ABSTRACT
To provide scientific and technological support for the smooth development of flowers, regulation of florescence, and cultural management, among others, this study analyzes the corresponding relationship between bioclimatology and the characteristics of growth, development, and reproductive biology of A. sellowiana. Berg (Feijoa). Therefore, through optimized paraffin sections, this study examined the floral bud differentiation in five-year-old A. sellowiana trees and analyzed the dynamic changes in carbohydrate and nitrogenous compound metabolism during floral bud differentiation and flower development under natural growth conditions. The results showed that there were six stages in the differentiation process of A. sellowiana floral buds: the initiation of differentiation, inflorescence primordium differentiation, sepal primordium differentiation, petal primordium differentiation, stamen primordium differentiation and pistil primordium differentiation. Differentiation of floral buds began in late March in the Mianyang region, China, and lasted approximately 30 days. Following bud differentiation, the growth enters a period of bud development lasting approximately five months. Throughout the entire differentiation process, the flower buds contained significantly higher levels of total sugar, starch, soluble sugar, and nucleic acid than those in the leaves, and a peak of total nitrogen, nitrate nitrogen, and sucrose during the physiological transition period. Total nucleic acid and RNA content both increased, with RNA content increasing more significantly.
Introduction
Feijoa, Acca sellowiana is a species from south of Brazil, Paraguay, northern Argentina and Uruguay (Belsham and Orlovich, Citation2003; Canhoto and Cruz, Citation1996) and belongs to the tribe Myrteae (Landrum, Citation1986) and the genus Feijoa, and has been introduced into China in recent years. It is also a vital horticultural crop bearing a delicious fruit, which is economically important in many regions worldwide (Pasquariello et al., Citation2015; Weston, Citation2010). Additionally, feijoa has strong medicinal and health value (Wang et al., Citation2015). Therefore, with the successful introduction of A. sellowiana and the creation of an industrial chain, ornamental plants and tree species can be diversified, and fruit farmers are able to increase their incomes.
In the case of A. sellowiana, flowering occurs only once a year in subtropical environments (Thorp, Citation2008). In contrast to this condition, in the tropics, A. sellowiana trees can flower once, twice or even more times (Fischer and Parra-Coronado, Citation2020; Ramírez and Kallarackal, Citation2018). The whole flowering phase of a single tree can last 4–6 weeks, and the opening period of each flower is 1–2 days. Flowers usually open sequentially from the base to the top of branches (Fachinello and Nachtigal, Citation1992). Javier Garcia et al. (Citation2008) studied the effect of potassium nitrate, potassium phosphate and ethephon in the floral induction of A. sellowiana. Sazima and Sazima (Citation2007) reported that the petals of A. sellowiana (Myrtaceae) are a food source for birds in an urban area in southern Brazil due to their medium size and the showy flowers, as well as their attractiveness to several bird species. Morphological and anatomical studies on the floral apparatus of myrtle, including A. sellowiana, were carried out and found that A. sellowiana has a special floral organ structure that can attract fruit-eating birds, and its possible pollination mechanism is discussed (G. Germán Roitman et al., Citation1997). Yang et al. (Citation2012) defined the pollen-ovule ratio by observing the pollen morphology of 3 A. sellowiana species with a scanning electron microscope and calculating the ovule numbers. Franzon R. C et al. (Citation2005) researched pollen germination of feijoa in vitro. Santos K. L. Santos et al. (Citation2007) found evidence of late self-incompatibility of A. sellowiana by observing and evaluating the development of pollen tubes. Correia and Canhoto (Citation2010) researched the characterization of somatic embryo-attached structures in A. sellowiana. Anomalous somatic embryos in A. sellowiana were studied by Pescador et al. (Citation2008). Pescador et al. (Citation2012) observed the cell structure of a zygote embryo after fertilization, and it was found to be the same as other myrtle plants. Harman (Citation1987) examined the growth and chemical composition of feijoa fruit harvested at different stages of maturity. Parra-Coronado et al. (Citation2015) determined the influence of weather conditions on the physicochemical properties of A. sellowiana fruit growth at two locations with different altitudes in Cundinamarca, Colombia.
As one of the most important stages of the development of advanced plants, flower bud differentiation is also a highly complex process that is influenced by both external environmental factors and internal factors, and understanding the formation mechanism of flower bud differentiation is of great importance for the management and regulation of plant flowering. Nitrogenous compounds and soluble sugars played a vital role in the morphological differentiation of floral buds after the onset of differentiation (Feng et al., Citation2021). Based on these results, it is possible to control the ratio of male to female flower buds in production to increase iron walnut yield (Zhang et al., Citation2022). An analysis of the soluble sugars and nitrogenous compounds contained in flower buds and leaves may provide insight into changes in physiological and biochemical indexes relating to flower bud differentiation in order to analyze the nutrient demand during each stage, thus enabling the regulation of nutrient supply to meet the varying needs of flower bud differentiation.
The differentiation of feijoa floral buds during winter is strictly correlated with flowering in the spring and, ultimately, with fruit production in autumn (Ramírez and Kallarackal, Citation2017). Thus, to determine the possible causes of alternate bearing and improve management practices to correct it, it is necessary to determine the time of flower bud induction. However, at present, few investigations have been conducted on the morphological anatomy and physiological mechanism of flower bud differentiation and flower and fruit development of A. sellowiana, especially in China, one of the new introducing sites of A. sellowiana. Therefore, the present study investigated the morphology of flower bud differentiation and its relationship with the nutrient metabolism of A. sellowiana in Mianyang, China, to lay a foundation for flowering regulation and cultivation management. In this study, the aim is to understand the dynamics of changes in the main physiological indicators of Feijoa fruit in each stage of flower bud differentiation and to determine whether the flower development of Feijoa fruit changes in time of occurrence and internal structure after it was introduced in Mianyang, Sichuan. Taking appropriate management measures and mastering the flowering and bud differentiation pattern will enable us to obtain an adequate quantity and quality flower buds, providing a solid foundation for a high yield.
Materials and Methods
Plant Materials
Inflorescence material was collected from the trees (5 years old) of the variety “unique” of A. sellowiana planted in the experimental orchard of the Southwest University of Science and Technology, Sichuan province, China. The orchard is located at 101° 41‘52“E and 31° 32’ 6” N, with an altitude of 519 m, in a humid subtropical monsoon climate. Its annual average temperature, sunshine, rainfall and frost-free period were 16.3°C, 1298.1 hours, 963.2 mm and 272 days, respectively. The soil was yellow loam with an organic matter content of 18.99% with a pH of 6.88.
The series of the bud and inflorescences were collected and kept in FAA fixative fluid in different developmental stages from January to March, once a time every five days at 9 a.m. The cell differentiation of meristem in the growth points was observed under the anatomic microscope with the paraffin section of the bud or inflorescences. The external morphology of flower buds in the different periods was observed simultaneously.
All the inflorescence or stem apex and leaf buds series (including 6 periods: vegetative growth, physiological transition, floral bud differentiation, floral bud development, beginning of flowering and full bloom stage) were collected from Jan 2, 2018, to May 15, 2018, and part of them was washed and wrapped with tin foil immediately, then put in the liquid nitrogen and save in the ultra-low temperature freezer(- 80°C) for determination of physiological index. The remaining material was heated at 105°C and then dried in the oven at 75°C to the constant weight, which was used to determine biochemical indexes. Each physiological and biochemical index is repeated three times.
Methods
The Observation of External Morphology of Flower Bud
The external morphological characteristics of floral bud development were observed, and floral bud diameter was measured by vernier calipers according to the time of sample taking.
Optimization Method for Paraffin Section Preparation of Flower Bud and Anatomical Study of Flower Bud Differentiation
The optimized method of paraffin section production of floral bud was adopted after the comparative experiment of the research group (Li et al., Citation2016). The trimming material was quickly put into FAA fixing liquid, treated with air extraction for 30 min and fixed for 24 h. The fixed material was soaked and softened in an aqueous solution of hydrochloric acid (V: V = 1:1) for 24 h and slushed off the softener after softening. Gradient dehydration with 70% ethanol (1.5 h), 85% ethanol (1.5 h), 95% ethanol (1.5 h) and anhydrous ethanol (1.5 h for 2 times) and transparent with anhydrous ethanol: xylene (V: V = 1:1) (1.5 h) and pure xylene (1.5 h) was performed. A small amount of paraffin powder of equal volume was added to xylene several times, and the mixed solution was placed in a 40°C oven for 48 h, then the 60°C oven for 2 h with the cap opening. At this stage, the material was placed in pure xylene in a 60°C oven for 3 d, and the pure wax was changed every 6 h. The material was transferred to a small paper box and buried to cool down. The trimmed wax blocks were fixed on the embedding box and sliced into a series of 10 μm thick. With hand wiping and diluting evenly, a drop of glue tablet was dropped on the glass slide, after which distilled water was added, and the wax ribbons were placed on it and stretched in the 40°C oven. The glass slide with the wax bands was dried in a 40°C oven and dyed with alum mordant dyeing of hematoxylin first, then 1% eosin redyeing. Microscopic observation and photography were taken finally. The optimized paraffin section technique obtained complete and continuous wax bands with clear section structure.
Study on the Physiological and Biochemical Indexes During Flower Bud Differentiation
Starch content was determined by the chromogenic iodine method, referring to Woodard (Citation1934). The content of soluble sugar, soluble protein, sucrose, total nitrogen, and nitrate nitrogen was determined by the anthrone colorimetry, coomassie bright blue colorimetry, anthracnose-sulfuric acid colorimetry, and nash colorimetry, salicylic acid colorimetry, respectively. The contents of nucleic acid, DNA and RNA were determined according to the method of Madison et al. (Citation1976). The mass fraction of total sugar was the sum of starch and total soluble sugar.
Results
Observation of the External Body of Floral Bud
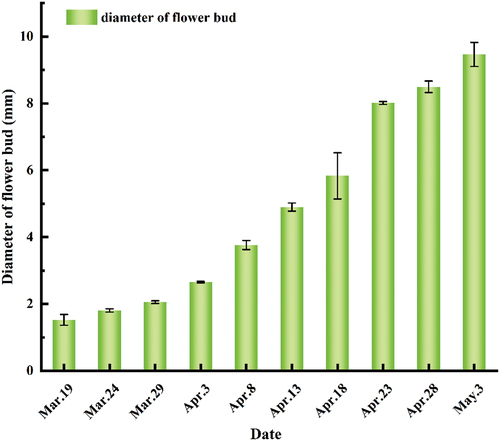
The floral bud differentiation of A. sellowiana began in March. It was found that there was no significant difference between floral bud and leaf bud in external morphology at the early stage of floral bud differentiation. But as the flower bud divided into flowers, their difference gradually increased. The leaf bud begins to grow long, and the top of the floral bud expands gradually as the flower bud develops and becomes circular at last. shows the external morphological measurement results of flower buds. The dynamic curve of floral bud growth showed a high- or low-speed trend. In the early stage of floral bud differentiation, the diameter of the floral bud did not increase significantly, and in the middle and late stages of flower bud development, there was a rapid growth period. That is, from Apr 3, the top of the floral bud significantly expanded and grew into the flower organ development stage until the buds appeared white.
Morphological Anatomy During Flower-Bud Differentiation
Based on the observation of flower bud differentiation sections of A. sellowiana, it was found that flower bud differentiation began in the middle of March in Mianyang city and could be divided into six stages: the initiation of differentiation, inflorescence primordium differentiation, sepal primordium differentiation, petal primordium differentiation, stamen primordium differentiation and pistil primordium differentiation. The specific characteristics in different stages were as follows:
(1) the initiation of differentiation
At the beginning of March, the A. sellowiana trees were still in the vegetative growth stage. The buds that had not begun differentiating were small and covered with scales and young leaves. During this period, anatomical observations of buds revealed that the growth cone was small and slightly pointed, and it was impossible to differentiate between leaf buds and flower buds by their external morphology ().
Figure 2. Flower bud development of A. sellowiana .

(2) inflorescence primordium differentiation
Due to external environmental conditions, the flower buds began to differentiate around Mar 19. The apical meristem underwent a series of morphological and physiological changes and formed the flower primordium. Anatomical observation showed that the growing points of buds began to form semicircular projections (). With the formation and differentiation of flower buds, there were differences in external morphology and anatomical structure. The top of the flower bud gradually expanded, while the leaf bud gradually became pointed, broadened near the base of the petiole, and grew wider than the length. However, the difference between the flower bud’s width and length is not apparent due to the small bud body.
(3) sepal primordium differentiation
Around Mar 24, the growth of the cone gradually flattens out and forms small protuberances of calyx primordium around the growth cone. Since it is a longitudinal section, only two can be seen (). Since the differentiation of the calyx primordium, the sepals developed by the sepals primordium were slightly enlarged with a diameter of about 1.78 mm.
(4) petal primordium differentiation
Around Mar 29, the section showed that the growth point of the bud top continued to widen and flatten. Inside the sepals’ primordium, the new protuberances were differentiated into petal primordium, which continued to rise and eventually developed into petals (). During this period, the top of the flower bud continued to expand, with a diameter of about 2.04 mm, and the leaf bud and flower bud could be clearly distinguished from each other in appearance.
(5) stamen primordium differentiation
Around Apr 3, the section showed many protuberances formed inside the petal primordium, namely the stamen primordium. With the growth of the stamen primordium, the stamen eventually developed ().
(6) pistil primordium differentiation
The tiny protuberances gradually appeared at the flower bud center, which was pistil primordium. Pistil primordium appeared later than stamen primordium and continued to elongate and eventually formed the pistil (). In this stage, the flower bud has grown to a diameter of 3.89 mm, and the differentiation process has been completed, followed by the development of the flower.
Dynamic of Physiological and Biochemical Indexes During Flower Bud Differentiation
Dynamic of Carbohydrate Content During Flower Bud Differentiation
The contents of total sugar, starch, and soluble sugar in the flower bud are significantly higher than in leaves during all flower bud development stages. But their change trend was different (). When flower buds differentiated, they contained a higher level of sugar than leaves, and their sugar content peaked during physiological transformation, which was 12.73% higher than that of the vegetative growth stage, and 2.41 times higher than that of leaves at the same time (). During the physiological transformation stage, both flower buds and leaf buds began to decrease gradually in sugar content, reached their lowest levels during flower bud differentiation, and then began to accumulate during the initial flowering bud development phase up to bloom.
Figure 3. Content of carbohydrates in Leaf and Stem in different development stages.
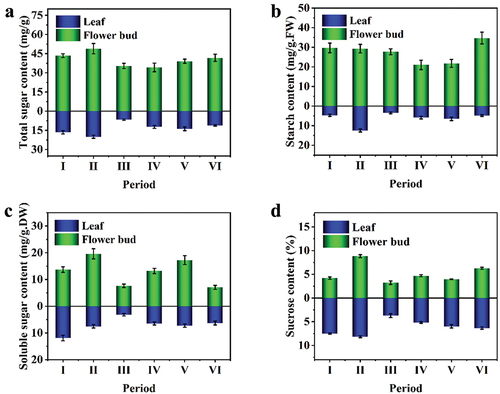
During flower development, the starch content of the leaves and flower buds differed significantly, with the flower buds containing more starch than the leaves (). There was a significant increase in the level of starch in the leaves from the vegetative phase to the physiological transformation phase (P < .05). Then the starch content in the leaves decreased significantly in the flower bud differentiation stage, 72% lower than that in the physiological transformation stage, and a little change from the flower bud differentiation stage to the bloom stage. The starch content in the flower bud decreased gradually from the vegetative growth stage to the flower bud development stage. It decreased to the trough in the flower bud development stage, and the starch content decreased by 24% compared with the flower bud differentiation stage. The starch content in the flower bud began to increase continuously in the flower bud development stage and reached the maximum at 34.68 mg/(g • FW) in the bloom stage ().
As shown in , soluble sugar content in the leaves and flower buds changed significantly during the flower bud development. The soluble sugar content in the leaves during flower bud development was the lowest, significantly different from that in other stages (P < .05). From the flower bud differentiation to the initial flowering stage, soluble sugar content in leaves gradually accumulated. The soluble sugar content in the flower bud was higher than that in the leaves in the same period during the flower development and got a maximum of 19.6 mg/g (DW) in the transition period. The soluble sugar content in flower buds gradually increased from the beginning of flower bud differentiation to the beginning of flowering, and a second peak appeared. The soluble sugar content in flower buds in the full bloom stage was significantly reduced, 59% lower than that at the beginning of the flowering stage.
As shown in , sucrose content in the leaves and flower buds changed significantly during flower bud development. During the vegetative growth stage, the sucrose content in the leaves was 7.48%, 1.78 times higher than that in the stem apex. Sucrose content in the flower bud was the highest (8.82%) at the physiological transformation stage and decreased to 3.24% at the differentiation stage of the flower bud and then increased gradually with the development of the flower buds.
Dynamic of the Nitrogenous Compound During Flower Bud Differentiation
As shown in , the total nitrogen content accumulates to the maximum in both flower buds and leaves during physiological transformation. With the process of flower bud differentiation, total nitrogen content gradually decreased during flower bud differentiation and development and then accumulated again during the flowering stage.
Figure 4. Content of nitrogen compounds in leaf and floral bud during different development stages.
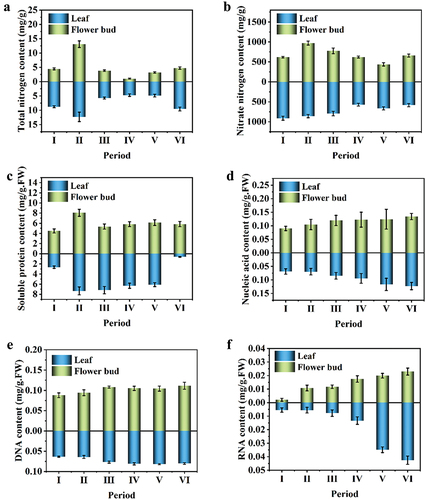
As shown in , the nitrate nitrogen content in leaves decreased continuously, and an accumulation peak did not appear until the beginning of the flowering stage with the development of flower bud differentiation. The nitrate nitrogen content in the flower bud reached the maximum at the physiological transition stage of 971.64 mg/g. Then it began to decrease, showing an upward trend until the flowering period. As shown in , soluble protein content in the leaves significantly increased in the physiological transition stage (P < .05), 80% higher than that in the vegetative growth stage, and there was no significant change in other stages. The soluble protein content in the stem apex obviously changed. At the vegetative growth stage, it was 2.91 mg/g (FW), but at the physical transformation stage, it reached 7.33 mg/g (FW), a 176% increase over the vegetative period. From the physiological transformation stage to the flowering stage, the soluble protein content in the stem apex gradually decreased, and the lowest value was 0.62 mg/g in the flowering stage.
As shown in , the total nucleic acid and DNA content increased continuously, and in flower buds was higher than that in the leaf. The increase of nucleic acid content was more significant in the process of flower bud formation. The content of RNA in leaves and flower buds also increased gradually, but the increase in leaves was more acute than that in floral buds, especially during periods Ⅳ, Ⅴ and Ⅵ.
Discussion
The Division of Flower Bud Stages
A rapid expansion of the apical growth cone of the A. sellowiana tree marks the transition from vegetative growth into reproductive growth. This is followed by an elliptical uplift. Then, at the top, the apex gradually widened and flattened, forming small projections surrounding it known as sepal primordium. The sepals of A. sellowiana usually were 3 or 4 pieces because of anatomical plates, and the two bumps in the slice were being seen. Then the sepals were initially elongated and curved inward and developed into sepals. A continuous process of flower bud differentiation resulted in the formation of petal primordium inside the sepals during the late development stage of the sepals. Its petals were usually 4 or 5 petals (Li et al., Citation2016). Since A. sellowiana was a polyandrous plant, it formed multiple stamen primordium projections inside the petals and eventually developed into stamen. The pistil of A. sellowiana is located in the center of the growth point, gradually protruding upward from the apical growth point.
Floral initiation in sweet cherry occurs after harvest. Sepals, petals, stamens, and pistils differentiate sequentially (Westwood, Citation1993). This is consistent with the sequence of flower bud differentiation of A. sellowiana. According to the morphological and anatomical observation, the flower bud differentiation of A. sellowiana can be divided into six stages: the initiation of differentiation, inflorescence primordium differentiation, sepal primordium differentiation, petal primordium differentiation, stamen primordium differentiation and pistil primordium differentiation. But it differs from Magnolias, which has 5 stages, including the initiation of differentiation, inflorescence primordium differentiation, petal primordium differentiation, stamen primordium differentiation, and pistil primordium differentiation (Fan et al., Citation2018). It began to differentiate flower buds in mid-to-late March. Following the completion of flower bud differentiation, the flower buds entered the development stage and bloomed in May.
The flower bud differentiation of A. sellowiana usually begins in mid-late March, which is closely related to the external environment. In early April, the flower bud enters the primordial differentiation stage of the stamen and pistil, and around mid-April, the ovary and anther are differentiated. In late April, the flower bud differentiation is completed. A. sellowiana generally takes 30 days from the initiation of bud differentiation to the completion of flower development.
It is generally believed that the external morphological sign of flower bud differentiation is the increase in bud size and the change of top shape, and the observation results of this experiment are consistent with this view. In Mianyang, China, a region in the northern hemisphere under subtropical conditions, A. sellowiana trees exhibit a synchronous growth habit that produces a single annual flowering and fruiting event. In the long-term A. sellowiana introduction experiment, we did not observe the phenomenon of flowering twice a year, consistent with New Zealand in the southern hemisphere, but flowers grow in the current season, with buds forming in September (Thorp, Citation2008). In the research of Fernando Ramírez and so on, they observed that the two major flowering events occurred during the two flowering seasons (February and September) in cultivar ‘Criolloʼ trees. However, about 10% of the canopy remained asynchronous throughout the year in Bogotá, Colombia (Ramírez and Kallarackal, Citation2018), indicating the flower bud differentiation of A. sellowiana trees occurs in spring in both the northern and southern hemispheres. Still, the times and time of flower bud differentiation were different in various cultivars and are greatly affected by the external environment, such as temperature, illumination and moisture.
The Relationship Between Sugar and Starch Metabolism and the Flower Development
Besides being a structural component, carbohydrates are also an energy source that plays an important role in the differentiation of flower buds (Buban and Faustt, Citation1982). Its accumulation is closely related to flower bud differentiation. A lot of research has been conducted in this area. Yahata et al. (Citation1995) found that the summer shoot of Wenzhou mandarin orange only accumulated starch but decreased soluble sugar before flower bud initiation. Eshghi et al. (Citation2007) found that sucrose in the shoot tips of induced strawberry plants at 42, 56 and 70 days after the start of the short-day treatment was significantly higher than the corresponding time in non-induced plants. Sugar concentration/metabolism has been reported to regulate the bud growth of Japanese pears in response to internal (genetic) and/or external (environmental) factors and probably make flower bud production modified as a consequence (Ito et al., Citation2004). Chaikiattiyos et al. (Citation1994) found no significant correlation between the flowering number and starch accumulation of lemon trees. The study of Rosa et al. (Citation2000) showed that the starch content in the flower bud of olive trees increased significantly during the flower bud incubation period. However, some experiments can correlate flower formation with the accumulation of carbohydrates (Goldschmidt and Golomb, Citation1982; Smith, Citation1976). Carbohydrate levels are not the sole factor regulating flower formation (Guardiola and García-Luis, Citation2000; Goldschmidt, Citation1999).
Our results also confirmed that carbohydrate is a critical factor in A. sellowiana flower bud differentiation. In all stages of flower bud differentiation, the total sugar content of the flower buds of the A. sellowiana tree was higher than that of the leaves. This peak occurred at physiological differentiation and decreased significantly at flower bud differentiation, indicating that much energy was required for flower buds’ smooth development. The starch content in the leaves reached the maximum during the physiological transformation stage of A. sellowiana and then decreased significantly at the flower bud differentiation stage. However, the starch content gradually rose when the flower bud of A. sellowiana entered the development stage. The starch content in the flower bud continued to decrease from the vegetative growth stage to the flower bud development stage and then increased in the initial flowering stage and the flowering stage, indicating that starch played an important role in the flower bud differentiation process. During flower bud differentiation, soluble sugar content in flower buds showed a double-peak phenomenon. A. sellowiana leaves show an upward trend in soluble sugar content during physiological transformation and initial flowering. During flower bud differentiation, sucrose content in the leaf and flower bud showed the same trend without apparent differences.
Relationship Between Nitrogenous Compounds and Flower Development
Nitrogen is essential to plants’ transformation from vegetative to reproductive growth. High nitrogen content can promote vegetative growth, and ensuring adequate nitrogen supply in soil is an important measure to ensure the healthy development of plants. For example, Phadung et al. (Citation2011) found that urea combined with water stress could induce pummelo tree flowering. However, the excessive nitrogen supply can delay the transformation of vegetative growth to reproductive growth. Many pieces of research have shown that excessive nitrogen supply during the physiological differentiation stage leads to less flowering and ultimately affects the yield of fruit trees. At the same time, nitrogen is the basic nutrient for flower formation. Too little nitrogen will result in the tree lacking nutrients, which will not allow flowers to develop. Proper foliar application of late-season mineral nutrition, including urea+ zinc sulfate+ boric acid improving nitrogenous reserves in kiwifruit vines can result in efficient growth and yield in the following season. The increase in flower N is determined mainly by late-season N foliar application (Ashouri Vajari et al., Citation2018). In this experiment, the content of total nitrogen, nitrate nitrogen and soluble protein in the flower bud of A. sellowiana was relatively high during the physiological differentiation stage of the flower bud. It gradually decreased with the progress of flower bud differentiation. The results were consistent with the above reports.
Protein is the executor of physiological function and the direct embodiment of life phenomenon of all creatures, which affects plant cell division, differentiation and enlargement. During flower induction of loquat, soluble protein content in the leaves on floral shoots rose, while during flower differentiation, it fell sharply. (Liu et al., Citation2011). Chacko (Citation1968) reported that total nitrogen content was higher in the stem and leaves of mango trees, which were expected to initiate flower buds irrespective of the cultivar.
Our results showed that the soluble protein content in the stem apex of A. sellowiana trees was the highest in the physiological transformation stage and gradually decreased with the development of flowers. Due to the fact that protein forms the basis of flower organ construction and plays a major role in flower differentiation and development, further research on protein components and contents in the differentiation process of flower buds of A. sellowiana will be of great significance in determining the molecular regulatory mechanisms responsible for flowering in the future.
Studies have shown that the content of metabolites such as total nitrogen and free amino acids will peak during flower bud differentiation, and the accumulation level of various substances will decline after morphological differentiation. Therefore, these substances have important effects on the flower bud differentiation of fruit trees, and their roles in the flower bud differentiation of A. sellowiana should be further studied.
The nucleic acid and protein content affect cell division; differentiation and enlargement determine plant growth, development and organ formation. The nucleic acid synthesis is the key to the transformation of stem apical meristem from vegetative growth to reproductive growth. There was a close relationship between nucleic acids and flower bud differentiation, which requires the participation of abundant nucleic acid. A study conducted in potted and field-grown loquat trees by Liu et al. (Citation2011) examined how nucleic acids and proteins vary during flower bud formation. Loquat flower buds contained higher amounts of nucleic acids, RNA, DNA, and RNA/DNA ratios than vegetative buds. Madhava Rao and Srinvasan (Citation1971) found that the content of the nucleic acids in grape buds showed a definite pattern of fluctuations during differentiation. Chacko (Citation1968) found no qualitative differences in the composition of free amino acids and amides in the stem and leaves of different mango cultivars during the different months of the study. Li (Citation2005) found that the concentration of DNA and RNA and the RNA/DNA ratio increased gradually in the period of floral bud differentiation of Mango, and the higher concentration of nucleic acid contributed to the floral bud differentiation of Mango.
The results of this study showed that the contents of total nucleic acid, DNA and RNA changed in both bud growing points and leaves. The content of total nucleic acid, DNA and RNA increased during flower bud differentiation, and the content of RNA reached a small peak at the physiological transformation stage, which was the flourishing stage of physiological differentiation. This indicated that DNA replication, mRNA transcription and protein synthesis flourished during this period. It was concluded that protein synthesis was beneficial to flower bud differentiation, and a large amount of protein needs to be consumed in the process of flower bud differentiation. Upon synthesis of protein in the leaves, it was continuously transferred to the flower buds.
Conclusion
The floral bud differentiation of A. sellowiana can be divided into six stages: the initiation of differentiation, inflorescence primordium differentiation, sepal primordium differentiation, petal primordium differentiation, stamen primordium differentiation and pistil primordium differentiation. The floral bud differentiation began in mid-to-late March and lasted about 30 days. After the completion of flower bud differentiation, the flower buds entered the development stage, around 5 months and bloomed around May.
The content of total sugar, starch, soluble sugar and nucleic acid in the flower buds was significantly higher than in the leaves during the flower buds differentiation, and there was an accumulation peak in the physiological transformation stage, as did total nitrogen, nitrate nitrogen and sucrose. Collectively, these results indicated that these substances played an important role in flower bud differentiation during the floral bud differentiation of A. sellowiana.
Acknowledgments
We gratefully acknowledge the financial support from the Breeding Platform of Radiation Mutagenesis Technology in Sichuan Province (2021YFYZ0011) and the Sichuan Province Key R&D Project(2023YFG035).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ashouri Vajari, M., J. Fatahi Moghadam, and S. Eshgh. 2018. Influence of late season foliar application of urea, boric acid and zinc sulfate on nitrogenous compounds concentration in the bud and flower of Hayward kiwifruit. Sci. Hortic. 242(242):137–145. doi: 10.1016/j.scienta.2018.07.029.
- Belsham, S.R., and D.A. Orlovich. 2003. Development of the hypanthium and androecium in South American Myrtoide-ae (Myrtaceae). New Zeal. J. Bot 41(1):161–169. doi: 10.1080/0028825X.2003.9512836.
- Buban, T., and M. Faustt. 1982. Flower bud induction in apple trees Int. Controland differentiation. Hort. Rev 4:174.
- Canhoto, J.M., and G.S. Cruz.1996. Feijoa sellowiana Berg (pineapple guava). In: pp. 155–171. In: ed. Y.P.S. Bajaj. Biotechnolo-gy in Agriculture and Forestry. Vol. 35, Trees IV Springer, Berlin Heidelberg.
- Chacko, E.K. 1968. Studies on the physiology of flowering and fruit growth in mango (Mangifera indica L.). Ph.D. thesis submitted to P. G. School of IARI.
- Chaikiattiyos, S., C.M. Menzel, and T.S. Rasmussen. 1994. Floral induction in tropical fruit trees: Effects of temperature and water supply. Hort. Sci 69(3):397–415. doi: 10.1080/14620316.1994.11516469.
- Correia, S.M., and J.M. Canhoto. 2010. Characterization of somatic embryo attached structures in Feijoa sellowiana Berg. (Myrtaceae). Protoplasma 242(1–4):95–107. doi: 10.1007/s00709-010-0130-z.
- C, F.R., C.E. R, and R.M.D.C. B. 2005. In vitro pollen germination of feijoa (Acca sellowiana (Berg) Burret). Crop Breed. Appl. Biotechnol 5(2):229–233. doi: 10.12702/1984-7033.v05n02a14.
- Eshghi, S., E. Tafazoli, S. Dokhani, M. Rahemi, and Y. Emam. 2007. Changes in carbohydrate contents in shoot tips, leaves and roots of strawberry (Fragaria×ananassa Duch.) during flower-bud differentiation. Sci. Hortic 113(3):255–260. doi: 10.1016/j.scienta.2007.03.014.
- Fachinello, J.C., and J.C. Nachtigal. 1992. Propagacao da goiabeira serrana Feijoa sellowiana Berg, através da mergulhia de- cepa[J]. Sci. Agr. 49(SPE):37–39. doi: 10.1590/S0103-90161992000400007.
- Fan, L., M. Chen, B. Dong, N. Wang, Q. Yu, X. Wang, L. Xuan, Y. Wang, S. Zhang, and Y. Shen. 2018. Transcriptomic Analysis of Flower Bud Differentiation in Magnolia sinostellata. Genes 9(4):212. doi: 10.3390/genes9040212.
- Feng, J.Q., Q. Xia, F.-P. Zhang, J.-H. Wang, and S.-B. Zhang. 2021. Is seasonal flowering time of Paphiopedilum species caused by differences in initial time of floral bud differentiation? AOB Plants. 13(5). doi: 10.1093/aobpla/plab053.
- Fischer, G., and A. Parra-Coronado. 2020. Influence of some environmental factors on the feijoa (Acca sellowiana [Berg] Burret): A review. Agronomía Colombiana 38(3):388–397. doi: 10.15446/agron.colomb.v38n3.88982.
- Goldschmidt, E.E. 1999. Carbohydrate supply as a critical factor for Citrus development and productivity. Hort. Sci 34(6):1020–1024. doi: 10.21273/HORTSCI.34.6.1020.
- Goldschmidt, E.E., and A. Golomb. 1982. The carbohydrate balance of alternate-bearing Citrus trees and the significance of reserves for flowering and fruiting. J. Am. Soc. Hortic. Sci. 107(2):206–208. doi: 10.21273/JASHS.107.2.206.
- Guardiola, J., and A. García-Luis. 2000. Increasing fruit size in Citrus. Thinning and stimulation of fruit growth. Plant Growth Regul. 31:121–132. doi: 10.1023/A:1006339721880.
- Harman, J. 1987. Feijoa fruit: Growth and chemical composition during development. N.Z.J. Exp. Agric 15(2):209–215. doi: 10.1080/03015521.1987.10425561.
- Ito, H., Y.K. Hayama, and Y. Kashimura. 2004. Possible roles of sugar concentration and its metabolism in the regulation of flower bud information in Japanese Pear (Pyrus pyrifolia). Inter. Horti. Congress: Key Processes Growth Cropping Deciduous Fruit Nut Trees 363(636):365–373. doi: 10.17660/ActaHortic.2004.636.44.
- Javier Garcia, O., E. Yiovani Dueñez, G. Fischer, B. Chaves, and O. Camilo Quintero. 2008. Efecto del nitrato de potasio, fosfato de potasio y ethephon en la inducción floral de la feijoa o goiabeira serrana (Acca sellowiana [O. Berg] Burret). Rev. Bras. Frutic. 30(3):577–584. doi: 10.1590/S0100-29452008000300003.
- Landrum, L.R. 1986. Camjomanesia, Pimenta, Blepharocalyx, Legrandia, Acca, Myrrhinium, and Luma (Myrtaceae) [M]. New York Botanical Garden, Bronx (N. Y.).
- Li, G.F. 2005. Studies on the regulation of mango flowering phase and flower bud differentiation[D]. Guangxi university, Nanning.
- Liu, Z.L., S.Q. Lin, and H.B. Chen. 2011. The relationships between nucleic acids, Proteins and flower bud formation in loquat. Acta. Hortic 887(887):197–202. doi: 10.17660/ActaHortic.2011.887.32.
- Li, Z.H., D. Wang, T.Y. Yao, and X.X. Qin. 2016. A Modified technique of paraffin section for feijoa flower buds. null 32(17):35–39.
- Madhava Rao, V.N., and C. Srinvasan. 1971. Nucleic acid composition in the developing buds and petioles of grapes. Vitis 10:210–214.
- Madison, J.T., J.F. Thompson, and A.-M.E. Menuster. 1976. Deoxyribon-ueleioaeid, ribonucleic acid, protein and uncombined amino- acid content of legume seeds during embryogeny. Ann.Bot 40(4):745一756. doi: 10.1093/oxfordjournals.aob.a085188.
- Parra-Coronado, A., G. Fischer, and J. Hernán Camacho-Tamayo. 2015. Development and quality of pineapple guava fruit in two locations with different altitudes in Cundinamarca, Colombia. Bragantia Campinas 74(3):359–366. doi: 10.1590/1678-4499.0459.
- Pasquariello, M.S., F. Mastrobuoni, D. Di Patre, L. Zampella, L.R. Capuano, M. Scortichini, and M. Petriccione. 2015. Agronomic, nutraceutical and molecular variability of feijoa (Acca sellowiana (O. Berg) Burret) germplasm. Sci. Hortic. 191:1–9. doi: 10.1016/j.scienta.2015.04.036.
- Pescador, R., K.G. B, V.L.L. D, L.L. Dal Vesco, H.P. de Freitas Fraga, and M.P. Guerra. 2012. Comparative study of reserve lipid accumulation during somatic and zygotic Acca sellowiana (O. Berg.) Burret embryogenesis. Acta Physiol. Plant. 34(2):771–778. doi: 10.1007/s11738-011-0877-7.
- Pescador, R., K.G. B, J.E. Kraus, W.M. Ferreira, M.P. Guerra, and R.C.L. Figueiredo-Riberio. 2008. Changes in soluble carbohydrates and starch amounts during somatic and zygotic embryogenesis of Acca sellowiana, (Myrtaceae). In vitro Cell. Dev. Biol. Plant 44(4):289–299. doi: 10.1007/s11627-008-9118-1.
- Phadung, T., K. Krisanapook, and L. Phavaphut-Anon. 2011. Paclobutrazol, Water stress and nitrogen induced flowering in ‘Khao Nam Phueng’ Pummelo. Kasets-art J. (Nat. Sci.) 45:189–200.
- Ramírez, F., and J. Kallarackal. 2017. Feijoa [Acca sellowiana (O. Berg) Burret] pollination: A review. Sci. Hortic. 226:333–341. doi: 10.1016/j.scienta.2017.08.054.
- Ramírez, F., and J. Kallarackal. 2018. Phenological growth stages of Feijoa [Acca sellowiana (O. Berg) Burret] according to the BBCH scale under tropical Andean conditions. Sci. Hortic. 232:184–190.
- Roitman, G., N.H. Montaldo, and D. Medan. 1997. Pollination Biology of Myrrhinium atropurpureum(Myrtaceae): Sweet, Fleshy Petals Attract Frugivorous Birds. Bio-tropica 29(2):162–168. doi: 10.1111/j.1744-7429.1997.tb00020.x.
- Rosa, R.D.L., L. Rallo, and H.F. Rapoport. 2000. Olive floral bud growth and starch content during winter rest and spring budbreak [J]. Hortscience 35(7):1223–1227. doi: 10.21273/HORTSCI.35.7.1223.
- Santos, K.L.D., M. Lenzi, C.C. A, A.C.D.M. Dantas, J.P.H.J. Ducroquet, R.O. Nodari, A.I. Orth, and M.P. Guerra. 2007. Evidência da atuação do sistema de auto-incompatibilidade tardia em Acca Sellowiana (berg) burret. (Myrtaceae). Revista Brasileira De Fruticultura 29(1):120–123. doi: 10.1590/S0100-29452007000100026.
- Sazima, I., and M. Sazima. 2007. Petiscos florais: pétalas de Acca sellowiana (Myrtaceae) como fonte alimentar para aves em área urbana no Sul do Brasil. Biota Neotrop. 7(2):307–311. doi: 10.1590/S1676-06032007000200035.
- Smith, P. 1976. Collapse of ‘Murcott’ Tangerine Trees1. J. Am. Soc. Hortic. Sci 101(1):23–25. doi: 10.21273/JASHS.101.1.23.
- Thorp, G. 2008. Feijoa Acca sellowiana (Berg) burret, my-rtaceae, In: pp. 526–533. In: J. Janick and R. Paull (eds.). Encyclopedia of Fruit and Nuts. CAB International, Wallingford.
- Wang, D., Y. X.H, X. S.L, et al. 2015. Nutritional and Bioactive Products from Feijoa (Feijoa sellowiana,Myrtaceae) and Their Application: A Review. J. Food Sci. Biotechnol. 04:337–348.
- Weston, R.J. 2010. Bioactive products from fruit of the feijoa (Feijoa sellowiana,Myrtaceae): a review. Food Chem. 121(4):923–926. doi: 10.1016/j.foodchem.2010.01.047.
- Westwood, M.N. 1993. Temperate-Zone Pomology: Physiology and Culture. 3rd ed. Timber press, Portland Oregon.
- Woodard, H.Q. 1934. Colorimetric Determination of Iodine by the Starch-Iodine Reaction. Ind. Eng. Chem. Anal. Ed. 6(5):331–333. doi: 10.1021/ac50091a014.
- Yahata, D., Y. Oba, and M. Kuwanhara. 1995. Changes in Carbohydrate Levels, .ALPHA.-Amylase Activity, Indoleacetic Acid and Gibberellin-like Substances in the Summer Shoots of Wase Satsuma Mandarin Trees Grown Indoors during Flower-Bud Differentiation. Japen Soc.Hort.Sci 64(3):527–533. doi: 10.2503/jjshs.64.527.
- Yang, X., D. Wang, and M. Zhang. 2012. Pollen-ovule ratio and scanning electron microscope observation to pollen morphology of Feijoa sellowiana. Guihai 32(5):599–602.
- Zhang, W., J. Li, W. Zhang, A. Njie, and X. Pan. 2022. The changes in C/N, carbohydrate, and amino acid content in leaves during female flower bud differentiation of Juglans sigillata. Acta Physiol. Plant. 44(2):19. doi: 10.1007/s11738-021-03328-9.