher altitude locality (Mj-3, 2300 m). These phenolic compounds are very common in berry fruits. While, comparing the phenolic compounds with other species of the genus, M. jaunsarensis exhibited higher content of chlorogenic acid than domesticated M. aquifolium (Coklar and Akbulut, Citation2017). Similarly, chlorogenic acid has been reported in many Himalayan berry fruit species such as Myrica esculenta (5.68 mg/g) (Rawat et al., Citation2011), Rubus ellipticus (5.34 mg/100 g) (Badhani et al., Citation2015) Likewise, caffeic acid (8.42 mg/g) and gallic acid (0.87 mg/g) have also been reported in B. jaeschkeana (Belwal et al., Citation2017).
Figure 2. A representative chromatogram of fruit sample of Mahonia jaunsarensis. ChA - Chlorogenic acid, CfA - Caffeic acid, SA - Syringic acid.
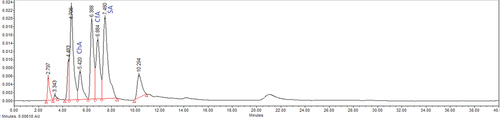
Table 5. Phenolic compounds (mg/100 g) identified and quantified in fresh fruits of Mahonia jaunsarensis along the altitudinal gradients in different localities.
As such, chlorogenic acid is reported to help reduce cardiovascular syndrome (CVS) by reducing the levels of free fatty acids and triglycerides, and plays a protective role in CVS by increasing NO production inhibition of platelet aggregation, and reduction in blood viscosity (Jiang et al., Citation2015; Zhang and Hu, Citation2016). Likewise, caffeic acid and its derivatives showed antioxidant, anti-inflammatory, and anti-carcinogenic activities. It also participated in the defense mechanism of plants against predators and pests, inhibition of growth of insects, fungi, and bacteria, and protects plants against ultraviolet radiation B (Gould et al., Citation2000). The other compounds like syringic acid showed anti-oxidant, anti-microbial, and anti-inflammatory activities and also for protecting the organs (i.e., heart, liver, brain) from free radicals (Badhani et al., Citation2015; Bhutia et al., Citation2021).
Antioxidant Activity
Antioxidant activity measured by in vitro assays revealed a significant variation (p < .05; i.e., ABTS 1.53–1.77 mM; DPPH 1.72–1.73 mM; FRAP 1.31–1.52 mM and OH· 1.48–1.72 mM AAE/100 g fresh weight) among studies localities (). The fresh berries collected from the locality of higher elevation (Mj-3, 2300 m) showed higher antioxidant activities (ABTS 1.77 mM; DPPH 1.73 mM; FRAP 1.52 mM; and OH· 1.72 mM AAE/100 g) as compared to others. Previously, a study on M. aquifolium showed the antioxidant activities as 35.26 mM for DPPH assay, 49.95 mM TE (Trolox equivalent)/kg for ABTS assay and 136.34 mM TE/kg for FRAP mM TE/kg fresh weight in the diverse extraction methods in methanol (Coklar and Akbulut, Citation2017). On contrary, Suyal et al. (Citation2020) studied diverse solvent extracts of M. jaunsarensis for antioxidant activities using the same methods and found relatively lower values of antioxidant activities as compared to the present results. However, such variation among different studies might be due to different ripening stages, time of collection, post-harvesting management, sample processing as well as the genetic makeup of different genotypes (Badhani et al., Citation2015; Bhutia et al., Citation2021).
Table 6. Antioxidant activities (in mM AAE/100 g fresh weight) in fresh fruits of Mahonia jaunsarensis along the altitudinal gradients in different localities.
Relationship Between Polyphenolic and Antioxidant Activity
Altitude has shown an important role in the accumulation of phytochemicals in M. jaunsarensis (). Altitude showed a significant (p < .01) positive relationship with phenolics (r = 0.903), tannins (r = 0.904), flavonoids (r = 0.891), proanthocyanidins (r = 0.918), antioxidant activities (ABTS r = 0.905; FRAP r = 0.890; OH· r = 0.892), nutritional contents (i.e., proteins r = 0.815, potassium r = 0.899, carotenoid r = 0.921) and vitamins (ascorbic acid r = 0.917; thiamine r = 0.918). The present study is in agreement with the earlier studies that the collection of berries from higher elevations (Mj-3, 2300 m) has a higher amount of natural bioactive constituents (Rawat et al., Citation2011). With the change in altitude, climatic condition changed drastically and it has been observed that with 100 m increase in elevation, about 0.6 to 0.7°C decrease in temperature, and with 1000 m increase in elevation, 10–12% increase in UV radiation has been recorded, which consequently impacted growth and physiological process of plant. Thus, increase in antioxidant compounds such as, anthocyanins and phenolics in peels of apples have been recorded to reduce the impact of UV radiation (Voronkov et al., Citation2019). The total titratable acidity represented by presence of organic acids have been recorded to increased in five species of berries (blackberry, raspberry, blueberry) with increasing altitude (Fischer et al., Citation2022). Similarly, total phenolic content showed a significant relationship with antioxidant activities (ABTS r = 0.997; FRAP r = 0.987; OH r = 0.991). In addition, total flavonoids, total flavonols total tannins, total anthocyanins, and proanthocyanidins positively (p < .01) correlated with antioxidant activities. Previous studies indicated that polyphenolics have the capability to scavenge free radicals and play an important role in the electron transfer chain (Prior et al., Citation2005). It has already been reported that antioxidants (viz. phenolic acids, carotenoids, tocopherols, flavonoids, etc.) rich fruits can reduce various metabolic disorders, hypertension, and scavenge or reduce the free radicals (Bhatt et al., Citation2012; Scalbert et al., Citation2005; Suleman et al., Citation2019). In addition, elevation range, habitat, environmental factors, temperature, rainfall, slope, aspects, light intensity, soil, and availability of nutrients or moisture retaining capacity of the soil, excess UV-B radiation, water availability, and other factors also influenced the accumulation of bioactive constituents in plants and protect against both biotic and abiotic factors, etc (Alonso-Amelot et al., Citation2004). Studies are available which indicated that environmental factors such as altitude influence the accumulation of diverse bioactive constituents and nutritional contents in plants, i.e., Valeriana jatamansi (Jugran et al., Citation2016); Coleus forskohlii; Mahonia jaunsarensis (Suyal et al., Citation2020); Mahonia leschenaultia, Mahonia aquifolium (Coklar and Akbulut, Citation2017; Gunduz, Citation2013 Karuppusamy et al., Citation2011; Fragaria nubicola (Bahukhandi et al., Citation2020), Nepeta septemcrenata, Origanum syriacum subsp. Sinaicum, Phlomisaurea, Rosa arabica, Silene schimperiana (Rawat et al., Citation2011).
Table 7. Relationship between analyzed parameters in fruits of in Mahonia jaunsarensis.
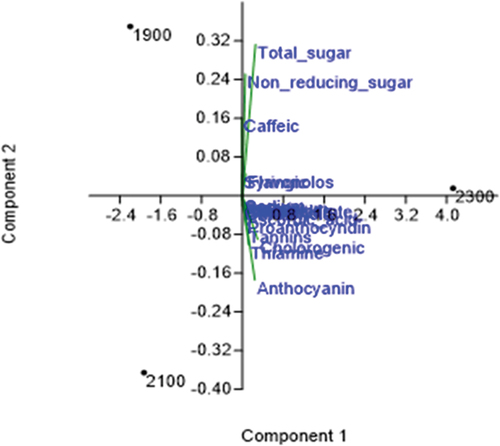
Principal component analysis (PCA) showed two components, component 1 (PC 1–99.007%) and component 2 (PC 2–0.99%) ().In the PCA analysis, flavonols, antioxidant compounds (ABTS; DPPH; OH· activity) and nutrients (non-reducing sugar and total sugar) were recorded high at 1900 m asl. However, phenolics, tannins, and antioxidant activity (FRAP; flavonoids activity) nutrients (carbohydrate, protein, sodium, potassium, anthocyanin, ascorbic acid, carotenoid, and thiamine) were recorded high in the locality of higher elevation (Mj-3, 2300 m). Thus overall, it can be concluded that various biochemical attributes of the species have different behavior toward the altitude and need specific climatic conditions for harnessing the optimum benefits.
Conclusion
M. jaunsarensis is one of the important species having the potential to be a source of natural bioactive and nutritional compounds, including, minerals, essential nutrients, vitamins, anthocyanins, phenolics, flavonoids, and antioxidant activity. In the present study, variation among physicochemical, biochemical, phytochemicals, and antioxidant activities is an indication that environmental factors do affect these contents and the location with high values may be considered for further investigation and need to be multiplied at a larger scale considering the endemic nature of the species. In addition, the consumption of berries and their processed form can fulfil the basic nutritional requirements of the body and will be useful for reducing nutritional security in the Himalayas. This information can be utilized for germplasm characterization, elite selection, quality control, and breeding programs in the future.
Acknowledgments
We thank the Director of GBPNIHE for providing the necessary facilities and continuous encouragement. Colleagues of CBCM are greatly acknowledged for their valuable inputs during the study.
Disclosure statement
The authors declared that there are no known conflicts of interest associated with this publication.
Data Availability Statement
Data sharing is not applicable to this article as all the datasets generated during the current study have been presented in the results.
References
- Ahmed, M., M.A. Anjum, R.M.M. Naz, M.R. Khan, and S. Hussain. 2013. Characterization of indigenous barberry germplasm in Pakistan: Variability in morphological characteristics and nutritional composition. Fruits 68(5):409–422. doi: 10.1051/fruits/2013085.
- Alonso-Amelot, M.E., A. Oliveros, and M.P. Calcagno-Pisarelli. 2004. Phenolics and condensed tannins in relation to altitude in neotropical Pteridium spp: A field study in the Venezuelan Andes. Biochem. Syst. Ecol. 32(11):969–981. doi: https://doi.org/10.1016/j.bse.2004.03.005.
- Andola, H.C., R.S. Rawal, and I.D. Bhatt. 2011. Comparative studies on the nutritive and anti-nutritive properties of fruits in selected Berberis species of West Himalaya, India. Food Res. Int. 44(7):2352–2356. doi: https://doi.org/10.1016/j.foodres.2010.07.017.
- Andreicut, A.D., A.E. Pârvu, A.C. Mot, M. Pârvu, E. Fischer Fodor, A.F. Cătoi, V. Feldrihan, M. Cecan, and A. Irimie. 2018. Phytochemical analysis of anti-inflammatory and antioxidant effects of Mahonia aquifolium flower and fruit extracts. Oxid Med. Cell Longev 2018:e2879793. doi: 10.1155/2018/2879793.
- Badhani, A., S. Rawat, I.D. Bhatt, and R.S. Rawal. 2015. Variation in Chemical Constituents and Antioxidant Activity in Yellow Himalayan (R ubus ellipticus Smith) and Hill Raspberry (R ubus niveus Thunb.). J. Food Biochem. 39(6):663–672. doi: 10.1111/jfbc.12172.
- Bahukhandi, A., A. Barola, and K.C. Sekar. 2020. Antioxidant activity and polyphenolics of Fragaria nubicola: A Wild Edible Fruit Species of Himalaya. Proceedings of the National Academy of Sciences, India, Section B: Biological Sciences 90: 761–767. 10.1007/s40011-019-01142-5
- Belwal, T., L. Giri, I.D. Bhatt, R.S. Rawal, and V. Pande. 2017. An improved method for extraction of nutraceutically important polyphenolics from Berberis jaeschkeana. C.K. Schneid. Fruits. Food Chemistry 230:657–666. doi: 10.1016/j.foodchem.2017.03.086.
- Bhatt, I.D., P. Dauthal, S. Rawat, K. Gaira, A. Jugran, R. Rawal, and U. Dhar. 2012. Characterization of essential oil composition, phenolic content, and antioxidant properties in wild and planted individuals of Valeriana jatamansi. Jones. Scientia Horticulturae 136:61–68. doi: 10.1016/j.scienta.2011.12.032.
- Bhatt, I.D., S. Rawat, A. Badhani, and R.S. Rawal. 2017. Nutraceutical potential of selected wild edible fruits of the Indian Himalayan region. Food Chem. 215:84–91. doi: 10.1016/j.foodchem.2016.07.143.
- Bhutia, P.O., P. Kewlani, A. Pandey, S. Rawat, and I.D. Bhatt. 2021. Physico-chemical properties and nutritional composition of fruits of the wild Himalayan strawberry (Fragaria nubicola Lindle.) in different ripening stages. Journal of Berry Research 11(3):481–496. doi: https://doi.org/10.3233/JBR-210742.
- Bilgi Boyaci, B., J.-Y. Han, M.T. Masatcioglu, E. Yalcin, S. Celik, G.-H. Ryu, and H. Koksel. 2012. Effects of cold extrusion process on thiamine and riboflavin contents of fortified corn extrudates. Food Chem. 132(4):2165–2170. doi: https://doi.org/10.1016/j.foodchem.2011.12.013.
- Chao, J., T.C. Lu, J.W. Liao, T.H. Huang, M.S. Lee, H.Y. Cheng, L.K. Ho, C.L. Cuo, and W.H. Peng. 2009. Analgesic and anti-inflammatory activities of ethanol root extract of Mahonia oiwakensis in mice. J. Ethnopharmacol 125(2):297–303. doi: 10.1016/j.jep.2009.06.024.
- Cirilli, M., D. Bassi, and A. Ciacciulli. 2016. Sugars in peach fruit: A breeding perspective. Horticulture Research 3(1):15067. doi: https://doi.org/10.1038/hortres.2015.67.
- Coklar, H., and M. Akbulut. 2017. Anthocyanins and phenolic compounds of Mahonia aquifolium berries and their contributions to antioxidant activity. J. Funct. Foods 35:166–174. doi: 10.1016/j.jff.2017.05.037.
- Coklar, H., and M. Akbulut. 2019. Bioactive compounds, antioxidant activity and some physicochemical properties of the seed and seed-oil of Mahonia aquifolium berries. Journal of Food Measurement and Characterization 13(2):1269–1278. doi: https://doi.org/10.1007/s11694-019-00042-6.
- Coklar, H., and M. Akbulut. 2021. Changes in phenolic acids, flavonoids, anthocyanins, and antioxidant activities of Mahonia aquifolium berries during fruit development and elucidation of the phenolic biosynthetic pathway. Horticulture, Environment, and Biotechnology 62(5):785–794. doi: https://doi.org/10.1007/s13580-021-00348-9.
- Dhyani, P., A. Bahukhandi, S. Rawat, I.D. Bhatt, and R. Rawal. 2018. Diversity of bioactive compounds and antioxidant activity in delicious group of apples in Western Himalaya. J Food Sci. Technol 55(7):2587–2599. doi: 10.1007/s13197-018-3179-x.
- Dutta, A.D., K. Pant, P. Kumar, and R. Singh. 2010. Impact of agro climatic and socio-economic variability on the nutritional status of inhabitants in the Garhwal Himalayas. Ecol. Food Nutr 43(5):409–420. doi: 10.1080/03670240490500325.
- Fischer, G., A. Parra-Coronado, and H.E. Balaguera-López. 2022. Altitude as a determinant of fruit quality with emphasis on the Andean tropics of Colombia. A review. Agronomía Colombiana 40(2):212–227. doi: https://doi.org/10.15446/agron.colomb.v40n2.101854.
- Gariglio, N.F., R.A. Pilatti, and M. Agustí. 2007. Requerimientos ecofisiológicos de los árboles frutales, p. 41–82.In: G.O. Sozzi (ed.). Árboles frutales. Ecofisiologia, cultivo y aprovechamiento. Editorial Facultad de Agronomia, Universidad de Buenos Aires, Buenos Aires.
- Gopalan, C., B.V. Ramasastri, and S.C. Balasubramanian. 2004. Nutritive value of Indian Foods, p. 59–67. National Institute of Nutrition (NIN). Indian Council of Medical Research. Hyderabad.
- Gould, K., K. Markham, R. Smith, and J. Goris. 2000. Functional role of anthocyanins in the leaves of Quintinia serrata. A. Cunn. Journal of Experimental Botany 51(347):1107–1115. doi: 10.1093/jexbot/51.347.1107.
- Gunduz, K. 2013. Morphological and phytochemical properties of Mahonia aquifolium from Turkey. Pakistan Journal of Agricultural Sciences 50:439–443.
- Haddy, F., P. Vanhoutte, and M. Feletou. 2006. Role of potassium in regulating blood flow and blood pressure. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology 290(3):R546–552. doi: 10.1152/ajpregu.00491.2005.
- Hammer, O., D. Harper, and P. Ryan. 2001. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontologia Electronica 4:1–9.
- He, J.-M., and Q. Mu. 2015. The medicinal uses of the genus Mahonia in traditional Chinese medicine: An ethnopharmacological, phytochemical and pharmacological review. J. Ethnopharmacol 175:668–683. doi: 10.1016/j.jep.2015.09.013.
- Hjartåker, A., M.D. Knudsen, S. Tretli, and E. Weiderpass. 2015. Consumption of berries, fruits and vegetables and mortality among 10,000 Norwegian men followed for four decades. Eur. J Nutr 54(4):599–608. doi: https://doi.org/10.1007/s00394-014-0741-9.
- Jiang, R., J. Hodgson, E. Mas, K. Croft, and N. Ward. 2015. Chlorogenic acid improves ex vivo vessel function and protects endothelial cells against HOCl-induced oxidative damage, via increased production of nitric oxide and induction of Hmox-1. J. Nutr. Biochem. 27:53–60. doi: 10.1016/j.jnutbio.2015.08.017.
- Jugran, A., W. Younis, A. Bahukhandi, I.D. Bhatt, R. Rawal, and P. Dhyani. 2016. Effect of Processing and Storage Methods on the Nutritional, Anti-nutritional, and Anti-oxidant Properties of Paeonia emodi, Wall. ex. Royle. Wall. Ex. Royle. Applied Biochemistry and Biotechnology 180(2):332–337. doi: 10.1007/s12010-016-2101-0.
- Kakar, M., M. Saeed, K. Luo, I. Suheryani, W. Shuang, Y. Deng, and R. Dai. 2019. Phytochemistry and medicinal values of Mahonia bealei: A review. Tropical Journal of Pharmaceutical Research 18(10):2219–2227. doi: 10.4314/tjpr.v18i10.31.
- Karuppusamy, S., G. Muthuraja, and K.M. Rajasekaran. 2011. Antioxidant activity of selected lesser known edible fruits from Western Ghats of India. Indian Journal of Natural Products and Resources. 2:174–178.
- Khoo, H.E., A. Azlan, S.T. Tang, and S.M. Lim. 2017. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food & Nutrition Research 61(1):1361779. doi: https://doi.org/10.1080/16546628.2017.1361779.
- Latha, R., J.R. Sagaya, and P. Agastian. 2015. Physico-chemical and phytochemical investigation on medicinal plants used by ethnic tribes of Nilgiri Mountains, South India. World Journal of Pharmaceutical Sciences 2015:632–644.
- Li, Y., X. Ji, H. Liu, Y. Yan, and J. Li. 2000. Characterization of 10 species of Mahonia by capillary electrophoresis. Chromatographia 51(5–6):357–361. doi: 10.1007/BF02490617.
- Liu, A.L., and S.Z. He. 2010. Research on species and geographical distribution of Mahonia resources in China. Traditional Chinese Medicine and Clinical Pharmacology 4:20–24.
- Nisha, A.P., and P.M. Radhamany. 2020. Evaluation of proximate and mineral constituents in different commercial cultivars and local varieties of Ananas comosus (L.) Merr. from Kerala. Int. J. Fruit Sci. 20(3):620–634. doi: 10.1080/15538362.2019.1628683.
- Parry, J., L. Su, M. Luther, K. Zhou, M.P. Yurawecz, P. Whittaker, and L. Yu. 2005. Fatty acid composition and antioxidant properties of cold-pressed marionberry, boysenberry, red raspberry, and blueberry seed oils. J. Agric. Food Chem. 53(3):566–573. doi: https://doi.org/10.1021/jf048615t.
- Paudel, K., A. Ramamurthy, and G. Sharma. 2022. Pharmacognostical and phytochemical standardization on stem bark of Mahonia nepalensis DC.: An extrapharmacopoeial plant. Journal of Indian System of Medicine 10(3):155. doi: 10.4103/jism.jism_52_22.
- Prior, R.L., X. Wu, and K. Schaich. 2005. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 53(10):4290–4302. doi: https://doi.org/10.1021/jf0502698.
- Qi, J., W. Liu, T. Jiao, A. Hamblin, and Y. Li. 2020. Variation in morphological and physiological characteristics of wildElymus nutans Ecotypes from different altitudes in the northeastern Tibetan Plateau. Journal of Sensors 2020:2869030. doi: 10.1155/2020/2869030.
- Rana, Y., O.P. Tiwari, and R. Krishan. 2018. Determination of nutritional potential of five important wild edible fruits traditionally used in Western Himalaya. International Journal Life Sciences 6:79–86.
- Rao, R.R., T. Husain, B. Datt, and A. Garg. 1998. Revision of the Family Berberidaceae of the Indian Region-II. Rheedea 8:109–143.
- Rawat, S., A. Jugran, L. Giri, I.D. Bhatt, and R.S. Rawal. 2011. Assessment of antioxidant properties in fruits of Myrica esculenta: A popular wild edible species in Indian Himalayan Region. Evidence-Based Complementary and Alternative Medicine 2011:812787. doi: 10.1093/ecam/neq055.
- Rounsaville, T.J., and T.G. Ranney. 2010. Ploidy levels and genome sizes of Berberis L. and Mahonia Nutt. species, hybrids, and cultivars. HortScience 45(7):1029–1033. doi: 10.21273/HORTSCI.45.7.1029.
- Scalbert, A., C. Manach, C. Morand, C. Rémésy, and L. Jiménez. 2005. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci Nutr 45(4):287–306. doi: https://doi.org/10.1080/1040869059096.
- Sood, P., R. Modgil, and M. Sood. 2010. Physico-chemical and nutritional evaluation of indigenous wild fruit Kasmal. Berberis Lycium Royle. Indian Journal of Natural Products and Resources 3:362–366.
- Suleman, M., A. Khan, and A. Baqi. 2019. Antioxidants, its role in preventing free radicals and infectious diseases in human body. Pure and Applied Biology 8:380–388.
- Suyal, R., A. Bahukhandi, R.S. Rawal, and S. Upadhyay. 2020. Polyphenolics and antioxidant activity of Mahonia jaunsarensis Ahrendt: A narrow endemic to west Himalaya. National Academy Science Letters 43(6):505–508. doi: https://doi.org/10.1007/s40009-020-00916-0.
- Tiwari, U., B. Adhikari, and G. Rawat. 2012. A checklist of berberidaceae in Uttarakhand, Western Himalaya, India. Check List 8(4):610. doi: 10.15560/8.4.610.
- Voronkov, A.S., T.V. Ivanova, E.I. Kuznetsova, and T.K. Kumachova. 2019. Adaptations of Malus domestica Borkh.(Rosaceae) fruits grown at different altitudes. Russian Journal of Plant Physiology 66(6):922–931. doi: https://doi.org/10.1134/S1021443719060153.
- Waterborg, J.H. 2009. The lowry method for protein quantitation, In: pp. 7–10. In: J.M. Walker (ed.). The Protein Protocols Handbook. Humana Press, Totowa, NJ.
- Zhang, M., and X. Hu. 2016. Mechanism of chlorogenic acid treatment on femoral head necrosis and its protection of osteoblasts. Biomedical Reports 5(1):57–62. doi: https://doi.org/10.3892/br.2016.679.