ABSTRACT
Chinese cherries are early flowering and early fruiting, with both edible and ornamental values. This study aims to explore the effects of pre-harvest spraying with different concentrations of CaCl2, amino acid calcium, and sugar alcohol calcium on the quality of Chinese cherry and the expression of genes related to cell wall softening. The goal is to identify suitable calcium-based agents for pre-harvest spraying on Chinese cherry. The results of the study demonstrated that all three exogenous calcium treatments significantly increased the mineral content compared to the control group. Moreover, these treatments also led to a significant increase in various fruit quality parameters such as hundred fruit weight, firmness, soluble solid, soluble sugar, titratable, vitamin C, soluble protein, β-Carotene content and superoxide dismutase, peroxidase activities. Additionally, they were found to effectively reduce rot rate, polyphenol oxidase activity, malondialdehyde content and relative conductivity. The activity of pectin methylesterase, polygalacturonase, cellulase and β-galactose was reduced by amino acid calcium and sugar alcohol calcium. All three types of exogenous calcium down-regulated the expression of PME and ChCaBP1. Additionally, amino acid calcium and sugar alcohol calcium decreased the expression of PG, Cx, and BGal. Exogenous calcium reduces the size of stomata in the fruit epidermis, leading to a decrease in both fruit respiration rate and transpiration. Of the three calcium preparations, amino acid calcium and sugar alcohol calcium were more effective than CaCl2, with 0.12 g·L−1sugar alcohol calcium and 0.06 g·L−1 amino acid calcium being the most effective.
Introduction
Chinese cherry (Cerasus pseudocerasus Lindl.), which belongs to Prunus of Rosaceae and is native to China, is one of the four main cherry species in the world (Wang et al., Citation2018, Citation2022). Chinese cherry is early flowering and early fruiting, with good adaptability and resistance, high yield, good fresh flavor quality, and both edible value and ornamental value (Guo et al., Citation2022). It plays an increasingly important role in the rural revitalization strategy and the development of a characteristic fruit industry in China (Zhang et al., Citation2018). According to statistics from the Chinese Horticultural Society’s Cherry Branch, the planting area of cherries in China has exceeded 6.67 × 104 ha and the yield has exceeded 5 × 105 tons as of 2020 (Wu et al., Citation2021). Chinese cherry is characterized by small size, thin peel, intolerant storage, high decay rate and short shelf life. Therefore, it is of high practical value to carry out research on safe, environmentally friendly and low-cost preservation technology of Chinese cherries.
Calcium is essential for fruit development and plays a crucial role in maintaining cell wall structure stability. It also enhances antioxidant capacity in both the cell wall and membrane structures (Mu et al., Citation2022). Ca2+ in cell tissue interacts with cell walls to synthesize Ca2+ glycan crosslinked polymers, which contribute to the formation of pectin polymers, form cell wall networks, enhance mechanical strength, and restrict cell wall hydrolase entry (Zhang et al., Citation2018, Citation2021). Calcium deficiency in fruit trees can result in stunted growth of stems, roots, and flowers. It can also cause chlorosis in young leaves, increase susceptibility to fruit diseases, and reduce the overall quality, yield, and resistance of the fruits. Apple bitter pox (Sun et al., Citation2022), cherry cracking (Michailidis et al., Citation2021), mango spongy tissue disease (Wainwright and Burbage, Citation2015) and tomato navel rot (Saravanan et al., Citation2021) are all related to calcium deficiency in fruit trees, so it is important to supplement calcium fertilizer in time.
Calcium fertilizer can improve the nutritional value of fruits and vegetables and has been widely used as a preservative and compactor for fruits and vegetables. Calcium fertilizer significantly increased the calcium content in fruit cell walls, the content of bound calcium in fruit cell walls, inhibited the degradation of pectin, cellulose and hemicellulose, reduced the content of arabinose and galactose, and increased the water-soluble pectin in fruit cell walls (Mohebbi et al., Citation2020; Xu et al., Citation2020). Spraying CaCl2 during grape fruit development up-regulated the expression of genes related to cell wall and pathogen defense, and reduced the decay of grape berries after harvest (Martins et al., Citation2021). Winkler and Knoche (Citation2019) reported that spraying 0.7% Ca (OH)2 and 0.5% CaCl2 before harvest reduced the fruit decay rate of sweet cherry. Dong et al. (Citation2019) indicated that spraying Ca (NO3)2 before harvest improved the fruit hardness, soluble solids and titratable acid content of sweet cherry.
Amino acid calcium and sugar alcohol calcium have been popularized and applied as new foliar fertilizers in fruit tree plantings, but there are few studies on the effects on fruit growth and development. Therefore, this study aimed to explore the effects of pre-harvest spraying of CaCl2, amino acid calcium and sugar alcohol calcium on the fruit quality, cell wall enzyme activity and expression of key genes of Chinese cherry, with a view of exploring the foliar calcium fertilizer most suitable for Chinese cherry preservation.
Materials and Methods
Fruit Materials and Postharvest Treatments
The experiment was conducted at the Cherry Base in Xiaba Town, Wudang District, Guiyang City, Guizhou Province (N26°50′2.15″, E106°53′24.01″). The test materials were 5-year-old Chinese cherries and the variety was “Black Pearl,” with a spacing of 3.5 m × 3.5 m.
The calcium preparations were CaCl2 (analytically pure), amino acid calcium water-soluble fertilizer (amino acid ≥100 g·L−1, Ca ≥30 g·L−1) and sugar alcohol calcium water-soluble fertilizer (saccharin ≥100 g·L−1, N ≥50 g·L−1, Ca ≥180 g·L−1). All preparations were from Sichuan Guoguang agrochemical Co., Ltd.
Three single factor treatments were set as shown in , with sprayed CaCl2, amino acid calcium, and sugar alcohol calcium as the test group, and water was sprayed as control (CK). For each treatment, 10 trees without pests or diseases and with consistent growth were selected. Spraying was conducted on the leaves and fruits at 7 d, 17 d, and 27 d after flowering, based on the presence of water droplets on the leaf surface and fruit surface. Cherry fruits were harvested when ripe (the color of the cherry fruit changes from yellow to red, and soluble solids are around 12% per cent) and immediately transported back to the laboratory of the College of Agriculture, Guizhou University after dissipating the field heat. The fruits with the same size, maturity and no mechanical damage were selected for the determination of relevant indicators. 3 kg of fruit per treatment and all treatments included three biological replicates.
Table 1. Experimental treatments and numbers.
Determination of Mineral Elements
The extraction and quantification of mineral elements in cherry fruits were conducted as in Wang et al. (Citation2022). The contents of N, P, K, Ca, Mg, Fe, Cu, and Zn were determined win an inductively coupled plasma emission spectrometer (ICP-AS) (OPTIMA3300DV, Perkin Elmer, Shelton, CT, USA). The argon flow rate was 15 L·min−1, and the injection volume was 1.5 mL·min−1.
Determination of Hundred Fruit Weight, Rot Rate and Firmness
Hundred Fruit Weight Was Measured Using an Electronic Balance
Cherry fruits were stored at 4 ± 1°C for one week to measure the rot rate. The rotting rate was defined as the proportion of fruits with a rotting area of more than 30%. Rot rate (%) = (number of rotting fruits/number of test fruits) × 100% calculation.
Firmness was measured by a hand-held hardness tester (GY-2, Yueqing adebao Instrument Co., Ltd.). One value was taken for each fruit, and six fruits were measured for each treatment. The firmness value was expressed in kg·cm−2.
Soluble solid (TSS), titratable acid (TA), soluble sugar (SS), vitamin C (Vc), β-Carotene and soluble protein (SP) content
TSS and TA were measured by a digital hand-held refractometer (PR101-α, Atago, Japan), and expressed as %. Three drops of juice from one segment were used and repeated twice per fruit, 10 single fruit replicates were performed.
SS was detected by anthrone colorimetry (Peng et al., Citation2023). A 0.5 mL sample extract was mixed with 1.5 mL distilled water, 0.5 mL ethyl anthranilate reagent, and 5.0 mL concentrated sulfuric acid by vortexing. The mixture was boiled in a water bath for 1 min, removed, and cooled to room temperature. The absorbance (OD) values were measured at 630 nm using a UV-5500 UV-Visible spectrometer (Shanghai Metash Instruments, Shanghai, China). The results were expressed as the mass fraction.
Vc was assayed by titration using a solution of 2, 6- dichlorophenol indophenol (Gao et al., Citation2018). A total of 10 mL of the sample extract in a 100 mL triangular flask was titrated with the calibrated 2,6-dichlorophenol indophenol solution until the color appeared red and did not fade for 15 s.
The content of β-Carotene was determined by U-3000 high performance liquid chromatograph (Thermo Fisher Scientific Co., Ltd.). 0.5 g of the fruit sample was accurately weighed after grinding with liquid nitrogen, and 5 mL of the extract containing 0.01% BHT (n-hexane: acetone: anhydrous ethanol = 2:1:1 mixture of extracts) was added, and the sample was centrifuged for 5 min at 12,000 r/min under the condition of 4°C with vortexing and shaking for 2 min at room temperature to collect supernatant, and then the extraction was repeated two times. The supernatant was blown to dryness with a nitrogen blower, and then reconstituted with 1 mL of the extract containing 0.01% BHT, and ultrasonicated for 10 min to dissolve it completely, filtered through a 0.22 μm membrane, and then stored in a brown injection bottle, and analyzed by liquid chromatography tandem mass spectrometry (LC-MS/MS). Chromatographic conditions: C6-100B (250 mm × 4.6 mm, 5 μm); column temperature 35°C; mobile phase methanol-hexane (60:40, v/v); injection volume 20 μL; flow rate 0.8 mL·min−1; detection wavelength 450 nm.
SP was detected by Coomassie brilliant blue G-250 staining. A 1 mL of sample extraction solution and 5 mL of Coomassie brilliant blue G-250 solution were mixed, left to stand for 2 min, and the absorbance value measured at 595 nm. The result was expressed as the soluble protein mass per gram of fruit (g·kg−1).
Superoxide Dismutase (SOD), Peroxidase (POD) and Polyphenol Oxidase (PPO) Activity
SOD and POD activity was evaluated following the method described by Zeng et al. (Citation2012) with minor modifications. For SOD determination, the reaction mixture (3 mL) contained 50 mmol·L−1 sodium phosphate buffer (pH 7.8), 130 mmol·L−1 methionine, 750 μmol·L−1 nitro-blue tetrazolium (NBT), 100 μmol·L−1 EDTA-Na2, 20 μmol·L−1 riboflavin and 0.1 mL of the enzyme extract. The mixtures were illuminated by light (4000 lx) for 20 min at 30°C and the absorbance was then determined at 560 nm. Identical solutions held in the dark served as blanks. For POD determination, 0.5 mL of enzyme extract was incubated in 2 mL buffered substrate (100 mM sodium phosphate, pH 6.4 and 25 mM guaiacol) for 5 min at 30°C and the increasing absorbance measured at 470 nm every 30 s for 180 s after adding 0.2 mL H2O2 (0.5 mol·L−1).
PPO activity was assayed according to Sofo et al. (Citation2004) with some modifications. The reaction mixture (3.0 ml final volume) consisted of 0.1 ml of 25 mM pyrogallol, 2.8 ml of 100 mM NaPi, pH 7.0, and 100 μl of the enzyme extract. The mixture was maintained at 30°C for 30 min and the activity was read at 420 nm.
Malondialdehyde (MDA) Content, Relative Conductivity (REC) and pH
MDA was assayed based on the method reported by Sofo et al. (Citation2004) with minor modifications. For this, 0.3 g of frozen fruit powder was used for MDA detection. The results are expressed on the basis of fresh weight as μmol·g−1.
REC was measured by the conductivity meter method (DDS-11A, INESA Scientific Instrument Co., Ltd.).
The pH was measured using an FE20 laboratory pH meter (Mettler Toledo instruments (Shanghai) Co., Ltd.).
Extraction and Assay of Cell Wall Degrading Enzymes
Take 1 g of fruit tissue, placed in a pre-cooled mortar, and 6 mL of pre-cooled 40 mM pH 5.5 sodium acetate buffer (containing 1 mol·L−1 NaCl and 10 g·L−1 PVPP) were added, ground in an ice bath, extracted at low temperature for 1 h, and then centrifuged at 12,000× g for 20 minutes at 4°C. The supernatant was collected to determine the activities of the pectin methyl esterase (PME), polygalacturonase (PG), cellulase (Cx), and β-galactose (β-Gal) maturation enzymes.
PME activity was assayed as described by Ren et al. (Citation2020) with some modification. The activity was measured in a mixture containing 0.1 mL of distilled water, 0.6 mL of 0.5% (w/v) pectin, 0.15 mL of 0.05% bromothymol blue and 0.1 mL of enzyme extract. The mixture was incubated at 37°C for 30 min and the absorbance at 620 nm was recorded. The decrease of 0.01 per minute at OD620 nm was taken as one enzyme activity unit, expressed in U·g−1.
PG activity was assayed according to the methods Ren et al. (Citation2020) with some modification. The reaction mixture was 0.2 mL of 40 mM sodium acetate buffer (pH 4.6), 0.2 mL of 1% (w/v) polygalacturonic acid and 0.1 mL of enzyme extract. They were incubated at 37°C for 1 h and the reaction was terminated by adding 0.5 mL of 3,5-dinitrosalicylic (DNS) acid and boiling for 5 min. The absorbance of the reaction solution was measured at a wavelength of 540 nm. The amount of enzyme required to produce 1 μmol of galacturonic acid per hour was used as one unit of enzyme activity, expressed as U·g−1.
Cx activity was measured by the method of Ren et al. (Citation2020) with slight modification. The assay mixture, including 0.5 mL of 1% (w/v) carboxy-methylcellulose and 0.1 mL of enzyme extract, was incubated at 37°C for 1 h. The reaction was terminated by adding 2.5 mL of DNS and boiling for 5 min. The absorbance was measured at 540 nm. The amount of enzyme used to produce 1 μmol of glucose per hour was used as one unit of enzyme activity, expressed as U·g−1.
β-Gal activity was measured by the method of Chen et al. (Citation2017). A mixture of 2 mL of 10 g·L−1 p-nitrophenyl-β-D-galactopyranoside and 0.2 mL of crude enzyme was held at 37°C for 1 h. After warming, 2 mL of 0.2 mol·L−1 Na2CO3 were added and mixed, then cooled, and the absorbance measured at 400 nm. The amount of enzyme used to generate 1 μmol of p-nitrophenol per hour was used as one enzyme activity unit U, expressed as U·g−1.
Detection of Epidermal Stomatal Structure
No more than 1 cm × 1 cm of area was cut from the fruit peel, the surface of which was sprayed with platinum after lyophilization, and the stomatal structure of the peel surface was observed using a biomicroscope (CX43, Olympus CO., LTD). Ten peel sections were evaluated for per treatment, with at least 10 fields of view selected for per treatment and five morphologically intact stomata and defense cells selected for measurement in per field of view.
Real-Time Fluorescence Quantitative PCR Detection of Gene Expression
A RNeasy Plant Mini Kit (Takara, Japan) was used to extract RNA from cherry fruit (1.0 g) in strict adherence to the manufacturer’s procedure. The first-strand cDNA synthesis was achieved from 1 μg total RNA, following the instructions provided by the manufacturer of Reverse-iT™ 1st Strand Synthesis Kit (Takara, Japan) was used.
A real-time quantitative PCR experiment system including 10 μL of SYBR™ Green qPCR Master Mix, 1 μL of template cDNA and 1 μL of 10 μM forward and reverse primers were added to each well in a 20 μL reaction volume. Fluorescent quantitative PCR results were analyzed by 2−ΔΔt method (Silva et al., Citation2021). 18sRNA was used as internal reference. Primers designed using Primer 5.0 software (Zhao et al., Citation2021) are presented in .
Table 2. Primers used in the study.
Data Analysis
SPSS (SPSS Inc., Chicago, IL, USA) was used to perform the analysis of data relating to relevant indicators, multiple comparisons were made using the ANOVA and Duncan multi-range test (p ≤ .05). Plots were drawn using Origin Pro 2018 (OriginLab Corporation., Northampton, Massachusetts, USA) to plot the graphs.
Results
Effects of Foliar Calcium Fertilizer on Mineral Elements in Fruit
Calcium treatment increased the contents of N, P, K, Ca, Mg, Fe, Mn and Zn in fruits, and had significant effects on N, P, Fe and Zn (). Different exogenous calcium treatments had different effects on different mineral elements. The CaCl2 treatment was more beneficial for the absorption of Mn, the amino acid calcium was more beneficial for the absorption of N and Ca, and the sugar alcohol calcium was more beneficial for the absorption of K and Mg. In addition, the Zn content in amino acid calcium and sugar alcohol calcium treatments was higher than that in the CaCl2 treatment, while there was no significant difference in P and Fe content among the three exogenous calcium treatments.
Table 3. Effects of foliar calcium fertilizer on mineral elements.
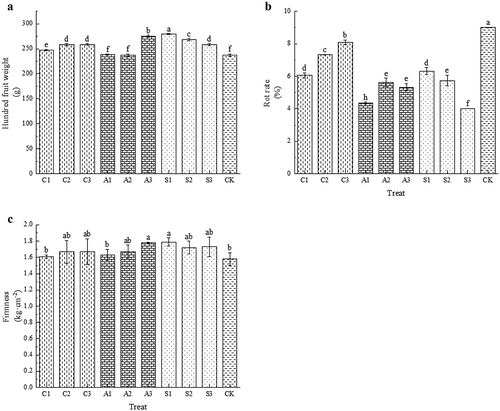
Effects of Foliar Calcium Fertilizer on Hundred Fruit Weight, Rot Rate and Firmness
Exogenous calcium effectively increased the hundred fruit weight of cherry. The hundred fruit weight of cherry treated with sugar alcohol calcium was higher than that treated with amino acid calcium and CaCl2. The hundred fruit weight treated with S1 was 1.18 times higher than that of CK (). The exogenous calcium treatment reduced the rot rate of fruit. The rot rate of each calcium treatment was 4.01%~8.10%, which was significantly lower than CK (p < .05). Among them, the amino acid calcium treatment and the sugar alcohol calcium treatment were lower than the CaCl2 treatment, and the lowest rot rate of S3 treatment was 4.01% (). The exogenous calcium treatment increased fruit firmness, with the sugar alcohol calcium treatment being more firm than CaCl2 and amino acid calcium treatment (). Exogenous calcium treatment improved the external quality of cherry fruit and had a positive effect on prolonging its postharvest preservation. Among them, the sugar alcohol calcium and amino acid calcium treatments had better effects than CaCl2, and S1 and A3 treatment had the best effect.
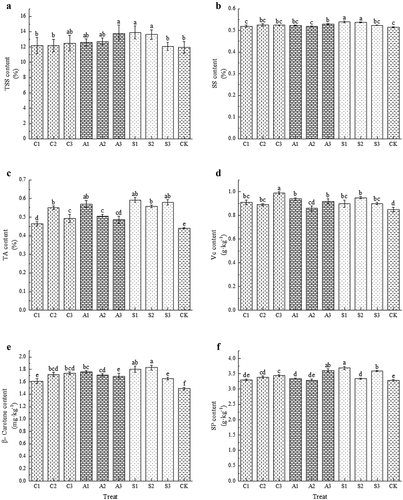
Effects of Foliar Calcium Fertilizer on the Flavor and Quality
Foliar calcium fertilization increased the TSS content, with A3, S1, and S2 showing significant differences compared to CK (p < .05), and the S1 treatment having the highest TSS content of 13.90% (). Foliar calcium spray increased SS content, with S1 and S2 treatments significantly higher than the other treatments (p < .05), and the S1 treatment having the highest SS content of 0.539% (). Calcium treatment significantly increased TA content (p < .05), with the highest TA content in the S1 treatment, which increased TA by 34.24% compared to CK (). Calcium treatment increased the Vc content, with the highest Vc content of 0.99 g·kg−1 in C3 treatment, which was 14.67% higher than CK (). Calcium treatment increased β-Carotene content, and all calcium treatments were significantly higher than CK (p < .05). The content of S2 treatment was the highest, and each calcium treatment increased 8.05%~22.82% compared with CK (). Calcium treatment increased SP content, with A1, S1, and S3 increasing SP by 10.06%, 12.50%, and 9.45% respectively compared to CK, and theS1 treatment having the highest SP content (). In conclusion, the three types of exogenous calcium treatments improved the flavor and quality of cherry fruits to some extent, with the S1 treatment showing the best results.
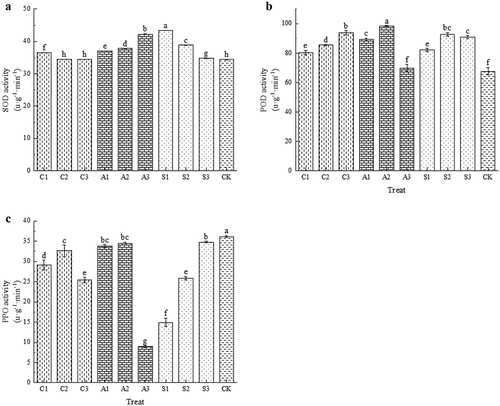
Effects of Foliar Calcium Fertilizer on SOD, POD and PPO Activities
Calcium treatment increased SOD activity, with the best effect observed in the S1 treatment, which showed a 26.10% improvement compared to CK (). Calcium treatment increased POD activity, and all treatments were significantly higher than CK except A3, among which A2 treatment had the best effect (). Calcium treatment significantly reduced PPO activity and varied significantly between different concentrations of the same treatment, with A3 and S1 treatments having significantly lower contents than the other treatments (). Exogenous calcium treatment increased the activity of SOD and POD, decreased the activity of PPO, and had the positive effects of reducing the damage of active oxygen to postharvest fruit and reducing fruit browning.
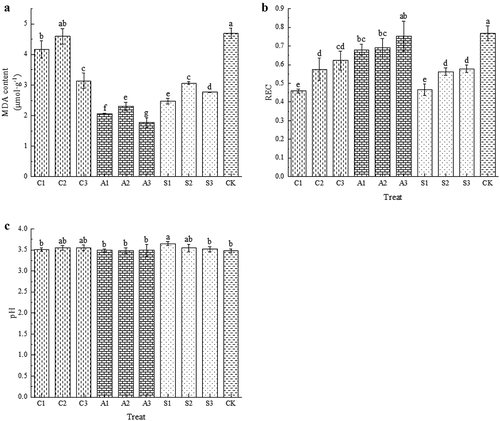
Effects of Foliar Calcium Fertilizer on MDA, REC and pH
There were significant differences in the effects of three types of exogenous calcium on MDA in fruits. Treatment with sugar alcohol calcium and amino acid calcium significantly reduced MDA content (p < .05), while CaCl2 treatment only reduced MDA content in C3 treatment, with A3 having the lowest MDA content (). The REC of fruits treated with exogenous calcium decreased compared to CK. Except for A3, all other treatments were significantly lower than CK (p < .05), with C1 and S1 having the lowest REC (). Calcium treatment had little effect on the pH, and the pH of each treatment was between 3.48 and 3.65 ().
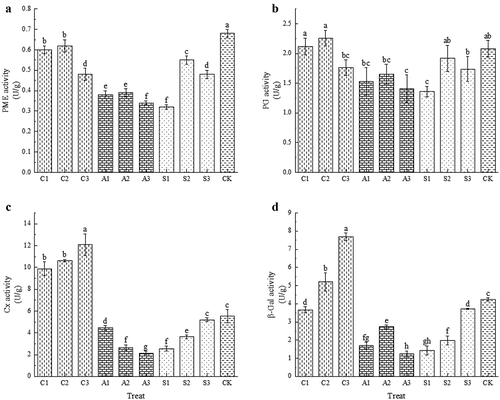
Effect of Exogenous Calcium Treatment on PME, PG, Cx and β-Gal Activities
All three exogenous calcium sources significantly reduced the PME activity (p < .05), with A1 and S1 having the lowest PME activity, 50.00% and 52.94% lower than CK, respectively (). The effect of the three exogenous calcium sources on PG activity was not significant, except for A3 and S1 which significantly reduced PG activity (p < .05) by 32.21% and 34.62%, respectively, compared to CK (). Both calcium amino acids and calcium glycolate effectively reduced Cx activity and β-Gal activity, with A3 having the lowest Cx activity and β-Gal activity, which were significantly reduced by 61.12% and 70.69%, respectively, compared with CK (). Thus, preharvest spraying of amino acid calcium and sugar alcohol calcium effectively reduced the activities of PME, PG, Cx and β-Gal, while spraying CaCl2 effectively reduced the activity of PME, but caused an increase in the activity of Cx and β-Gal.
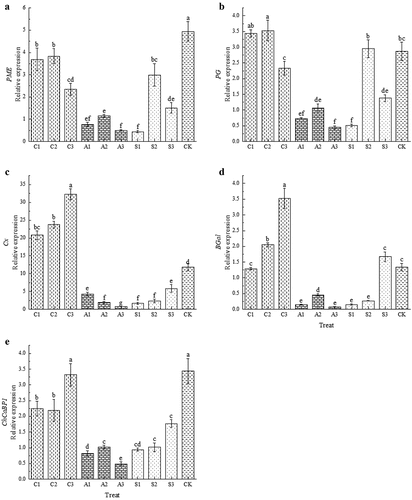
Effect of Foliar Calcium Fertilizer on Gene Expression Related to Fruit Cell Wall Softening
Three different calcium treatments significantly reduced the expression of PME. Among these treatments, CaCl2 showed higher expression of PME compared to sugar alcohol calcium and amino acid calcium treatments. The lowest expression level of PME was observed in S1 treatment, which was only 0.09 times that of CK (). Except for C1, C2, and S2 treatments, all other treatments down-regulated the expression of PG. A3 and S1 treatments had lower expressions of PG at 0.45 and 0.51 respectively (). The CaCl2 treatment up-regulated the expression of Cx, with increasing concentration leading to increased expression levels; on the other hand, amino acid calcium and sugar alcohol calcium treatments down-regulated the expression of Cx. In the case of the calcium amino acid treatment, as concentration increased, the expression level decreased while it increased in the sugar alcohol calcium treatment. Among them, the expression of A3 was the lowest at 0.063 times that of CK (). The expression of BGal was up-regulated by the CaCl2 treatment, and its expression increased with increasing concentration. However, the expression of BGal was down-regulated by the amino acid calcium and sugar alcohol calcium treatments, except for S3. Among all treatments, the lowest expression of BGal was observed in A3, which was only 0.06 times that of CK (). On the other hand, three types of exogenous calcium treatments resulted in a down-regulation of ChCaBP1 expression. Specifically, both amino acid calcium and sugar alcohol calcium significantly decreased the expression of ChCaBP1. Compared to CaCl2 and sugar alcohol calcium, the calcium amino acid treatment led to even lower expression levels of ChCaBP1, with the A3 treatment showing the lowest level at only 0.14 times that of CK ().
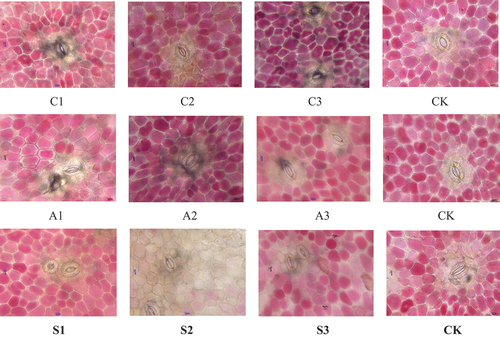
Effects of Foliar Calcium Fertilizer on Cherry Fruit Epidermis
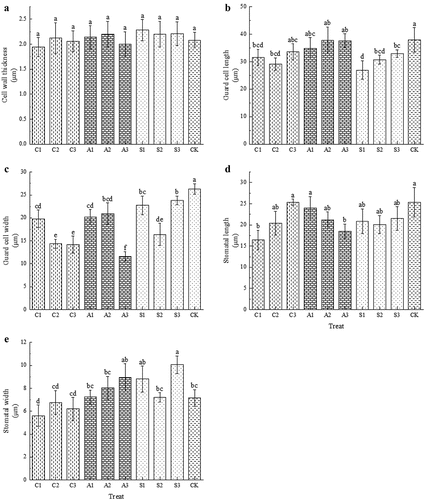
Spraying exogenous calcium before harvest had a certain effect on the epidermal structure of “Black Pearl” cherry fruit (). There was no significant effect of any treatment on cell wall thickness (). The S1 treatment significantly reduced the length and width of guard cell, but had no significant effect on the length and width of stomata (). C1 significantly reduced the length and width of stomata ().
Discussion
Mineral elements have an important role in tree growth, fruit ripening, and storage, as well as fruit growth and quality. Calcium is an essential nutrient for fruit growth and development. Calcium influences not just the photosynthetic physiology of plants, but also their mineral intake and transportation (Maryam and Rayhaneh, Citation2021: 2023; Wang et al., Citation2022). The absorption of various elements has both synergistic and antagonistic effects. This study discovered that applying CaCl2, amino acid calcium, and sugar alcohol calcium to cherries before harvest increased the content of N, P, K, Ca, Mg, Fe, Mn, and Zn in fruit, and had a significant effect on N, P, Fe, and Zn. Wang et al. (Citation2022) found in Fuji apple that calcium spraying promoted the accumulation of Ca, Mg, and B mineral elements in the fruit, thereby improving fruit quality. The results of this study are similar to those of previous studies.
After harvest, cherry fruits undergo various physiological and metabolic processes. However, the quality of the fruit deteriorates during preservation as it solely relies on its own stored nutrients and water to sustain its life outside of the tree. To prevent browning, deterioration, and slow down physiological abnormalities in cherries, applying calcium fertilizer to fruit trees is recommended (Ban et al., Citation2021; Madani et al., Citation2014). Soaking in 1% CaCl2 reduced the decay and weight loss of strawberries (Shafiee et al., Citation2010). Spraying CaCl2 (0.5%) on pepper before harvest enhanced the level of VC, TSS, and phenolic compounds while decreasing the occurrence of chilling injury during storage (Bagnazari et al., Citation2018). Our research showed that CaCl2, amino acid calcium and sugar alcohol calcium effectively increased the fruit weight, fruit hardness, TSS, SS, TA, Vc, SP, β- Carotene content and SOD and POD activities decreased the decay rate, PPO activity, MDA content and REC. This may be due to the fact that calcium is involved in the physiological process of cherry fruit and the regulation of enzyme activity, which can make the cell wall structure more compact, inhibit the depolymerization of cell wall substances, reduce the consumption of nutrients, delay fruit ripening, organ senescence and abscission, enhance the plant’s resistance to disease, and improve the quality of the fruit (Wang et al., Citation2014). Calcium plays an extremely important role in maintaining cell wall structure and strength, cell membrane structure and integrity, and intracellular signaling responses (Mu et al., Citation2022). Calcium is able to reduce pectin solubility, enhance cell wall strength, maintain fruit firmness and delay fruit softening by interacting with the carboxyl group of glucuronic acid on the pectin polysaccharide chain in the cell wall (Chen et al., Citation2011).
Stomata are tiny pores on the leaf surface that play an important role in gas exchange. Plant transpiration and photosynthesis are heavily influenced by stomatal number, size, and aperture, and variations in these traits can have an impact on plant growth and productivity (Pathoumthong et al., Citation2023). The turgor changes of plant guard cells cause stomatal movement, and osmotic ions flow into and out of the guard cells through ion channels and transporters, resulting in the turgor changes of the guard cells (Qi et al., Citation2018). Exogenous calcium ions caused cytosolic Ca2+ to increase and induce stomatal movement. Ca2+ plays an important role in the interaction between different signal pathways (Qi et al., Citation2018). This study found that the length and width of guard cells in the pericarp of fruit treated with calcium were smaller than CK, and the stomatal opening of pericarp was also smaller than CK. However, different forms of exogenous calcium had different effects on the stomatal opening of cherry fruit. Among them, S1 was the most effective at increasing cell thickness and decreasing guard cell length, while C1 was the most effective at decreasing stomatal length and width. Reduced transpiration and respiration were aided by smaller plant guard cells.
The components of the cell wall are catalyzed by a series of cell wall modifying enzymes, mainly including PG, PME, β-Gal and Cx degrading enzymes (Payasi et al., Citation2009). Calcium treatment changed fruit ripening, prevented normal degradation of fruit cell walls, and significantly reduced the transcription levels of key cell wall degradation genes (Figueroa et al., Citation2012; Zudaire et al., Citation2019). Calcium treatment also down-regulated the expression of pectin decomposition related genes in strawberries (Langer et al., Citation2019), reduced the PME, PG, β-Gal and other activities of peach (Zhi et al., Citation2017) and apricots (Liu et al., Citation2021), and delayed the degradation of cell wall substances, thereby delaying fruit aging. This experiment found that pre-harvest application of amino acid calcium and sugar alcohol calcium effectively reduced the activity of PME, PG, Cx and β-Gal, with 0.06 g·L−1 amino acid calcium and 0.12 g·L−1 sugar alcohol calcium being more effective. To further understand the softening of Chinese cherry fruit, the expression of PME, PG, Cx, BGal and ChCaBP1, key genes for fruit softening, was explored in this experiment. In this study, the expression of PME and ChCaBP1 in cherry fruit treated with three kinds of exogenous calcium were significantly down-regulated. Compared with CaCl2 treatment, the down-regulation of PME and ChCaBP1 in cherry fruit treated with amino acid calcium and sugar alcohol calcium were greater; the expression of PG, Cx and BGal was down-regulated by amino acid calcium and sugar alcohol calcium treatments. In the present experiment, the expression trends of PME, PG, Cx, BGal and ChCaBP1 genes were consistent with the changes in PME, PG, Cx and β-Gal activities, respectively. Therefore, this study showed that amino acids calcium and sugar alcohol calcium treatments down-regulated the expression of genes related to fruit cell wall softening, reduced enzyme activity, decreased degradation of cell wall material, slowed down softening and decay of harvested fruit, and prolonged their shelf life.
In this study, calcium amino acids and calcium sugar alcohols were generally more effective treatments than CaCl2. Sugar alcohols are naturally extracted from plant phloem and are nontoxic and harmless to the human body, combining with mineral nutrients to form stable low molecular compounds (Li et al., Citation2020). Both a calcium fertilizer and an amino acid nitrogen fertilizer, amino acid calcium helps plants grow and develop by increasing the amount of chlorophyll and photosynthesis in their leaves (Saftner et al., Citation2003). Compared with CaCl2, amino acid calcium and sugar alcohol calcium are potentially easier to absorb, more effective regulators of osmosis and enzymes, and likely improve the physical durability of the fruit, which has a significant effect on the preservation of Chinese cherry.
Conclusion
In conclusion, the results of this study demonstrated that preharvest application of exogenous calcium had several positive effects on fruit quality and shelf life. Firstly, it increased the uptake of mineral elements in the fruit. Secondly, it reduced stomatal opening, resulting in a slower exchange rate of gas and water and decreased respiratory consumption. Thirdly, it improved fruit quality by reducing cell wall softening enzyme activity and inhibiting the expression of genes related to cell wall softening. As a result, fruit softening was delayed and the shelf life of Chinese cherry was prolonged. Among the three types of exogenous calcium tested (CaCl2, amino acid calcium, sugar alcohol calcium), amino acid calcium and sugar alcohol calcium treatments were found to be more effective than CaCl2. Specifically, 0.12 g·L−1 sugar alcohol calcium treatment and 0.06 g·L−1 amino acid calcium treatment yielded the best results.
Author Contribution
Yongfei Wu: Conceptualization; Writing original draft; Formal analysis; Data curation; Software; Methodology; Validation. Xuelian Yang: Conceptualization; Funding acquisition; Project administration; Resources; Supervision; Writing-review & editing. Xia Wang: Software; Methodology. Li Yan: Methodology; Validation. Xiaojing Hu: Supervision; Resources; Funding acquisition. Nanjing Lian: Software; Investigation.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (31860225); Guizhou Provincial Science and Technology Projects (Qian Kehe Foundation [2019] 1408; Qian Kehe platform Personne [2018] 5781)
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bagnazari, M., M. Saidi, M. Mohammadi, O. Khademi, and G. Nagaraja. 2018. Pre-harvest CaCl2 and GA3 treatments improve postharvest quality of green bell peppers (capsicum annum L.) during storage period. Sci. Hortic. 240:258–267. doi: 10.1016/j.scienta.2018.06.043.
- Ban, Q.Y., T.J. Liu, K. Ning, J. Fan, Q. Cui, Y. Guo, and X.M. Zai. 2021. Effect of calcium treatment on the browning of harvested eggplant fruits and its relation to the metabolisms of reactive oxygen species (ROS) and phenolics. Food Sci. Nutr. 9(10):5567–5574. doi: 10.1002/fsn3.2517.
- Chen, Y.H., Y.C. Hung, M.Y. Chen, and H.T. Lin. 2017. Effects of acidic electrolyzed oxidizing water on retarding cell wall degradation and delaying softening of blueberries during postharvest storage. LWT 84:650–657. doi: 10.1016/j.lwt.2017.06.011.
- Chen, F.S., H. Liu, H.S. Yang, S.J. Lai, X.L. Cheng, Y. Xin, B. Yang, H.J. Hou, Y.Z. Yao, S.B. Zhang, et al. 2011. Quality attributes and cell wall properties of strawberries (Fragaria annanassa Duch.) under calcium chloride treatment. Food Chem. 126(2):450–459. doi: 10.1016/j.foodchem.2010.11.009.
- Dong, Y., H.H. Zhi, and Y. Wang. 2019. Cooperative effects of pre-harvest calcium and gibberellic acid on tissue calcium content, quality attributes, and in relation to postharvest disorders of late-maturing sweet cherry. Sci. Hortic. 246:123–128. doi: 10.1016/j.scienta.2018.10.067.
- Figueroa, C.R., M.C. Opazo, P. Vera, O. Arriagada, M. Diaz, and M.A. Moya-Leon. 2012. Effect of postharvest treatment of calcium and auxin on cell wall composition and expression of cell wall-modifying genes in the Chilean strawberry (Fragaria chiloensis) fruit. Food Chem. 132(4):2014–2022. doi: 10.1016/j.foodchem.2011.12.041.
- Gao, Y., C.N. Kan, C.P. Wan, C.Y. Chen, M. Chen, and J.Y. Chen. 2018. Quality and biochemical changes of navel orange fruits during storage as affected by cinnamaldehyde -chitosan coating. Sci. Hortic. 239:80–86. doi: 10.1016/j.scienta.2018.05.012.
- Guo, K.B., L. Peng, Y. Hong, and G. Qiao. 2022. Optimizing nitrogen, phosphorus, and Potassium Fertilization Rates for Fruit Performance of Chinese Cherry (Prunus pseudocerasus Lindl.). Int. J. Fruit Sci. 22(1):769–778. doi: 10.1080/15538362.2022.2129551.
- Langer, S.E., M. Marina, J.L. Burgos, G.A. Martinez, P.M. Civello, and N.M. Villarreal. 2019. Calcium chloride treatment modifies cell wall metabolism and activates defense responses in strawberry fruit (Fragaria × ananassa Duch). J. Sci. Food Agric. 99(8):4003–4010. doi: 10.1002/jsfa.9626.
- Li, P.C., C.Z. Geng, L.Y. Li, Y.P. Li, T.S. Li, Q.Q. Wei, and D.Y. Yan. 2020. Calcium-sorbitol chelating technology and application in potatoes. Am. J. Biochem. Biotechnol 16(1):96–102. doi: 10.3844/ajbbsp.2020.96.102.
- Liu, M.P., J. Li, W. Zong, W.W. Sun, W.J. Mo, and S.F. Li. 2021. Comparison of calcium and ultrasonic treatment on fruit firmness, pectin composition and cell wall-related enzymes of postharvest apricot during storage. J. Food Sci. Technol 59(4):1–10. doi: 10.1007/s13197-021-05170-w.
- Madani, B., M.T.M. Mohamed, C.B. Watkins, J. Kadir, Y. Awang, and T.R. Shojaei. 2014. Preharvest calcium chloride sprays affect ripening of eksotika II’papaya fruits during cold storage. Sci. Hortic. 171(1):6–13. doi: 10.1016/j.scienta.2014.03.032.
- Martins, V., C. Soares, S. Spormann, F. Fidalgo, and H. Gerós. 2021. Vineyard calcium sprays reduce the damage of postharvest grape berries by stimulating enzymatic antioxidant activity and pathogen defense genes, despite inhibiting phenolic synthesis. Plant Physiol. Biochem. 162:48–55. doi: 10.1016/j.plaphy.2021.02.025.
- Maryam, V., and A. Rayhaneh. 2021. Foliar spray with sodium hydrosulfide and calcium chloride advances dynamic of critical elements and efficiency of nitrogen metabolism in Cucurbita pepo L. under nickel stress. Sci. Hortic. 283:110052. doi: 10.1016/j.scienta.2021.110052.
- Michailidis, M., C. Polychroniadou, M. Kosmidou, D. Petraki-Katsoulaki, E. Karagiannis, A. Molassiotis, and G. Tanou. 2021. An early calcium loading during cherry tree dormancy improves fruit quality features at harvest. Hortic 7(6):135. doi: 10.3390/horticulturae7060135.
- Mohebbi, S., M. Babalar, Z. Zamani, and M.A. Askari. 2020. Influence of early season boron spraying and postharvest calcium dip treatment on cell-wall degrading enzymes and fruit firmness in ‘starking delicious’ apple during storage. Sci. Hortic. 259:108822. doi: 10.1016/j.scienta.2019.108822.
- Mu, B.Y., J.X. Xue, S.J. Zhang, and Z.Z. Li. 2022. Effects of the use of different temperature and calcium chloride treatments during storage on the quality of fresh-cut “xuebai” cauliflowers. Foods 11(3):442. doi: 10.3390/foods11030442.
- Pathoumthong, P., Z. Zhang, S.J. Roy, and H.A. El. 2023. Rapid non-destructive method to phenotype stomatal traits. Plant Methods 19(1):36–36. doi: 10.1186/s13007-023-01016-y.
- Payasi, A., N.N. Mishra, A.L.S. Chaves, and R. Singh. 2009. Biochemistry of fruit softening: An overview. Physiol. Mol. Biol. Pla 15(2):103–113. doi: 10.1007/s12298-009-0012-z.
- Peng, J.S., S.L. Zhu, X. Lin, X. Wan, Q. Zhang, N. Alagie, D.C. Luo, Y.H. Long, R. Fan, and X.Q. Dong. 2023. Evaluation of preharvest melatonin on soft rot and quality of kiwifruit based on principal component analysis. Foods 12(7):1414. doi: 10.3390/foods12071414.
- Qi, G.N., F.Y. Yao, H.M. Ren, S.J. Sun, J. Hussain, and Y.F. Wang. 2018. Constitutive activation of calcium-dependent protein kinase 3 confers a drought tolerance by inhibiting inward K+ channel KAT1 and stomatal opening in Arabidopsis. Sci. Bull 63(16):1037–1039. doi: 10.1016/j.scib.2018.07.011.
- Ren, Y.Y., P.P. Sun, X.X. Wang, and Z.Y. Zhu. 2020. Degradation of cell wall polysaccharides and change of related enzyme activities with fruit softening in Annona squamosa during storage. Postharvest Biol. Tec 166(3):111203. doi: 10.1016/j.postharvbio.2020.111203.
- Saftner, R.A., J. Bai, J.A. Abbott, and Y.S. Lee. 2003. Sanitary dips with calcium propionate, calcium chloride, or a calcium amino acid chelate maintain quality and shelf stability of fresh-cut honeydew chunks. Postharvest Biol. Tec 29(3):257–269. doi: 10.1016/S0925-5214(03)00041-3.
- Saravanan, S., M. Nithyakumar, V. Mana, S. Sangavi, S.N. Saran, A.M.S. Lakshmi, and X.Z. Gao. 2021. Blossom end rot disease tracking and prevention: A smart approach. Int. J. Inf. Technol 13(2):1–6. doi: 10.1007/s41870-021-00636-8.
- Shafiee, M., T.S. Taghavi, and M. Babalar. 2010. Addition of salicylic acid to nutrient solution combined with postharvest treatments (hot water, salicylic acid, and calcium dipping) improved postharvest fruit quality of strawberry. Sci. Hortic. 124(1):40–45. doi: 10.1016/j.scienta.2009.12.004.
- Silva, F.L.B., T.B.D. Santos, M.D.O.V. Figueiredo, V. Cacefo, L.G.E. Vieira, and A.F. Ribas. 2021. Validation of reference genes for real-time quantitative PCR in Brachiaria grass under salt stress. Plant Gene 27:100319. doi: 10.1016/j.plgene.2021.100319.
- Sofo, A., B. Dichio, C. Xiloyannis, and A. Masia. 2004. Effects of different irradiance levels on some antioxidant enzymes and on malondialdehyde content during rewatering in olive tree. Plant Sci. 166(2):293–302. doi: 10.1016/j.plantsci.2003.09.018.
- Sun, C., W.W. Zhang, H.Y. Qu, L.F. Yan, L.X. Li, Y.Q. Zhao, H.Q. Yang, H. Zhang, G.F. Yao, and K.D. Hu. 2022. Comparative physiological and transcriptomic analysis reveal MdWRKY75 associated with sucrose accumulation in postharvest ‘honeycrisp’ apples with bitter pit. BMC Plant Biol. 22(1):71–71.
- Wainwright, H., and M.B. Burbage. 2015. Physiological disorders in mango (Mangifera indica L.) fruit. J. Hortic. Sci. 64(2):125–135. doi: 10.1080/14620316.1989.11515936.
- Wang, Y., H.M. Du, J. Zhang, T. Chen, Q. Chen, H.R. Tang, and X.R. Wang. 2018. Ploidy level of Chinese cherry (cerasus pseudocerasus Lindl.) and comparative study on karyotypes with four cerasus species. Sci. Hortic. 232:46–51. doi: 10.1016/j.scienta.2017.12.065.
- Wang, Y., G.P. Hu, Z.S. Liu, J. Zhang, L. Ma, T. Tian, H. Wang, T. Chen, Q. Chen, W. He, et al. 2022. Phenotyping in flower and main fruit traits of Chinese cherry [cerasus pseudocerasus (Lindl.) G.Don]. Sci. Hortic. 296:110920. doi: 10.1016/j.scienta.2022.110920.
- Wang, G.P., J.Z. Wang, X.P. Han, R. Chen, and X.M. Xue. 2022. Effects of spraying calcium fertilizer on photosynthesis, mineral content, sugar–acid metabolism and fruit quality of Fuji apples. Agronomy 12(10):2563–2563. doi: 10.3390/agronomy12102563.
- Wang, Y., X.B. Xie, and E.L. Long. 2014. The effect of postharvest calcium application in hydro-cooling water on tissue calcium content, biochemical changes, and quality attributes of sweet cherry fruit. Food Chem. 160:22–30. doi: 10.1016/j.foodchem.2014.03.073.
- Winkler, A., and M. Knoche. 2019. Calcium and the physiology of sweet cherries: A review. Sci. Hortic. 245:107–115. doi: 10.1016/j.scienta.2018.10.012.
- Wu, Y.J., Q.Q. Song, Y. Yuan, F.Q. Guo, K.X. Wu, and M.M. Dong. 2021. In vitro efficiency of embryo rescue of intra- and interspecific hybrid crosses of sweet cherry and Chinese cherry cultivars. Sci. Hortic. 275:109716. doi: 10.1016/j.scienta.2020.109716.
- Xu, H.S., Y.R. Wang, S.H. Ding, H. Zhou, L.W. Jiang, and R.R. Wang. 2020. Effect of hydrothermal-calcium chloride treatment on pectin characteristics and related quality in green peppers during storage. J. Food Sci. Technol 58(10):1–13. doi: 10.1007/s13197-020-04829-0.
- Zeng, R., A.S. Zhang, J.Y. Chen, and Y.Q. Fu. 2012. Postharvest quality and physiological responses of clove bud extract dip on ‘Newhall’ navel orange. Sci. Hortic. 138:253–258. doi: 10.1016/j.scienta.2012.02.036.
- Zhang, J., T. Chen, Y. Wang, Q. Chen, B. Sun, Y. Luo, Y. Zhang, H.R. Tang, and X.R. Wang. 2018. Genetic diversity and domestication footprints of Chinese cherry [cerasus pseudocerasus (Lindl.) G.Don] as revealed by nuclear microsatellites. Front. Plant Sci 9:238. doi: 10.3389/fpls.2018.00238.
- Zhang, C., L.W. Cui, P.A. Zhang, T.Y. Dong, and J.G. Fang. 2021. Transcriptome and metabolite profiling reveal that spraying calcium fertilizer reduces grape berry cracking by modulating the flavonoid biosynthetic metabolic pathway. Food Chem. 2:100025. doi: 10.1016/j.fochms.2021.100025.
- Zhang, L.F., S.N. Zhao, S.J. Lai, F.S. Chen, and H.S. Yang. 2018. Combined effects of ultrasound and calcium on the chelate-soluble pectin and quality of strawberries during storage. Carbohyd. Polym. 200:427–435. doi: 10.1016/j.carbpol.2018.08.013.
- Zhao, H.D., M.R. Fu, Y.M. Du, F. Sun, Q.M. Chen, T. Jin, Q. Zhang, and B.D. Liu. 2021. Improvement of fruit quality and pedicel color of cold stored sweet cherry in response to pre-storage 1-methylciclopropene and chlorine dioxide treatments: Combination treatment of 1-MCP plus ClO2 improves post-harvest quality of sweet cherry fruit. Sci. Hortic. 277:109806. doi: 10.1016/j.scienta.2020.109806.
- Zhi, H.H., Q.Q. Liu, Y. Dong, M.P. Liu, and W. Zong. 2017. Effect of calcium dissolved in slightly acidic electrolyzed water on antioxidant system, calcium distribution, and cell wall metabolism of peach in relation to fruit browning. J. Hortic. Sci. Biotech. 92(6):621–629. doi: 10.1080/14620316.2017.1309994.
- Zudaire, L., I. Viñas, M.B. Iglesias, L. Plaza, M. Abadias, and I. Aguiló-Aguayo. 2019. Evaluation of Pseudomonas graminis CPA-7 as a biopreservation method for fresh-cut pear: Physicochemical, enzymatic, and nutritional quality. Food Sci. Technol. Int. 25(4):271–281. doi: 10.1177/1082013218816483.