ABSTRACT
China has abundant germplasm resources of pomegranate. Previous studies have analyzed the fruit quality of the cultivated and wild pomegranates collected from different regions of China. In this study, 45 pomegranate cultivars from Yicheng, Shandong province, China were used as materials. Principal component and cluster analysis was performed based on 18 fruit quality traits. There were significant differences in 18 fruit traits among 45 pomegranate cultivars. Nine principal components (PCs) were extracted by principal component analysis, and the cumulative variance contribution rate was 84.9%. PC1 and PC2 represented 20.9%, 14.3% of total variance respectively. The former was highly positively contributed by total flavonoid and phenolic content of juice, pericarp (FCJ, PCJ, FCP and PCP), and Vitamin C content of juice, while the latter was highly positively correlated with proanthocyanidins and total flavonoid content of seeds (PAS and FCS) and titratable acid content. According to the comprehensive scores, Yushiliu, Gangliu, Mantianhongsuan, Tianlvzi, Xinjianghongpi, Huaiyuanerbenzi, Xiaoqingpi, Damaya, Yichengheshunzhuangwuci, Taishanhong ranked as the top ten. The 45 pomegranate cultivars were clustered into four categories according to 18 fruit quality indicators, each group had unique characteristics. Most cultivars of group III had higher PCJ and proanthocyanidins content of pericarp, while most members of group Ⅳ had higher total anthocyanin contents, FCP and PCP, which suggested that the two groups were rich in functional substances and can be used for further processing. Therefore, the results would provide a method to comprehensively evaluate different cultivars and provide a reference for agricultural cultivation, industrial utilization and breeding.
Introduction
As an important fruit crop, pomegranate (Punica granatum L.) is adaptable to a wide range of agro-climatic conditions and is mainly cultivated in Iran, Afghanistan, India, Tunisia, Turkey, Egypt, Spain, Morocco, USA, China, Israel and South Africa (Kahramanoglu and Usanmaz, Citation2016). China is one of the biggest pomegranate producers in the world. Pomegranates grow in many regions of China, particularly in Shandong, Henan, Shaanxi, Sichuan, Anhui provinces and Chongqing City (Karimi et al., Citation2017; Sarig and Galili, Citation2012).
Pomegranate fruits attract attention not only for their rich nutrients, such as vitamins, carbohydrates and minerals, but also for being rich in many positive health bioactive compounds such as ellagic acid, ellagitannins, punicic acid, anthocyanins, punicalagin, flavonols, flavan−3-ols, flavones, etc (Fuhrman et al., Citation2005; Khan et al., Citation2018; Lansky and Newman, Citation2007; Viuda-Martos et al., Citation2010; Xiao et al., Citation2016). The fruit’s composition comprises 50% edible portion, which includes 40% arils and 10% seeds (Viuda-Martos et al., Citation2010). The health benefits of pomegranate fruits are not only limited to the edible part (arils) but also extend to the non-edible parts, mainly the peel, which contains more biologically active compounds than the edible part (Abid et al., Citation2017; Akhtar et al., Citation2015). Products derived from pomegranate peels can be used for medical treatments for a wide range of diseases and ailments in humans (Hussein and Gouda, Citation2018).
Fruit quality is an important index that includes external characteristics such as size, shape, color and uniformity, as well as internal indicators such as soluble solids, sugar acids, Vitamin C, anthocyanins, flavonoids and other functional components (Nuncio-Jáuregui et al., Citation2014). Previous studies on pomegranate fruit quality mainly focused on comparing the size, weight, edible rate, grain size, grain hardness and intrinsic quality (soluble solids, titratable acid, Vitamin C, total sugar) of different cultivars or clones (Alcaraz-Mármol et al., Citation2017; Chater et al., Citation2018; Chen et al., Citation2022; Melgarejo-Sánchez et al., Citation2015; Nuncio-Jáuregui et al., Citation2014; Zhao et al., Citation2013, Citation2014). Several studies have also analyzed the fruit quality of cultivated and wild pomegranates in China (Chen et al., Citation2022; Guo et al., Citation2022), revealing that China has abundant pomegranate germplasm resources and the fruit quality varied widely by geographic region. Therefore, this study focuses on the fruit quality of 45 pomegranate cultivars in Yicheng, Zaozhuang City, which is one of the eight major pomegranate production areas in China. Cluster analysis and principal component analysis were carried out on 18 quality parameters to provide theoretical references for selecting high-quality pomegranate cultivars and to guide agricultural cultivation, industrial utilization and breeding.
Materials and Methods
Plant Material
Fruits of forty-five cultivars were collected from the China Pomegranate Germplasm Resource Nursery located in Yicheng District, Zaozhuang City, China. The analyzed cultivars included: “Taiansanbaitian,” “Miliu,” “Yichengsanbaitian,” “Lintongsanbai,” “Huaiyuanbaiyushizi,” “Huaibeisanbai,” “Damaya,” “Chaodabaipitian,” “Chaohong,” “Yichengdahongpitian,” “Huaibeihongpitian,” “Linxuan No.2,” “Yushiliu,” “Jingpitian,” “Xinjianghongpi,” “Yushiliu No.3,” “Huaibeihongpiruanzi,” “Henandahongpitian,” “Damantianhongtian,” “Luoke No.4,” “Taihanghong,” “Huanglihongpi No. 1,” “Yushiliu,” “Huaiyuandaqingpitian,” “Mengyanghong,” “Huaibeitashanhong,” “Taishanhong,” “Wanheizipitian No.1,” “Biliu,” “Heyinbopi,” “Mantianhongsuan,” “Lintongqingpitian,” “Huangliqingpi No.2,” “Yichengheshunzhuangwuci,” “Huaiyuanerbenzi,” “Xiaoqingpi,” “Tianlvzi,” “Sichuanqingpi,” “Qiuyan,” “Gangliu,” “Yichengdahongpisuan,” “Huangliqingpi No.1,” “Huanglihongpi No.2,” “Xiaomantianhongtian,” “Yihong No.1.” The trees were all 5 years old, trained in the vase-shaped system and planted with a spacing of 3 m between plants and 5 m between rows. Fifteen fruits per cultivar (3 fruits from each biological replication) were selected at the commercial mature stage and harvested at the beginning of October in 2019, 2020 and 2021. After harvesting, the fruits were immediately transported to the laboratory at Zaozhuang University, Zaozhuang, China.
Physical Characteristics
The fruits were weighed using an electronic balance with an accuracy of ±0.01 g. The husks were carefully cut at the calyx zone and longitudinally cut 5–6 times on the fruit with sharp knives. Then, the arils were manually separated and mixed to allow for the calculation of aril yields (AY). The hundred-aril weight (HAW) were weighted and repeat three times. The juice yield (JY, %) was the weight of juice obtained by pressing 100 g of fleshy arils in a domestic squeezer and filtered through a cloth tissue. The skin, juice and seeds were collected, flash-frozen with liquid nitrogen and stored in a refrigerator at −20°C.
Titratable Acid, Total Soluble Solids, Soluble Sugar and Vitamin C
The titratable acid (TA) of juice was determined by titration to pH 8.1 with 0.1 M NaOH solution and expressed as citric acid percentage according to Chater et al. (Citation2018). The soluble solids content (SSC) was determined with a digital refractometer (PAL−1, Shanghai Xinji Instrument Co., LTD, China, calibrated using distilled water), and results were reported as 0 Brix at 21°C.
The soluble sugar content (SS) of juice was determined using Yemm and Willis’s (Citation1954). Two-milliliter juice was placed into a 100 mL volumetric flask, added 50 mL deionized water, boiled for 30 min, repeated 2 times, then fixed to 100 mL. Two milliliter extracts were collected and placed in a 100 mL volumetric flask. After settling, 0.5 mL extracts were mixed with the anthrone reagent (0.5 mL) (0.5 g anthrone dissolved in 100 mL ethyl acetate) and vitriol (84% cm3) (5 mL). The absorbance was recorded at 620 nm.
The content of Vitamin C was determined by a UV-visible method according to Peng et al. (Citation2020).
Phenolics Extract and Determination
The total phenol extract in the peel, juice and seeds was extracted according to the method of Zhang et al. (Citation2020). The contents of total phenols, total flavonoids and proanthocyanidins were determined using the Folin-Ciocalteu method (Tian et al., Citation2011), aluminum chloride colorimetric method (Qi et al., Citation2001) and n-butanol-hydrochloric acid colorimetric method (Waterhouse et al., Citation2000), respectively.
To extract anthocyanins, 25 milliliters of pomegranate juice was added to a 50 mL brown volumetric flask, then 20 mL of 1% hydrochloric acid and anhydrous methanol extract were added. The mixture was extracted in the dark for 12 h at room temperature, and the volume was fixed to 50 mL. The total anthocyanin content was determined according to the pH differential method described by Lako et al. (Citation2007).
Statistical Analysis
The data was processed using Excel 2013. Standardized data were subjected to principal component analysis and cluster analysis using SAS. Hierarchical associations were obtained by employing the Squared Euclidean distance method as a dissimilarity measure.
Results and Discussion
Physical Characteristics
Fruit size is one of the most important characteristics that influence consumer preferences in pomegranates (Holland et al., Citation2009), with commercially valuable fresh market fruit typically weighing over 400 g (Blumenfeld et al., Citation2000). In , the fruit weight (FW) of 45 pomegranate cultivars ranged from 177.8 g (Tianlvzi) to 689.2 g (Damaya) (311.3 g mean value), similar to the range of 196 to 674 g for 30 pomegranate accessions (363 g mean value) in Tunisia (Hasnaoui et al., Citation2011). The FW was generally higher than in previous reports, such as 106.6 ~ 496.9 g for 100 pomegranate genotypes in Iran (Khadivi et al., Citation2018), 168.9 ~ 574.9 g for eight ones in Italy (Ferrara et al., Citation2011), but lower than in previous reports, such as 289.25 g to 1072.35 g for 20 cultivars in China (Chen et al., Citation2022), 300.10 ~ 854.63 g for six clones grown in Spain (Melgarejo-Sánchez et al., Citation2015). The largest number of cultivars (42.2%) had a weight in the range of 200 ~ 300 g, while 20.0% weighted 400 g. The wide variation in FW may fluctuate depending on climatic and agricultural conditions except for genotypes (Ferrara et al., Citation2011; Melgarejo-Sánchez et al., Citation2015).
Table 1. Fruit characteristics of 45 pomegranate cultivars.
As the key trait for evaluating the edible portion, the hundred-aril weight (HAW) varied from 25.6 g (Lintongqingpitian) to 78.2 g (Huaiyuanbaiyushizi) with a mean of 41.0 g (). This was similar to previous research from Tunisian that reported a HAW of 34.8 g to 70.3 g (Hasnaoui et al., Citation2011) and from China that reported a HAW of 24.26 g to 68.40 g (Chen et al., Citation2022). The HAW of most cultivars ranged from 30 g to 40 g accounted for 53.3%, while those over 50 g accounted for 17.8%. The average HAW of 41.0 g in our present study was lower than 49.8 g for 30 Tunisia accessions (Hasnaoui et al., Citation2011) and 47.3 g for 20 Chinese cultivars (Chen et al., Citation2022), but much higher than 35.4 g for 100 Iranian genotypes (Khadivi et al., Citation2018).
The aril yields (AY) ranged from 31.3% to 56.1% with a mean of 42.1% (), which was lower than 59.7% for Taifi cultivars (Al-Maiman and Ahmad, Citation2002), 41.1 ~ 65% for Iranian cultivars (Tehranifar et al., Citation2010), 52.67 ~ 65.87% for Spanish accessions (Hernández et al., Citation2014), 41.5 ~ 60.1% for California cultivars (Chater et al., Citation2018). The AY of cultivars ranged from 35% to 45%, accounting for 60.0% of all cultivars, while cultivars with AY over 50% accounted for only 8.9%.
The juice yield (JY) of 100 g arils ranged from 68.1% to 78.8% (), with a minimum coefficient of variation (3.99%). The JY of most cultivars was more than 70%, accounting for 88.9% of all cultivars.
Chemical Parameters of Pomegranate Fruits
The soluble solid content (SSC) ranged from 11.2 to 18.8 °Brix (14.28% of the mean value) (), which was similar to the reported 13.7 ~ 19.1°Brix in other reports (Alcaraz-Mármol et al., Citation2017; Dafny-Yalin et al., Citation2010; Ferrara et al., Citation2011, Citation2014; Khadivi et al., Citation2018; Martínez et al., Citation2012). The coefficient of variation was 8.28%, and the SSC of most cultivars ranged from 14.0 to 16.0 °Brix, accounting for 51.1% of all cultivars.
The content of titratable acid (TA) varied from 0.25% to 4.97% (), which was similar to 0.37 ~ 4.6% reported in the northern regions (e.g., Turkey, Russia, Georgia and Macedonia), but higher than the result reported by Chen et al. (Citation2022). The coefficient of variation was maximal (145.72%). The TA of 42 cultivars was distributed in the range of 0.24%~0.65%, while that of the other 3 cultivars was higher than 3.5%.
The soluble sugar content (SS) ranged from 46.9 to 99.9 mg·L−1 (68.0 mg·L−1 of the mean value) (). The SS of 29 cultivars ranged from 50 mg·L−1 to 80 mg·L−1, accounting for 64.4% of all cultivars, while 11 cultivars had SS levels exceeding 80 mg·L−1.
As a nutritional parameter, Vitamin C (VC) content of juice was concentrated in 0.953 ~ 9.980 mg·100 g−1 (5.183 mg·100 g−1 FW of mean value) (), which was lower than 9.91 ~ 20.92 mg ·100 g−1 reported in 20 Iranian pomegranate genotypes (Tehranifar et al., Citation2010) and the 4.60 ~ 10.10 mg ·100 g−1 reported in 20 Chinese cultivars (Chen et al., Citation2022). VC content of 30 cultivars was more than 4.0 mg·100 g−1, accounting for 66.7% of all cultivars.
Content of Phenolic Substances in Pomegranate Fruits
Phenolic compounds have been extensively investigated in many fruits (Djeridane et al., Citation2006). In fruits, they are associated with color, sensory characteristics (flavor, astringency and hardness), nutritional characteristics and antioxidant activity (Robbins, Citation2003).
Singh et al. (Citation2018) collected total phenol content (TPC) data of pomegranate varieties from different geographical locations and various reports. The TPC in the peel of different varieties/cultivars varied, and the TPC of the extractions with different solvents was also different. Malviya et al. (Citation2014) reported that the water-ethanol extract of the peel contained 297.5 mg tannic acid equivalents (TAE) ·g−1 phenolics. In our study, we used 70% ethanol to extract the phenolics, and the results showed that TPC in the peel (PCP) varied from 17.70 to 131.53 mg TAE·g−1FW (), which was lower probably because of fresh weight. The PCP of 37 cultivars varied from 30.0 to 90.0 mg·g−1 FW, accounting for 82.2% of all cultivars, while the cultivars with content higher than 90.0 mg·g−1 FW accounted for 11.1% of all cultivars.
Table 2. Fruit phenolic characteristics of 45 pomegranate cultivars.
The flavonoid content of peel (FCP) ranged from 2.973 to 21.585 mg catechin equivalents (CE) g −1FW (), while Guo et al. (Citation2022) showed that it varied from 16.11 to 72.50 mg·g−1 DW of 50 wild and cultivated pomegranates in China. Previous studies showed FCP of pomegranate varieties in different geographical locations from different reports varied from 5.83 mg to 257 mg CE g−1 (Singh et al., Citation2018). FCP of 32 cultivars ranged from 6.0 to 13 mg·g−1FW, accounting for 71.1% of all cultivars.
The proanthocyanidins content of peel (PAP) ranged from 0.049 to 1.982 mg·g−1 FW (). The PAP of 22 cultivars ranged from 0.30 to 0.60 mg·g−1 FW, accounting for 48.9% of all cultivars.
The TPC in the juice (PCJ) ranged from 0.692 to 2.643 mg·g−1FW (), which was less than in the peel, consistent with previous studies (Elfalleh et al., Citation2012). PCJ of 27 cultivars ranged from 0.80 to 1.60 mg·g−1FW, accounting for 60.0% of all cultivars.
The TFC of the juice (FCJ) ranged from 0.038 to 0.348 mg (catechin) ·g−1 FW (), with the minimum coefficient of variation among the phenolic indexes. FCJ of 34 cultivars was lower than 0.25 mg·g−1 FW, accounting for 75.6% of all cultivars, while those with a content higher than 0.25 mg·g−1 FW accounted for 24.4%.
The PAC of juice (PAJ) varied from 0.007 mg·g−1FW to 0.362 mg·g−1 FW (). The PAJ of 32 cultivars distributed in 0.04 ~ 0.12 mg·g−1 FW, accounting for 71.1% of all cultivars, while those higher than 0.12 mg·g−1 FW accounted for 15.6%.
Anthocyanins are indicator of color. Among the 45 pomegranate cultivars, the juice of seven white cultivars had no anthocyanins. The total anthocyanin contents (TAC) of the remaining 38 cultivars ranged from 0.91 mg·L−1 to 103.09 mg·L−1 (), which was lower than 4.90 ~ 255.80 mg·L−1 in 20 Chinese cultivars (Chen et al., Citation2022), and 1.8 ~ 175 mg·L−1 in Tunisia and Iranian pomegranate cultivars, respectively (Akhavan et al., Citation2015; Hasnaoui et al., Citation2011). The TAC of 18 cultivars ranged from 20 mg·L−1 to 40 mg·L−1 accounted for 40.0%, while 6 cultivars had a TAC higher than 40 mg·L−1.
The TPC, TFC and PAC of seeds (PCS, FCS and PAS) varied from 0.045 to 0.973 mg·g−1 FW, 0.032 mg·g−1 FW to 0.210 mg·g−1 FW, 0.004 to 0.059 mg·g−1 FW, respectively (). There were 36 cultivars with PCS, FCS and FAS lower than 0.60 mg·g−1 FW, 0.15 mg·g−1 FW, and 0.025 mg·g−1 FW, accounting for 80.0% of all cultivars.
Feng et al. (Citation2016) analyzed TPC and TFC in different parts of eight cultivars in China, which showed that they were lower in seeds than that in juice. However, our results showed that, except for Xiaomantianhongtian, Huangliqingpi No. 1 and Huanglihongpi No.2, PCS of other cultivars was lower than PCJ; except for Sichuanqingpi, Yichengdahongpisuan, Huanglihongpi No. 2, Xiaomantianhongtian, Yushiliu and Yihong No. 1, FCS of other cultivars was lower than FCJ; while except for Tianlvzi and Yichengdahongpisuan, PAS was lower than PAJ in other cultivars.
Variations of Quality Indexes
The variation coefficients of fruit quality indexes of 45 pomegranate cultivars varied greatly, ranging from 3.99% to 145.72%. As shown in , the coefficient of variation of SSC, JY and ER was small and below 20%, especially the one for JY, which was the smallest (3.99%). The coefficient of variation of TA was higher than 100%. The coefficients of PCS, FCS and PAS were higher than the ones of PCP, FCP, PAP, while the latter was higher than the ones of PCJ, FCJ and PAJ ().
Principal Component Analysis
Principal component analysis (PCA) can simplify multiple indexes into a few comprehensive indexes, and it has been used to evaluate the principal components of various comprehensive indexes of pomegranates (Alcaraz-Mármol et al., Citation2017; Chen et al., Citation2022; Melgarejo-Sánchez et al., Citation2015). In this study, PCA was conducted on 18 fruit quality indexes after standardized processing. As shown in , the cumulative contribution rate of the first 9 principal components (PCs) reached 84.9%, indicating that these 9 PCs could reflect most of the information of the 18 quality indexes. Therefore, these 9 PCs can be selected as the comprehensive evaluation indexes of pomegranate fruit quality.
As shown in and , PC1 represented 20.9% of the total variance and was highly positively contributed by FCJ, FCP, PCJ, PCP, VC content of juice, but it was highly negatively contributed by PCS and HAW. PC2 accounted for 14.3% of the total variance, and was highly positively correlated with PAS, FCS and TA, but negatively correlated with HAW ( and . PC3 accounted for 11.3% of the total variance and was highly positively linked to ER and JY, but highly negatively with SS and SSC. PC4 accounted for 9.2% of the total variance. Among the total variance, the PCP and VC had the largest positive and negative loading values, respectively. PC5 accounted for 8.0% of the total variance and was highly positively correlated with PAJ and FW, but highly negatively correlated with ER. PC6 accounted for 7.0% of the total variance and was more highly positively linked to SS, FCJ, TAC while more highly negatively linked to PAP and TA. PC7, PC8 and PC9 respectively accounted for 5.1%, 4.9% and 4.2% and were more highly positively linked to SSC, PAP, and TA.
Figure 1. (A) Plot of contribution of the physico-chemical investigated traits into the first and second principal components. (B) Plot of the first and second principal components resulting from a PCA of the pomegranate cultivars using physico-chemical characters. 1, Mantianhongsuan;2, Damaya; 3, Yichengdahongpisuan; 4, Taihanghong; 5, Heyinbopi; 6, Yichengdahongpitian;7, Xinjianghongpi; 8, Huaibeitashanhong; 9, Xiaomantianhongtian;10, Yushiliu No. 3; 11, Huaibeisanbai;12, Yichengheshunzhuangwuci;13, Biliu; 14, Huangliqingpi No. I ; 15, Huaibeihongpitian;16, Taiansanbaitian;17, Lintongqingpitian;18, Damantianhongtian; 19, Henandahongpitian; 20, Yihong No.1; 21, Yichengsanbaitian; 22, Luoke No. 4; 23, Chaodabaipitian; 24, Chaohong; 25, Jingpitian; 26, Linxuan No.2; 27, Gangliu; 28, Lintongsanbai;29, Mengyanghong; 30, Huaibeihongpiruanzi;31, Tianlvzi; 32, Huaiyuanbaiyushizi; 33, Sichuanqingpi; 34, Taishanhong; 35, Huanglihongpi No.2; 36, Wanheizipitian No.1; 37, Miliu; 38, Qiuyan; 39,Huangliqingpi No. 2; 40, Xiaoqingpi; 41, Huaiyuanerbenzi; 42, Huaibeiqingpitian; 43, Huaiyuandaqingpitian; 44, Huanglihongpi No.1; 45, Yushiliu.
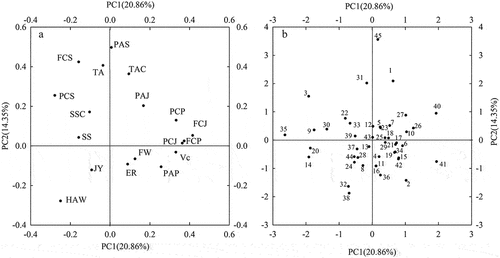
Table 3. Eigenvalues and proportion of variation associated with each principal component in 45 pomegranates.
The biplot () showed that Xiaoqingpi and Huaiyuanerbenzi had large positive scores on the PC1, while Huanglihongpi No.2 had the largest negative score on the PC1 axis. Yushiliu had largest positive score on the PC2 axis, which was opposed to Huaiyuanbaiyushizi and Qiuyan. In , there were 13 cultivars with both positive PC1 and PC2 values, including Mantianhongsuan, Heyinbopi, Xinjianghongpi, Yushiliu No.3, Yichengheshunzhuangwuci, Damantianhongtian, Chaodabaipitian, Jingpitian, Linxuan No.2, Gangliu, Xiaoqingpi, Huaiyuandaqingpitian, Yushiliu. There were 8 cultivars with a negative PC1 value and a positive PC2 value, including Yichengdahongpisuan, Xiaomantianhongtian, Luoke No.4, Huaibeihongpiruanzi, Tianlvzi, Sichuanqingpi, Huanglihongpi No.2 and Huangliqingpi No. 2. There were 10 cultivars with both negative PC1 and PC2 values, including Huaibeitashanhong, Biliu, Huangliqingpi No.1, Yihong No.1, Chaohong, Lintongsanbai, Huaiyuanbaiyushizi, Miliu, Qiuyan and Huanglihongpi No.1. There were 8 cultivars with negative PC1 value and positive PC2 value, including Yichengdahongpisuan, Xiaomantianhongtian, Luoke No.4, Huaibeihongpiruanzi, Tianlvzi, Sichuanqingpi, Huanglihongpi No.2 and Huangliqingpi No. 2. There were 14 cultivars with both positive PC1 value and negative PC2 value, including Damaya, Taihanghong, Yichengdahongpitian, Huaibeisanbai, Huaibeihongpitian, Taiansanbaitian, Lintongqingpitian, Henandahongpitian, Yichengsanbaitian, Mengyanghong, Taishanhong, Wanheizipitian No.1, Huaiyuanerbenzi and Huaibeiqingpitian.
A Comprehensive Evaluation of Utilization Value of 45 Pomegranate cultivars’ Fruits
Based on the functional expression of each principal component, the score value for each principal component of 45 pomegranate cultivars was calculated (). The cultivars with highest scores of PC1~PC9 were Huaiyuanerbenzi, Yushiliu, Mantianhongsuan, Yushiliu No.3, Yushiliu, Mengyanghong, Huaiyuandaqingpitian, Damaya and Mantianhongsuan. These cultivars were suggested to have an advantage as parent material for pomegranate quality breeding. The synthetical score was calculated as the accumulated sum of the factors’ score of each sample and the weight value of each principal component (Nie et al., Citation2019). The 45 cultivars were ranked according to their comprehensive scores. The higher the score, the better the overall comprehensive characteristics of fruit quality. The top ten cultivars were Yushiliu, Gangliu, Mantianhongsuan, Tianlvzi, Xinjianghongpi, Huaiyuanerbenzi, Xiaoqingpi, Damaya, Yichengheshunzhuangwuci and Taishanhong. This indicated that these varieties have great potential as parent materials for quality breeding.
Table 4. Principal component scores and comprehensive scores of the fruit quality of 45 pomegranate cultivars.
Cluster Analysis
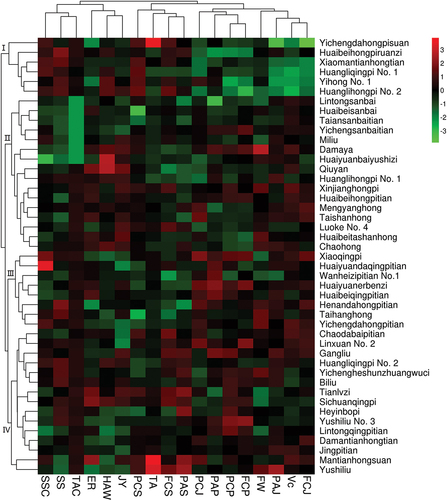
All data were normalized before cluster analysis. The heat map showed that 45 pomegranate cultivars could be divided into four subcategories (). The first category (Yichengdahongpisuan, Huaibeihongpiruanzi, Xiaomantianhongtian, Huangliqingpi No.1, Huanglihongpi No.2 and Yihong No.1) consisted of six pomegranate cultivars. This group had low VC, FCJ, PAJ contents and high PCS contents, which were different from the other three groups. The second category had 16 pomegranate cultivars, including 7 white ones. Most cultivars had SS content lower than 60 mg/L, and Vc content higher than 5 mg/100gFW, accounting for 84.5% and 75%, respectively. The third category (Xiaoqingpi, Huanyuandaqingpitian, Wanheizipitian No.1, Huaiyuanerbenzi, Huaibeiqingpitian, Henandahongpitian, Taihanghong and Yichengdahongpitian) had eight pomegranates. Most cultivars had higher PCJ than 1.50 mg·g−1FW and higher PAP than 0.80 mg·g−1FW, accounting for 87.5% and 75.0%, respectively. The fourth category (Chaodabaipitian, Linxuan No.2, Gangliu, Huangliqingpi No.2, Yichengheshunzhuangwuci, Biliu, Tianlvzi, Heyinbopi, Sichuanqingpi, Yushiliu No.3, Lintongqingpitian, Damantianhongtian, Jingpitian, Mantianhongsuan and Yushiliu) had fifteen pomegranates. Most had higher TAC than 18 mg·L−1, higher FCP than 10 mg·g−1FW, and higher PCP than 50 mg·g−1FW, accounting for 80.0%, 73.3% and 100%, respectively.
Figure 2. Clustering heat map of 45 pomegranates. FW, fruit weight ; HAW, hundred-aril Weight;ER, edible rate; JY, juice yield; SSC, soluble solid content; TA, titratable acid content; SS, contents of soluble sugar; Ve, Ve content; TAC, total anthocyanins content of juice; PCP, total phenolic content of pericarp; PCJ, total phenolic content of juice; PCS, total phenolic content of seeds; FCP, total flavonoid content of pericarp; FCJ, total flavonoid content of juice; FCS, Total flavonoid content of seeds; PAP, proanthocyanidins content of pericarp; PAJ, proanthocyanidins content of juice; PAS, proanthocyanidins content of seeds. Each small square shows the quality index of the pomegranates, and the values were standardized.
Conclusion
Fruit quality is the main factor that determines the competitiveness of the fruit market. There were significant differences in 18 quality indexes among 45 pomegranate cultivars, with their variation coefficients ranging from 3.99% to 145.72%. Principal component analysis of these 18 quality indicators concluded that the first 9 principal component factors contribute 84.9%, representing the majority of pomegranate quality information. This information can be used for comprehensive and objective evaluation of pomegranate fruit quality. The contribution value of PC1 was the largest, which mainly reflected the content of phenolic substances and Vc in juice and should be paid more attention. The 10 cultivars including Yushiliu, Gangliu, Mantianhongsuan, Tianlvzi, Xinjianghongpi, Huaiyuanerbenzi, Xiaoqingpi, Damaya, Yichengheshunzhuangwuci, and Taishanhong can be used as parental materials for quality breeding.
Acknowledgments
We thank Mr. Zhaoxiang Hao from Yicheng Pomegranate Research Institute (Zaozhuang Pomegranate National Tree Germplasm Resources Bank) for providing pomegranate fruits.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Abid, M., H. Yaich, S. Cheikhrouhou, I. Khemakhem, M. Bouaziz, H. Attia, and M.A. Ayadi. 2017. Antioxidant properties and phenolic profile characterization by LC–MS/MS of selected Tunisian pomegranate peels. J Food Sci Technol 54(9):2890–2901. doi: 10.1007/s13197-017-2727-0.
- Akhavan, H., M. Barzegar, H. Weidlich, and B.F. Zimmermann. 2015. Phenolic compounds and antioxidant activity of juices from ten Iranian pomegranate cultivars depend on extraction. J. Chem. 2015:1–7. doi: 10.1155/2015/907101.
- Akhtar, S., T. Ismail, D. Fraternale, and P. Sestili. 2015. Pomegranate peel and peel extracts: Chemistry and food features. Food Chem. 174:417–425. doi: 10.1016/j.foodchem.2014.11.035.
- Alcaraz-Mármol, F., N. Nuncio-Jáuregui, F. García-Sánchez, J.J. Martínez-Nicolás, and F. Hernández. 2017. Characterization of twenty pomegranate (Punica granatum L.) cultivars grown in Spain: Aptitudes for fresh consumption and processing. Sci. Hortic. 219:152–160. doi: 10.1016/j.scienta.2017.03.008.
- Al-Maiman, S.A., and D. Ahmad. 2002. Changes in physical and chemical properties during pomegranate (Punica granatum L.) fruit maturation. Food Chem. 76(4):437–441. doi: 10.1016/S0308-8146(01)00301-6.
- Blumenfeld, A., F. Shaya, and R. Hillel. 2000. Cultivation of pomegranate. Options méditerranéennes. Série A, Séminaires méditerranéens 42:143–147.
- Chater, J.M., D.J. Merhaut, Z.Y. Jia, P.A. Mauk, and J.E. Preece. 2018. Fruit quality traits of ten California-grown pomegranate cultivars harvested over three months. Sci. Hortic. 237:11–19. doi: 10.1016/j.scienta.2018.03.048.
- Chen, Y.H., H.F. Gao, S. Wang, X.Y. Liu, Q.X. Hu, Z.H. Jian, R. Wan, J.H. Song, and J.L. Shi. 2022. Comprehensive evaluation of 20 pomegranate (Punica granatum L.) cultivars in China. Journal Of Integrative Agriculture 21(2):434–445. doi: 10.1016/S2095-3119(20)63389-5.
- Dafny-Yalin, M., I. Glazer, I. Bar-Ilan, Z. Kerem, D. Holland, and R. Amir. 2010. Color, sugars and organic acids composition in aril juices and peel homogenates prepared from different pomegranate accessions. J. Agr. Food Chem. 58(7):4342–4352. doi: 10.1021/jf904337t.
- Djeridane, A., M. Yousfi, B. Nadjemi, N. Vidal, J.F. Lesgards, and P. Stocker. 2006. Screening of some Algerian medicinal plants for the phenolics compounds and their antioxidant activity. Eur. Food Res. Technol. 224(6):801–809. doi: 10.1007/s00217-006-0361-6.
- Elfalleh, W., H. Hannachi, N. Tlili, Y. Yahia, N. Nasri, and A. Ferchichi. 2012. Total phenolic contents and antioxidant activities of pomegranate peel, seed, leaf and flower. J. Med. Plants Res. 6(32):4724–4730. doi: 10.5897/JMPR11.995.
- Feng, L.J., Y.L. Yin, Q.Q. Jiao, X.M. Yang, and C. Wu. 2016. “Study on the phenolic compounds and antioxidant activity in fruits of different pomegranate cultivars.” Journal Of Nuclear Agricultural Science 30 (4):0710–0718. In Chinese.
- Ferrara, G., I. Cavoski, A. Pacifico, L. Tedone, and D. Mondelli. 2011. Morpho-pomological and chemical characterization of pomegranate (Punica granatum L.) genotypes in Apulia region, Southeastern Italy. Sci. Hortic. 130(3):599–606. doi: 10.1016/j.scienta.2011.08.016.
- Ferrara, G., A. Giancaspro, A. Mazzeo, S.L. Giove, A.M.S. Matarrese, C. Pacucci, R. Punzi, A. Trani, G. Gambacorta, A. Blanco, et al. 2014. Characterization of pomegranate (Punica granatum L.) genotypes collected in Puglia region, Southeastern Italy. Sci. Hortic. 178:70–78. doi: 10.1016/j.scienta.2014.08.007.
- Fuhrman, B., N. Volkova, and M. Aviram. 2005. Pomegranate juice inhibits oxidized LDL uptake and cholesterol biosynthesis in macrophages. J. Nutr. Biochem. 16(9):570–576. doi: 10.1016/j.jnutbio.2005.02.009.
- Guo, L.H., D.P. Ge, Y. Ren, J.M. Dong, X.Q. Zhao, X.Q. Liu, and Z.H. Yuan. 2022. The comparative analysis and identification of secondary metabolites between Tibet wild and cultivated pomegranates (Punica granatum L.) in China. Journal Of Integrative Agriculture 21(3):736–750. doi: 10.1016/S2095-31192163642-0.
- Hasnaoui, N., R. Jbir, M. Mars, M. Trifi, A. Kamal-Eldin, P. Melgarejo, and F. Hernandez. 2011. Organic acids, sugars, and anthocyanins contents in juices of Tunisian pomegranate fruits. Int. J. Food Prop. 14(4):741–757. doi: 10.1080/10942910903383438.
- Hernández, F., P. Legua, R. Martínez, P. Melgarejo, and J.J. Martínez. 2014. Fruit quality characterization of seven pomegranate accessions (Punica granatum L.) grown in Southeast of Spain. Sci. Hortic. 175:174–180. doi: 10.1016/j.scienta.2014.05.035.
- Holland, D., K. Hatib, and I. Bar-Ya’akov. 2009. Pomegranate: Botany, horticulture, breeding. Hortic Rev (Am Soc Hortic Sci) 35:127–191.
- Hussein, L., and M.E.L. Gouda. 2018. Pomegranate: Cultivation, pomological properties, processing, global market and health benefits. pomegranate: Cultivation, antioxidant and health benefits; Food science and technology. Nova Science Publisher, New York, NY, USA.
- Kahramanoglu, I., and S. Usanmaz. 2016. Pomegranate production and marketing. CRC Press.
- Karimi, M., R. Sadeghi, and J. Kokini. 2017. Pomegranate as a promising opportunity in medicine and nanotechnology. Trends Food Sci. Tech. 69:59–73. doi: 10.1016/j.tifs.2017.08.019.
- Khadivi, A., D. Ayenehkar, M. Kazemi, and A. Khaleghi. 2018. Phenotypic and pomological characterization of a pomegranate (Punica granatum L.) germplasm collection and identification of the promising selections. Sci. Hortic. 238:234–245. doi: 10.1016/j.scienta.2018.04.062.
- Khan, H., M. Jawad, M.A. Kamal, A. Baldi, J. Xiao, S.M. Nabavi, and M. Daglia. 2018. Evidence and prospective of plant derived flavonoids as antiplatelet agents: Strong candidates to be drugs of future. Food Chem. Toxicol. 119:355–367. doi: 10.1016/j.fct.2018.02.014.
- Lako, J., V.C. Trenerry, M. Wahlqvist, N. Wattanapenpaiboon, S. Sotheeswaranc, and R. Premier. 2007. Phytochemical flavonols, carotenoids and the antioxidant properties of a wide selection of Fijian fruit, vegetables and other readily available foods. Food Chem. 101(4):1727–1741. doi: 10.1016/j.foodchem.2006.01.031.
- Lansky, E.P., and R.A. Newman. 2007. Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J Ethnopharmacol 109(2):177–206. doi: 10.1016/j.jep.2006.09.006.
- Malviya, S., A. Jha, A. Jha, and N. Hettiarachchy. 2014. Antioxidant and antibacterial potential of pomegranate peel extracts. J. Agric. Sci. Technol. 51(12):4132–4137. doi: 10.1007/s13197-013-0956-4.
- Martínez, J.J., F. Hernández, H. Abdelmajid, P. Legua, R. Martínez, A.E. Amine, and P. Melgarejo. 2012. Physico-chemical characterization of six pomegranate cultivars from Morocco: Processing and fresh market aptitudes. Sci. Hortic. 140:100–106. doi: 10.1016/j.scienta.2012.04.002.
- Melgarejo-Sánchez, P., J.J. Martínez, P. Legua, R. Martínez, F. Hernández, and P. Melgarejo. 2015. Quality, antioxidant activity and total phenols of six Spanish pomegranates clones. Sci. Hortic. 182:65–72. doi: 10.1016/j.scienta.2014.11.020.
- Nie, Z., C. Wan, C. Chen, and J. Chen. 2019. Comprehensive evaluation of the postharvest antioxidant capacity of Majiayou pomelo harvested at different maturities based on PCA. Antioxidants 8(5):136. doi: 10.3390/antiox8050136.
- Nuncio-Jáuregui, N., A. Calín-Sánchez, A.A. Carbonell-Barrachina, and F. Hernández. 2014. Changes in quality parameters, proline, antioxidant activity and color of pomegranate (Punica granatum L.) as affected by fruit position within tree, cultivar and ripening stage. Sci. Hortic. 165:181–189. doi: 10.1016/j.scienta.2013.11.021.
- Peng, Y.S., G.B. Wang, F.L. Cao, and F.F. Fang‑Fang Fu. 2020. Collection and evaluation of thirty‑seven pomegranate germplasm resources. Applied Biological Chemistry 63(1):15. doi: 10.1186/s13765-020-00497-y.
- Qi, X.Y., X.H. Wang, and J.H. Rong. 2001. “Study on the effect of apple polyphenol extracts on scavenging hydroxyl radicals.” Science And Technology Of Food Industry 22 (4):7–9. In Chinese.
- Robbins, R.J. 2003. Phenolic acids in foods: An overview of analytical methodology. J. Agr. Food Chem. 51(10):2866–2887. doi: 10.1021/jf026182t.
- Sarig, Y., and A. Galili. 2012. The pomegranate industry in China - current status and future challenges, In: pp. 261–264. In: P. Melgarejo and D. Valero (eds.). II international symposium on the pomegranate. CIHEAM/Universidad Miguel Hernández, Zaragoza.
- Singh, B., J.P. Singh, A. Kaur, and N. Singh. 2018. Phenolic compounds as beneficial phytochemicals in pomegranate (Punica granatum L.) peel: A review. Food Chem. 261:75–86. doi: 10.1016/j.foodchem.2018.04.039.
- Tehranifar, A., M. Zarei, Z. Nemati, B. Esfandiyari, and M.R. Vazifeshenas. 2010. Investigation of physico-chemical properties and antioxidant activity of twenty Iranian pomegranate (Punica granatum L.) cultivars. Sci. Hortic. 126(2):180–185. doi: 10.1016/j.scienta.2010.07.001.
- Tian, S.F., Y. Wang, G. Du, and Y.X. Li. 2011. Changes in contents and antioxidant activity of phenolic compounds during gibberellin- induced development in Vitis vinifera L. ‘Muscat’. Acta Physiol. Plant. 33(6):2467–2475. doi: 10.1007/s11738-011-0791-z.
- Viuda-Martos, M., J. Fernández-López, and J.A. Pérez-Álvarez. 2010. Pomegranate and its many functional components as related to human health. Comprehensive Reviews In Food Science And Food Safety 9(6):635–654. doi: 10.1111/j.1541-4337.2010.00131.x.
- Waterhouse, A.L., S. Ignelzi, and J.R. Shirley. 2000. A comparison of methods for quantifying oligomeric proanthocyanidins from grape seed extracts. Am. J. Enol. Vitic. 51(4):383–389. doi: 10.5344/ajev.2000.51.4.383.
- Xiao, J., E. Capanoglu, A.R. Jassbi, and A. Miron. 2016. Advance on the Flavonoid C -glycosides and Health Benefits. Crit Rev Food Sci Nutr 56(sup1):S29–S45. doi: 10.1080/10408398.2015.1067595.
- Yemm, E.W., and A.J. Willis. 1954. Then estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 57(3):508–514. doi: 10.1042/bj0570508.
- Zhang, Y., Q.Y. Zou, S. Wu, Z.L. Zhao, X. Yan, L.H. Zhang, and W. Tan. 2020. “Comparison of phenolic content and antioxidant capacity in fruit parts of different pomegranate varieties.” Journal Of Agriculture 10 (12):57–67. In Chinese.
- Zhao, X., Z. Yuan, Y. Fang, Y. Yin, and L. Feng. 2013. Characterization and evaluation of major anthocyanins in pomegranate (Punica granatum L.) peel of different cultivars and their development phases. Eur. Food Res. Technol. 236(1):109–117. doi: 10.1007/s00217-012-1869-6.
- Zhao, X., Z. Yuan, Y. Fang, Y. Yin, and L. Feng. 2014. Flavonols and flavones changes in pomegranate (Punica granatum L.) fruit peel during fruit development. J. Agric. Sci. Technol. 16:1649–1659.
