ABSTRACT
Developing new strawberry varieties with high level of disease resistance and superior fruit quality is critical for strawberry production. Despite the complexity of the octoploid strawberry genome, recent advances in genomics and DNA sequencing technologies have allowed us to identify causal sequence variants associated with specific phenotypes. These have enabled the development of new and improved DNA markers for integration in marker-assisted breeding (MAB) efforts. The use of MAB has significantly improved the efficiency of stacking loci responsible for disease resistance and fruit quality characteristics. The “Strawberry DNA Testing Handbook” is currently accessible in the Genome Database for Rosaceae and has been an essential resource for the national and international strawberry research community. Based on the information provided in the handbook, the UF strawberry breeding program implemented seedling selection from 2021 to 2023, retaining 25.3%, 19.2%, and 37.9% of seedlings for further evaluation in each respective year. In this report, we provide updates on DNA markers associated with disease resistance and fruit quality traits. Additionally, we demonstrate the practical implementation of these markers in high-throughput marker-assisted selection and their potential to enhance strawberry breeding.
Introduction
Strawberry is a popular fruit crop enjoyed by people all around the world. The garden strawberry (Fragaria ×ananassa Duchesne ex Rozier) is an allo-octoploid (2n = 8× = 56) arising from two wild octoploid progenitors, F. virginiana and F. chiloensis, approximately around 300 years ago (Whitaker et al., Citation2020). The strawberry has distinctive taste and aroma and is a valuable source of essential dietary components like ascorbic acid, potassium, and dietary fiber (Khan et al., Citation2014). In addition, strawberries are abundant in secondary metabolites, including various vitamins and phenolics (Capocasa et al., Citation2008; Hernández-Martínez et al., Citation2023). Strawberry production has been steadily increasing to satisfy consumers’ growing demand, and the United States currently holds the position as the global leader in strawberry production (Wu et al., Citation2020). Within the United States, California is the predominant strawberry producer, accounting for 91% of the total production (Hernández-Martínez et al., Citation2023). Florida contributes 8% of the overall production, with a specific emphasis on fulfilling the demands of the winter market (Hernández-Martínez et al., Citation2023).
Because of the complexities of the F. ×ananassa genome, many researchers initially focused on the genome of diploid wild strawberries. Consequently, the genome sequence of the diploid wild strawberry, F. vesca, was first elucidated and published in 2011 (Shulaev et al., Citation2011). Supplementing this achievement, the RosBREED consortium subsequently developed a high-throughput genotyping platform in 2015, constructing a 90K single nucleotide polymorphism (SNP) array based on the F. vesca assembly and resequencing data from octoploids (Bassil et al., Citation2015). However, on average, IStraw90 yields 50–60% usable data points classified as poly-high resolution (PHR) and no minor homozygote (NMH) marker categories (Verma et al., Citation2016). This result highlights the requirement for the development of more efficient genotyping arrays, capable of increasing the percentage of usable data while decreasing the associated cost. Addressing these limitations, the count of markers was scaled down from 90K to 35K in the 2016 and assembled as AxiomⓇ IStraw35 384HT array. By implementing this modification, a cost-effective and high-quality genome scanning approach was achieved, resulting in the identification of more than 87% high-quality polymorphic markers that are specific to octoploid strawberry (Verma et al., Citation2016).
The IStraw35 and IStraw90 SNP arrays were designed using probes based on the F. vesca reference genome sequence (Bassil et al., Citation2015; Verma et al., Citation2017). Consequently, this poses significant hurdles for anchoring sub-genome-specific physical markers to the genome of the octoploid strawberry (Whitaker et al., Citation2020). This limitation impeded the precise localization of QTL and physical markers within homoeologous subgenome regions of the octoploid genome (Hardigan et al., Citation2020). The advancements in next generation sequencing (NGS) technology facilitated the generation of the first draft of a chromosome-scale octoploid reference genome in 2019 (Edger et al., Citation2019). Based on the octoploid strawberry genome, the SNP array with 50K SNPs (FanaSNP) was newly anchored, allowing us to discover causal variants associated with the target traits and develop more accurate DNA markers for octoploid strawberry breeding (Hardigan et al., Citation2020). This advancement has significantly enhanced the efficiency of variety improvement via marker-assisted seeding selection (MASS).
Recently, two high-quality haplotype phased genomes of octoploid strawberry have been published from two commercial cultivars, “Florida Brilliance” (FaFB1) (Han et al., Citation2022) and “Royal Royce” (FaRR1) (Hardigan et al., Citation2021), using long-read sequencing (HiFi) data. The availability of these reference genomes has enabled us to understand the diverse genomic structures comprehensively and perform comparative analysis for identifying candidate genes associated with traits of interest. All the existing “Camarosa” v1 reference-based SNP array probes were aligned to both high-quality haplotype-phased reference genomes for genetic studies. This information is available at a Dryad repository (https://doi.org/10.25338/B86057) (Pincot et al., Citation2022).
In this report, we provide updates on the current available and recently developed strawberry DNA markers for fruit quality, flavor, and disease resistance. These markers can be effectively used in marker-assisted breeding to enhance the improvement of strawberry varieties.
Strawberry DNA Markers
A workflow for locus discovery, validation and DNA marker development is shown in . Molecular marker development involves utilizing the Axiom™ Strawberry FanaSNP 50k Genotyping Array (Applied Biosystems™) to conduct genotyping and identify QTL from strawberry breeding populations (Hardigan et al., Citation2020). Based on the genotyping results, mapping populations that align with the breeding objectives are selected, and various analyses, including linkage mapping, phenotyping, QTL analysis, genome-wide association study (GWAS) analysis, and linkage disequilibrium (LD) analysis, are performed to identify loci associated with target traits. Subsequently, high-quality haplotype-phased genome data (FaRR1 and FaFB1) are utilized to identify significant SNPs or Indels, which serve as the basis for designing DNA markers for fine-mapping of QTL within the identified candidate genic regions. Kompetitive Allele-Specific PCR (KASP) or Polymerase Allelic Competitive Extension (PACE) employs allele-specific primers based on SNP and InDel polymorphisms to conduct PCR, which is then measured using an endpoint fluorescent method (Kalendar et al., Citation2022). Additionally, High-Resolution Melting (HRM) curve analysis, based on fluorescence changes during the melting of DNA duplexes, is a robust, cost-effective, and high-throughput method (Akey et al., Citation2001). Furthermore, probe-based TaqMan SNP genotyping, which attaches a fluorescence dye to SNP polymorphism-specific probes, could be another genotyping method for differentiating SNP markers at specific locations (Francisco et al., Citation2005). With these tools, we can develop various types of markers for targeted experiments based on regions where polymorphisms are distinguished and areas where 50K SNP locations and linkage disequilibrium (LD) are identified. If there is sequencing data of paternal and maternal resources or other germplasm with contrasting phenotypes, such as resistance or susceptibility to the target trait, the sequencing data can lead to more rapid and accurate marker development. Consequently, SNPs or Indel around the target genomic location are identified, and the primer designing tools such as PolyOligo (http://ec2-52-52-41-39.us-west-1.compute.amazonaws.com/), Primer Design (www.geneious.com), and IDT (https://sg.idtdna.com) can be used to design primers encompassing a range of 50–100 base pair amplicons, including variants.
Figure 1. A schematic overview of the QTL identification of disease resistance and fruit quality traits, genomic studies, and marker-assisted seedling selection in octoploid strawberry at UF strawberry breeding program.
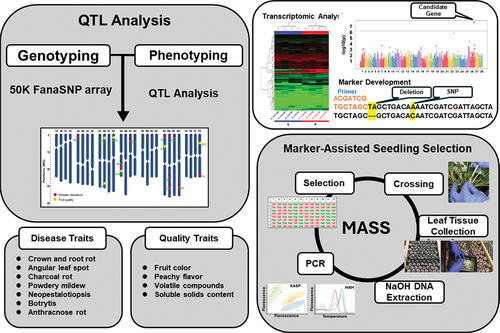
Subsequently, the proof of the polymorphism is verified by employing the maternal and paternal plants that harbor the SNP or Indel variant pertinent to the target trait, using the prepared primer. The marker’s applicability is assessed across a broader mapping population if the polymorphism is discernible. Finally, primer sets that demonstrate proven marker applicability undergo rigorous validation to ensure that the polymorphisms are accurately identified through HRM genotyping using crude DNA obtained via low-cost DNA extraction before their ultimate integration into MASS protocols. Furthermore, in the case of KASP or PACE technologies enabling genotype analysis through allele-specific PCR technology, approximately 300 base pairs of the nucleotide sequence of interest encompassing the target variant are inputted using PolyOligo (Ledda et al., Citation2020). Another option is to submit the sequence to 3CR Bioscience through their website (3CR Bioscience, Essex, UK; https://3crbio.com/). Subsequently, two allele-specific forward primers and one common reverse primer per variant are designed and supplied for allele-specific PCR analysis. Whole-genome transcriptome analysis can then be conducted to identify candidate genes that exhibit significant correlations with the target traits in the refined mapping regions, thereby facilitating the development of additional gene-specific functional markers. Throughout this entire process, the acquired markers are instrumental in selecting high-throughput seedlings, ultimately pinpointing the candidate region based on specific traits. This approach facilitates the discovery of genes and variants associated with target traits for breeding, encompassing the entirety of our methodology.
DNA markers have been successfully applied for improving strawberry varieties through MAB. For example, in 2015, when the marker was initially employed for selecting fruity aroma flavor, we screened approximately 20,000 seedlings and subsequently narrowed down the selection to 12,000 for field evaluation. From 2021 to 2023, the University of Florida (UF) strawberry breeding program subsequently increased the intensity of seedling selection and number of DNA markers. In 2021, the program retained 18,625 seedlings out of 73,500 for further field evaluation. In 2022, we screened approximately 80,000 and selected 15,425 seedlings. In 2023, due to disease incidence in the greenhouse, a significant number of our seedlings perished, leading to the screening of only 42,201 seedlings. However, we managed to select approximately 16,000 seedlings using 12 different DNA markers for further field evaluation (Supplementary Table S2).
Pursuant to the comprehensive process illustrated in , a collection of fourteen DNA markers have been effectively employed for MASS within the UF strawberry breeding program (). The corresponding sequence information for each marker, in conjunction with the precise locations of traits relevant to both disease resistance and fruit quality, are displayed in and Supplementary Table S1. Although the markers FaRMp1 (Nelson et al., Citation2021), FanOMT (Barbey et al., Citation2021; Fan et al., Citation2022), FanQR, FaSSC1 (Fan et al., Citation2023), and FaSSC2 (Fan et al., Citation2023) have been utilized for seedling selection in 2023, it is noteworthy that additional refinement and updates will be required to further enhance their effectiveness.
Table 1. Characteristics, quantitative trait loci (QTL) associations, and citations of DNA markers in strawberry (F. ×ananassa).
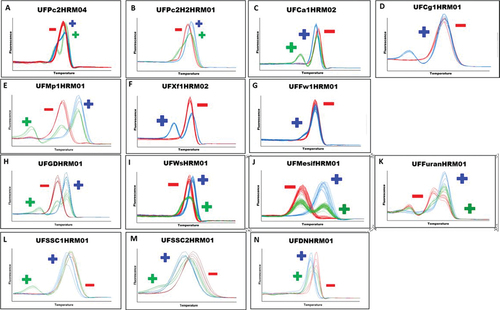
A detailed overview of marker melting patterns, including newly updated SNP-based HRM markers are shown in . Comprehensive genomic information for all DNA markers can be found in the “Strawberry DNA Testing Handbook” available at https://www.rosaceae.org/, as detailed in and Supplementary Table S1. These markers are adaptable for various genotyping assays, including KASP, PACE, and TaqMan, depending on the genomic region of interest. The University of Florida’s strawberry breeding program has adopted the HRM assay genotyping system to facilitate crude extract DNA-based high-throughput genotyping. As documented by Oh et al. (Citation2019), markers utilized for disease resistance traits such as Phytophthora cactorum (UFPc2HRM04), Colletotrichum acutatum (UFCa1HRM02), Colletotrichum gloeosporioides (UFCg1HRM01), and Xanthomonas fragariae (UFXf1HRM02), along with fruit quality traits such as γ-Decalactone production (UFGDHRM01) and perpetual flowering (UFDNHRM01). Additional DNA markers, derived from genomic locations in previously published studies, are newly included for Phytophthora cactorum (named QTL; FaRPc2-H2, UFPc2H2HRM01), Fusarium oxysporum f. sp. fragariae (UFFw1HRM01), and white fruit color (UFWsHRM01) as detailed in and Supplementary Table S1. Also, in this report, we included new DNA markers for resistance to Macrophomina phaseolina (Nelson et al., Citation2021), furaneol, mesifurane (Barbey et al., Citation2021; Fan et al., Citation2022), and soluble solids content (Fan et al., Citation2023).
Figure 2. HRM based DNA markers and melting curve patterns for traits associated with disease resistance and fruit quality in octoploid strawberry.
Efforts of Marker-Assisted Breeding for Strawberry Improvement
MAB capitalizes on genetic diversity and unique DNA markers to distinguish between plant varieties and to streamline the selection process. Using DNA markers established from foundational genetic data, we can efficiently select desired traits like disease resistance, fruit quality, and productivity (Das et al., Citation2017; Whitaker, Citation2011; Zhou et al., Citation2007). MAB in strawberry breeding can reduce time, labor, and resources for selection and can dramatically increase selection intensity at the seedling stage. While purified DNA from laborious extraction methods is needed for foundational genetic analyses, such methods limit throughput. An NaOH-based high-throughput DNA extraction method has been employed, effectively reducing time, cost, and labor during the MASS process (Noh et al., Citation2017). This high-throughput DNA extraction and MASS has also been applied to other breeding programs of Rosaceae crops (Lee et al., Citation2016).
The practical application of DNA markers has been implemented in the development of new strawberry varieties. For example, genetic and genomic studies confirmed that white color is conferred by a mutation in one of the three homeologs of a MYB transcription factor regulating anthocyanin biosynthesis in fruit, namely FaMYB10-2 (Castillejo et al., Citation2020). In the same study, a gene-specific DNA marker was developed that has since been utilized for the white strawberry breeding efforts (Whitaker et al., Citation2023). Additionally, DNA markers have accelerated the breeding process of disease resistance in strawberry. For example, charcoal rot, caused by the soil-borne fungal pathogen Macrophomina phaseolina, is a major constraint in many strawberry production areas worldwide, including Florida and California, the two largest producers in the United States (Nelson et al., Citation2021). The resistance to charcoal rot is conferred by three QTLs, designated as FaRMp1, FaRMp2, and FaRMp3 with variable effects against the pathogen (Nelson et al., Citation2021). The marker for FaRMp1 was developed, while the development of FaRMp2 and FaRMp3 markers is currently in progress. The marker-assisted pyramiding of FaRMp1, FaRMp2, and FaRMp3 would be an ideal approach for achieving durable resistance to charcoal rot disease in strawberry. It has been often demonstrated that combinations of quantitative resistance from different sources in the same variety has achieved increased and more durable resistance to pathogens in various crops (Boyd et al., Citation2013; Hasan et al., Citation2021; Nelson et al., Citation2018).
Improving fruit flavor is one of the most important breeding objectives in strawberry. Due to the highly complex nature of strawberry flavor, it has been a challenge to effectively select for improved flavor at a population level compared with more easily measured traits. Discovering the chemical drivers of strawberry flavor requires a large investment into sensory panels and metabolomic analysis which is costly and not feasible for routine screening within large breeding populations. DNA markers are the most effective way to implement knowledge from discovery populations into breeding programs at an early stage to select for aroma compounds that enhance strawberry flavor. A DNA marker for γ-Decalactone previously discovered in our program (Oh et al., Citation2021) has been implemented for multiple years of selection in the Florida breeding program. Its value was realized in the recently released cultivar “Florida Medallion” which exhibits the characteristic peach/fruity aroma conferred by the volatile compound (Oh et al., Citation2021). Given that over 30 volatile compounds have been shown to enhance consumer liking and sweetness perception (Fan et al., Citation2021), there is great potential for using many markers in combination to select for desired flavor profiles.
Furthermore, as shown in , the currently available DNA markers can effectively predict the presence and absence of target traits in the US breeding germplasm due to their generic relevance and similarity. However, most of these markers are not gene specific, thus they may not co-segregate in the genetically diverse cultivated strawberry varieties outside of US breeding programs. It would be important to test these markers in a more diverse breeding germplasm globally.
Table 2. DNA markers screening results for strawberry varieties originating from the US and Asia.
A total of 18 strawberry cultivars were included as a representative DNA testing set, including ten UF varieties, two University of California Davis (UC Davis), three Korean varieties, and three Japanese varieties (). This result implies that the markers presented in this report can be effectively integrated into various breeding materials with different genetic backgrounds.
In conclusion, we have defined strawberry genotypes with various combinations of marker alleles for disease resistance, fruit characteristics, and flowering time traits. This report including the updated Strawberry DNA Testing Handbook also provides comprehensive details regarding genomic information and the physical locations of QTL and markers based on recent studies. These findings would be greatly valuable for the strawberry breeding community and other Rosaceae research communities.
Supplemental Material
Download MS Word (52.2 KB)Acknowledgments
We thank for the technical support provided by the members of the University of Florida Strawberry Breeding Program (Strawberry Molecular Genetics and Genomics Lab; Strawberry Genetics and Breeding Lab). This research is supported by grants from the United States Department of Agriculture National Institute of Food and Agriculture (NIFA) Specialty Crops Research Initiative (#2022-51181-38328), and this work was carried out with the support of ‘Cooperative Research Program for Agriculture Science and Technology Development’ (Project No: PJ01698901), Rural Development Administration, Republic of Korea.
Disclosure Statement
No potential conflict of interest was reported by the author(s).
Supplementary Material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15538362.2024.2365683
Additional information
Funding
References
- Akey, J., D. Sosnoski, E. Parra, S. Dios, K. Hiester, B. Su, C. Bonilla, L. Jin, and M. Shriver. 2001. Melting curve analysis of SNPs (McSNP®): A gel-free and inexpensive approach for SNP genotyping. BioTechniques 30(2):358–367. doi: 10.2144/01302tt05.
- Anciro, A., J. Mangandi, S. Verma, N. Peres, V.M. Whitaker, and S. Lee. 2018. FaRCg1: A quantitative trait locus conferring resistance to Colletotrichum crown rot caused by Colletotrichum gloeosporioides in octoploid strawberry. Theor. Appl. Genet. 131(10):2167–2177. doi: 10.1007/s00122-018-3145-z.
- Barbey, C.R., M.H. Hogshead, B. Harrison, A.E. Schwartz, S. Verma, Y. Oh, S. Lee, K.M. Folta, and V.M. Whitaker. 2021. Genetic analysis of methyl anthranilate, mesifurane, linalool, and other flavor compounds in cultivated strawberry (Fragaria× ananassa). Front. Plant Sci. 12:615749. doi: 10.3389/fpls.2021.615749.
- Bassil, N.V., T.M. Davis, H. Zhang, S. Ficklin, M. Mittmann, T. Webster, L. Mahoney, D. Wood, E.S. Alperin, and U.R. Rosyara. 2015. Development and preliminary evaluation of a 90 K Axiom® SNP array for the allo-octoploid cultivated strawberry Fragaria× ananassa. BMC. Genomics 16(1):1–30. doi: 10.1186/s12864-015-1310-1.
- Boyd, L.A., C. Ridout, D.M. O’Sullivan, J.E. Leach, and H. Leung. 2013. Plant–pathogen interactions: Disease resistance in modern agriculture. Trends Genet. 29(4):233–240. doi: 10.1016/j.tig.2012.10.011.
- Capocasa, F., J. Scalzo, B. Mezzetti, and M. Battino. 2008. Combining quality and antioxidant attributes in the strawberry: The role of genotype. Food Chem. 111(4):872–878. doi: 10.1016/j.foodchem.2008.04.068.
- Castillejo, C., V. Waurich, H. Wagner, R. Ramos, N. Oiza, P. Muñoz, J.C. Triviño, J. Caruana, Z. Liu, and N. Cobo. 2020. Allelic variation of MYB10 is the major force controlling natural variation in skin and flesh color in strawberry (Fragaria spp.) fruit. Plant Cell 32(12):3723–3749. doi: 10.1105/tpc.20.00474.
- Chandler, C.K. 2005. ‘Sweet Charlie’Strawberry. J. Am. Pomol. Soc. 59:67.
- Chandler, C.K., D. Legard, D. Dunigan, T. Crocker, and C. Sims. 2000. ‘Strawberry festival’ strawberry. HortScience. 35(7):1366–1367. doi: 10.21273/HORTSCI.35.7.1366.
- Chandler, C.K., B.M. Santos, N.A. Peres, C. Jouquand, and A. Plotto. 2009. ‘Florida Elyana’Strawberry. HortScience. 44(6):1775–1776. doi: 10.21273/HORTSCI.44.6.1775.
- Chandler, C.K., B.M. Santos, N.A. Peres, C. Jouquand, A. Plotto, and C.A. Sims. 2009. ‘Florida radiance’strawberry. HortScience. 44(6):1769–1770. doi: 10.21273/HORTSCI.44.6.1769.
- Das, G., J.K. Patra, and K.-H. Baek. 2017. Insight into MAS: A molecular tool for development of stress resistant and quality of rice through gene stacking. Front. Plant Sci. 8:985. doi: 10.3389/fpls.2017.00985.
- Edger, P.P., T.J. Poorten, R. VanBuren, M.A. Hardigan, M. Colle, M. Mr, R.D. Smith, S.J. Teresi, A.D. Nelson, and C.M. Wai. 2019. Origin and evolution of the octoploid strawberry genome. Nat. Genet. 51(3):541–547. doi: 10.1038/s41588-019-0356-4.
- Fan, Z., T. Hasing, T.S. Johnson, D.M. Garner, M.L. Schwieterman, C.R. Barbey, T.A. Colquhoun, C.A. Sims, M.F. Resende, and V.M. Whitaker. 2021. Strawberry sweetness and consumer preference are enhanced by specific volatile compounds. Hortic. Res. 8(1). doi: 10.1038/s41438-021-00502-5.
- Fan, Z., D.M. Tieman, S.J. Knapp, P. Zerbe, R. Famula, C.R. Barbey, K.M. Folta, R.R. Amadeu, M. Lee, and Y. Oh. 2022. A multi‐omics framework reveals strawberry flavor genes and their regulatory elements. New Phytol. 236(3):1089–1107. doi: 10.1111/nph.18416.
- Fan, Z., S. Verma, H. Lee, Y.J. Jang, Y. Wang, S. Lee, and V.M. Whitaker. 2023. Strawberry soluble solids QTL with inverse effects on yield. Hortic. Res. 11(2):uhad271. doi: 10.1093/hr/uhad271.
- Francisco, M., K.D. Lazaruk, M.D. Rhodes, and M.H. Wenz. 2005. Assessment of two flexible and compatible SNP genotyping platforms: TaqMan® SNP genotyping assays and the SNPlex™ genotyping system. Mutat. Res-Fund. Mol 573(1–2):111–135. doi: 10.1016/j.mrfmmm.2005.01.008.
- Han, H., C.R. Barbey, Z. Fan, S. Verma, V. Whitaker, and S. Lee. 2022. Telomere-to-telomere and haplotype-phased genome assemblies of the heterozygous octoploid′ Florida brilliance′ strawberry (Fragaria× ananassa). BioRxiv. 2022.2010. 2005.509768.
- Hardigan, M.A., M.J. Feldmann, A. Lorant, K.A. Bird, R. Famula, C. Acharya, G. Cole, P.P. Edger, and S.J. Knapp. 2020. Genome synteny has been conserved among the octoploid progenitors of cultivated strawberry over millions of years of evolution. Front. Plant Sci. 10:1789. doi: 10.3389/fpls.2019.01789.
- Hardigan, M.A., M.J. Feldmann, D.D. Pincot, R.A. Famula, M.V. Vachev, M.A. Madera, P. Zerbe, K. Mars, P. Peluso, and D. Rank. 2021. Blueprint for phasing and assembling the genomes of heterozygous polyploids: Application to the octoploid genome of strawberry. BioRxiv. 2021.2011. 2003.467115.
- Hasan, N., S. Choudhary, N. Naaz, N. Sharma, and R.A. Laskar. 2021. Recent advancements in molecular marker-assisted selection and applications in plant breeding programmes. J. Genet. Eng. Biotechnol. 19(1):1–26. doi: 10.1186/s43141-021-00231-1.
- Hernández-Martínez, N.R., C. Blanchard, D. Wells, and M.R. Salazar-Gutiérrez. 2023. Current state and future perspectives of commercial strawberry production: A review. Sci. Hortic. 312:111893. doi: 10.1016/j.scienta.2023.111893.
- Jiménez, N.P., M.J. Feldmann, R.A. Famula, D.D. Pincot, M. Bjornson, G.S. Cole, and S.J. Knapp. 2023. Harnessing underutilized gene bank diversity and genomic prediction of cross usefulness to enhance resistance to Phytophthora cactorum in strawberry. Plant Genome. 16(1):e20275. doi: 10.1002/tpg2.20275.
- Kalendar, R., A.V. Shustov, I. Akhmetollayev, and U. Kairov. 2022. Designing allele-specific competitive-extension PCR-based assays for high-throughput genotyping and gene characterization. Front. Mol. Biosci. 9:773–956. doi: 10.3389/fmolb.2022.773956.
- Khan, A., B. Shamrez, U. Litaf, A. Zeb, Z. Rehman, R. Naz, S.H. Khan, and A.S. Shah. 2014. Effect of sucrose solution and chemical preservatives on overall quality of strawberry fruit. J. Food Process. Technol 6:2.
- Ledda, M., N. Cobo, A. Lorant, M. Hardigan, and S. Knapp (2020) Polyoligo: A bioinformatic platform for identifying target DNA sequences for the development of sub-genome specific DNA markers in polyploid/complex genomes. Annual conference of the American Society of Horticultural Sciences (2019), Las Vegas, NV, USA, p. 21–25
- Lee, S., Y.-H. Noh, J.A. Roach, J. Mangandi, S. Verma, V.M. Whitaker, and K.R. Cearley (2016) A high-throughput genotyping system combining rapid DNA extraction and high-resolution melting analysis in allo-octoploid strawberry. Acta. Hortic. 1156:89–94.
- Mangandi, J., S. Verma, L. Osorio, N.A. Peres, E. van de Weg, and V.M. Whitaker. 2017. Pedigree-based analysis in a multiparental population of octoploid strawberry reveals QTL alleles conferring resistance to Phytophthora cactorum. G3. Genes, Genomes, Genet. 7(6):1707–1719. doi: 10.1534/g3.117.042119.
- Nelson, J.R., S. Verma, N.V. Bassil, C.E. Finn, J.F. Hancock, G.S. Cole, S.J. Knapp, V.M. Whitaker, and E. Huang. 2021. Discovery of three loci increasing resistance to charcoal rot caused by macrophomina phaseolina in octoploid strawberry. G3 Genes|genomes|genet. 11(3):jkab037. doi: 10.1093/g3journal/jkab037.
- Nelson, R., T. Wiesner-Hanks, R. Wisser, and P. Balint-Kurti. 2018. Navigating complexity to breed disease-resistant crops. Nat. Rev. Genet. 19(1):21–33. doi: 10.1038/nrg.2017.82.
- Noh, Y.-H., S. Lee, V.M. Whitaker, K.R. Cearley, and J.-S. Cha. 2017. A high-throughput marker-assisted selection system combining rapid DNA extraction high-resolution melting and simple sequence repeat analysis: Strawberry as a model for fruit crops. J. Berry. Res. 7(1):23–31. doi: 10.3233/JBR-160145.
- Oh, Y., C.R. Barbey, S. Chandra, J. Bai, Z. Fan, A. Plotto, J. Pillet, K.M. Folta, V.M. Whitaker, and S. Lee. 2021. Genomic characterization of the fruity aroma gene, FaFAD1, reveals a gene dosage effect on γ-decalactone production in strawberry (Fragaria× ananassa). Front. Plant Sci. 12:639345. doi: 10.3389/fpls.2021.639345.
- Oh, Y., S. Chandra, and S. Lee. 2020. Development of subgenome-specific markers for FaRXf1 conferring resistance to bacterial angular leaf spot in allo-octoploid strawberry. Int. J. Fruit. Sci. 20(sup2):S198–S210. doi: 10.1080/15538362.2019.1709116.
- Oh, Y., J.D. Zurn, N. Bassil, P.P. Edger, S.J. Knapp, V.M. Whitaker, and S. Lee. 2019. The strawberry DNA testing handbook. HortScience. 54(12):2267–2270. doi: 10.21273/HORTSCI14387-19.
- Pincot, D.D., M.J. Feldmann, M.A. Hardigan, M.V. Vachev, P.M. Henry, T.R. Gordon, M. Bjornson, A. Rodriguez, N. Cobo, and R.A. Famula. 2022. Novel Fusarium wilt resistance genes uncovered in natural and cultivated strawberry populations are found on three non-homoeologous chromosomes. Theor. Appl. Genet. 135(6):2121–2145. doi: 10.1007/s00122-022-04102-2.
- Roach, J.A., S. Verma, N.A. Peres, A.R. Jamieson, W.E. van de Weg, M.C. Bink, N.V. Bassil, S. Lee, and V.M. Whitaker. 2016. FaRXf1: A locus conferring resistance to angular leaf spot caused by Xanthomonas fragariae in octoploid strawberry. Theor. Appl. Genet. 129(6):1191–1201. doi: 10.1007/s00122-016-2695-1.
- Salinas, N., Z. Fan, N. Peres, S. Lee, and V.M. Whitaker. 2020. FaRCa1 confers moderate resistance to the root necrosis form of strawberry anthracnose caused by Colletotrichum acutatum. HortScience. 55(5):693–698. doi: 10.21273/HORTSCI14807-20.
- Shulaev, V., D.J. Sargent, R.N. Crowhurst, T.C. Mockler, O. Folkerts, A.L. Delcher, P. Jaiswal, K. Mockaitis, A. Liston, and S.P. Mane. 2011. The genome of woodland strawberry (Fragaria vesca). Nat. Genet. 43(2):109–116. doi: 10.1038/ng.740.
- Verma, S., N.V. Bassil, E. van de Weg, R.J. Harrison, A. Monfort, J.M. Hidalgo, I. Amaya, B. Denoyes, L. Mahoney, T.M. Davis , et al. (2016) Development and evaluation of the Axiom® IStraw35 384HT array for the allo-octoploid cultivated strawberry Fragaria× ananassa. Acta. Hortic. 1156:75–82. doi: 10.17660/ActaHortic.2017.1156.10.
- Verma, S., J.D. Zurn, N. Salinas, M.M. Mathey, B. Denoyes, J.F. Hancock, C.E. Finn, N.V. Bassil, and V.M. Whitaker. 2017. Clarifying sub-genomic positions of QTLs for flowering habit and fruit quality in U.S. strawberry (Fragaria×ananassa) breeding populations using pedigree-based QTL analysis. Hortic. Res. 4(1): Res. 4. doi: 10.1038/hortres.2017.62.
- Whitaker, V.M. 2011. Applications of molecular markers in strawberry. J. Berry. Res. 1(3):115–127. doi: 10.3233/BR-2011-013.
- Whitaker, V.M., C.K. Chandler, N. Peres, M.C. Do Nascimento Nunes, A. Plotto, and C.A. Sims. 2015. Sensation™ ‘Florida127’ strawberry. HortScience. 50(7):1088–1091. doi: 10.21273/HORTSCI.50.7.1088.
- Whitaker, V.M., C. Dalid, L.F. Osorio, N.A. Peres, S. Verma, S. Lee, and A. Plotto. 2023. Florida pearl® ‘FL 16.78-109’ pineberry. HortScience. 58(1):143–146. doi: 10.21273/HORTSCI16951-22.
- Whitaker, V.M., S.J. Knapp, M.A. Hardigan, P.P. Edger, J.P. Slovin, V. Bassil, H.T. N, K.K. Mackenzie, S. Lee, S. Jung, et al. 2020. A roadmap for research in octoploid strawberry. Hortic. Res. 7(1). doi: 10.1038/s41438-020-0252-1.
- Whitaker, V.M., L.F. Osorio, N.A. Peres, Z. Fan, M. Herrington, M.C. Do Nascimento Nunes, A. Plotto, and C.A. Sims. 2017. ‘Florida beauty’strawberry. HortScience. 52:1443–1447. doi: 10.21273/HORTSCI12281-17.
- Whitaker, V.M., N.A. Peres, L.F. Osorio, Z. Fan, M.C. Do Nascimento Nunes, A. Plotto, and C.A. Sims. 2019. ‘Florida brilliance’strawberry. HortScience. 54(11):2073–2077. doi: 10.21273/HORTSCI14327-19.
- Wu, F., Z. Guan, and A.J. Whidden. 2020. An overview of the US and Mexico strawberry industries: FE971, 6/2015. EDIS 2016(1):4–4. doi: 10.32473/edis-fe971-2015.
- Zhou, L., J.-K. Wang, Q. Yi, Y.-Z. Wang, Y.-G. Zhu, and Z.-H. Zhang. 2007. Quantitative trait loci for seedling vigor in rice under field conditions. Field Crops Res. 100(2–3):294–301. doi: 10.1016/j.fcr.2006.08.003.
