Abstract
We have recently shown that the Epstein Barr virus (EBV) incorporates the autophagic membrane label LC3B-II into mature virus particles. Upon EBV production, autophagic membranes are stabilized and infectious viral particle production is dependent on these, because ATG protein-deficiency dampens, whereas rapamycin induces, infectious particle production. Moreover, viral DNA accumulates in the cytosol when macroautophagy is impaired. We therefore conclude that EBV needs autophagic membranes for efficient enveloping during infectious viral particle production. Here, we discuss how EBV might incorporate lipidated LC3B (LC3B-II) into the viral envelope and how other viruses as well as cellular processes customize the macroautophagy machinery for exocytosis in the context of unconventional secretion.
Autophagy is a highly conserved, lysosome-based cellular degradation system found in all eukaryotic cells and is regulated by at least 30 autophagy-related (ATG) gene products. Autophagy is fundamental in maintaining cellular homeostasis and survival, especially upon nutrient deprivation, which is a classical inducer of autophagy. However, it has also become apparent that ATG proteins can regulate endocytosis. Furthermore, we will focus in this punctum on the even more recent evidence that the same molecular machinery modifies exocytosis.
Unconventional secretion with autophagic proteins
Secretion in its conventional form occurs via the endoplasmic reticulum (ER)-to-Golgi pathway, involving signal-peptide recognition and translocation into the ER, from where vesicles bud to the Golgi and are then targeted to the plasma membrane. In contrast, autophagy has been linked to signal-peptide-independent exocytosis (). As the most prominent example, AcbA/Acb1 (acyl-Co enzyme A-binding protein or ACBD) is secreted in an unconventional manner in the amoeba Dyctostelium discoideum and the yeast Pichia pastoris during starvation. Here, autophagy gene products are shown to be involved in this process, along with factors that are required for the endosomal sorting complexes required for transport (ESCRT), proteins of the multivesicular body (MVB) pathway, the t-SNARE Sso1, and the Golgi stacking protein GORASP2/GRASP55. In addition, some proteins that are important for immune function are proposed to follow the same pathway. The mammalian pro-inflammatory cytokine IL1B/IL-1β was described to be secreted via ATG-dependent exocytosis. Similar to AcbA/Acb1, GORASP2/GRASP55 has also been implicated in IL1B secretion, next to the GTPase RAB8A. Furthermore, secretory lysosomes of osteoclasts, which in cytotoxic lymphocytes deliver target cell death-inducing molecules, seem to be released via the same pathway. However, the regulation of this process and how autophagosomes or amphisomes decide to fuse with lysosomes or the cell membrane, require further investigation.
Figure 1. Autophagic membranes participate in unconventional secretion. (A) Dual-function lysosomes, called secretory lysosomes, contain the relevant degradative proteins and can store newly synthesized cell-specific secretory proteins, e.g. cathepsins, PRF1/perforin, and granzymes. (B) During exophagy, ACBD is engulfed by autophagic membranes, which then fuse with MVBs. These subsequently fuse with the plasma membrane and release ACBD into the extracellular milieu. t-SNAREs are required for exophagy, whereas lysosomal fusion is dispensable. (C) During viral exophagy, the EBV nucleocapsid exits the nucleus through the acquisition of an envelope at the inner nuclear membrane (INM). This primary envelope is retained at the outer nuclear membrane (ONM), leading to the release of unenveloped capsid into the cytosol. The cytosol-located capsid undergoes a secondary envelopment step by fusion of vesicles decorated with viral glycoproteins and LC3B-II. Fusion of vesicles, containing the viral particles, with the plasma membrane releases the mature virion into the extracellular milieu.
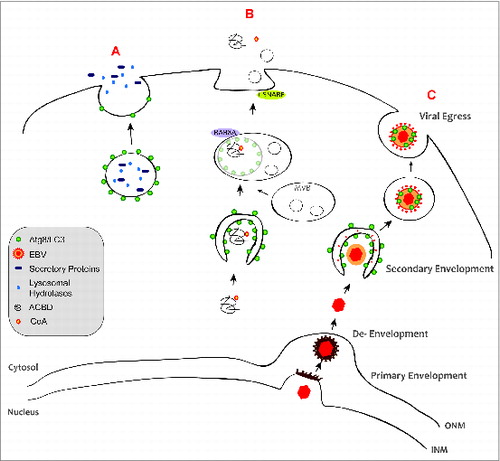
Viral Release with Autophagic Membranes
The herpesvirus EBV was discovered 50 years ago as the first human tumor virus and is a ubiquitous human pathogen. EBV's DNA is encased in an icosahedral capsid, surrounded by a protein tegument layer and further by an envelope. The source of the final secondary envelope is thought to be of perinuclear origin, including Golgi membranes. This requires membrane remodeling with a topology similar to autophagosomes (). Our study demonstrated, that the membrane-coupled form of the essential autophagy protein LC3B (LC3B-II) can be found in viral particles and that autophagic membranes seem to contribute to envelope acquisition of EBV in the cytosol. Accordingly, autophagic membranes are stabilized during EBV replication and without autophagic membrane generation, viral DNA accumulates in the cytosol. How LC3B-II-coupled membranes are recruited to the viral particle and prevented from fusing with lysosomes, remains to be elucidated.
Other Viruses Employ the Autophagy Machinery to Exit Cells
Various viruses of distinct families have been described to modify the autophagic machinery in order to egress from their host cells. Influenza A virus induces the inhibition of autophagosome-to-lysosome fusion by the virus matrix protein M2. M2 contains an LC3 interacting region (LIR), which enables the redirection of these LC3B-II coupled membranes to the plasma membrane during virus production. This mobilization to the plasma membrane is important for the filamentous budding and stability of viral particles in the extracellular milieu, presumably by delivering lipid resources to the plasma membrane. However, LC3B-II-coupled membranes could not be found in influenza virus particles. Similarly, human immunodeficiency virus (HIV) blocks autophagosome fusion with lysosomes via its Nef protein. This increases viral yields in myeloid cells. Furthermore, infection with poliovirus induces autophagic membrane accumulation, and the formation of double-membrane vesicles surrounding viral particles, which strongly resemble autophagosomes. These autophagosome-like structures seem to serve as a scaffold for membrane-associated replication. In addition, autophagy proteins have been described to be required for efficient nonlytic poliovirus egress, which is thought to involve unconventional secretion. As shown for the related Coxsackievirus, this might even result in nonenveloped viral particles being surrounded by autophagic membranes in the supernatant of producer cells. Thus, the release of several RNA viruses seems to benefit from autophagic membrane remodeling, but the DNA virus EBV seems to be the first example for autophagic membrane incorporation into the viral envelope.
Conclusions
The molecular machinery of autophagy likely provides a membrane source for EBV envelopes. LIR-mediated recruitment of LC3B-II to EBV potentially assists in the delivery of appropriate membrane resources to the maturing virion during second and final envelope acquisition in the cytosol. In light of our results, we speculate that viral proteins of the tegument or envelope interact with LC3B-II and recruit autophagic membranes via LIR motifs to the maturing viral particle. These interactions might offer new targets for interference with the assembly of this important human tumorvirus. Moreover, these findings suggest that the interaction of LIR-containing proteins with the autophagic membrane tag can serve a multitude of membrane recruitment functions. Classically, these LIR-containing receptors deliver substrates for sequestration by phagophores, but they seem to also influence fusion factor recruitment to phagosomes and, as shown in our study, might select cargo for unconventional secretion. Thus, LC3B and the other mammalian Atg8 orthologs seem to constitute membrane anchors for protein complex recruitment similar to phosphoinositides. These functional modules, although initially discovered in macroautophagy, might fulfill a variety of biological functions in eukaryotic cells.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
