Abstract
The induction of autophagy usually requires the activation of PIK3C3/VPS34 (phosphatidylinositol 3-kinase, catalytic subunit type 3) within a multiprotein complex that contains BECN1 (Beclin 1, autophagy related). PIK3C3 catalyzes the conversion of phosphatidylinositol into phosphatidylinositol 3-phosphate (PtdIns3P). PtdIns3P associates with growing phagophores, which recruit components of the autophagic machinery, including the lipidated form of MAP1LC3B/LC3 (microtubule-associated protein 1 light chain 3 β). Depletion of BECN1, PIK3C3 or some of their interactors suppresses the formation of MAP1LC3B+ phagophores or autophagosomes elicited by most physiological stimuli, including saturated fatty acids. We observed that cis-unsaturated fatty acids stimulate the generation of cytosolic puncta containing lipidated MAP1LC3B as well as the autophagic turnover of long-lived proteins in the absence of PtdIns3P accumulation. In line with this notion, cis-unsaturated fatty acids require neither BECN1 nor PIK3C3 to stimulate the autophagic flux. Such a BECN1-independent autophagic response is phylogenetically conserved, manifesting in yeast, nematodes, mice and human cells. Importantly, MAP1LC3B+ puncta elicited by cis-unsaturated fatty acids colocalize with Golgi apparatus markers. Moreover, the structural and functional collapse of the Golgi apparatus induced by brefeldin A inhibits cis-unsaturated fatty acid-triggered autophagy. It is tempting to speculate that the well-established health-promoting effects of cis-unsaturated fatty acids are linked to their unusual capacity to stimulate noncanonical, BECN1-independent autophagic responses.
In a screen designed to identify novel inducers of autophagy, we assessed the capacity of several fatty acids to induce cytosolic puncta containing a green fluorescent protein (GFP)-tagged variant of MAP1LC3B, which localizes to expanding phagophores upon lipidation, in human osteosarcoma U2OS cells. To discriminate between an increase in autophagic flux and a blockade in the degradation of autophagosomes (two situations that lead to the accumulation of MAP1LC3B+ puncta), we performed these experiments both in the absence and in the presence of lysosomal inhibitors. We found that the capacity of fatty acids to boost the autophagic flux is strongly influenced by the length of their carbon chains. In particular, we observed that saturated fatty acids with side chains containing 15 to 18 carbon atoms as well as cis-unsaturated fatty acids with side chains containing 14 to 20 carbon atoms efficiently stimulate the autophagic flux, whereas shorter and longer fatty acids fail to do so. This suggests that the physicochemical properties of fatty acids that are the determined by the length of their carbon chains (e.g., solubility, volubility) dictate, at least in part, their biological activity. Notably, autophagic responses elicited by saturated fatty acids (exemplified by palmitate and stearate) and their cis-unsaturated counterparts (exemplified by oleate and arachidonate) indistinguishably rely on downstream components of the autophagic machinery, such as ATG5 (autophagy related 5) and ATG7. However, an in-depth analysis revealed major functional differences between the autophagy-inducing potential of saturated and cis-unsaturated fatty acids.
The first hint in favor of such heterogeneity was obtained by observing the subcellular localization of GFP-MAP1LC3B+ puncta induced by saturated versus cis-unsaturated fatty acids. Indeed, at odds with their saturated counterparts, cis-unsaturated fatty acids promote the formation of GFP-MAP1LC3B+ puncta that colocalize with markers of the Golgi apparatus, resulting in a progressive change in its morphology and eventually in its fragmentation. Subsequent experiments involved brefeldin A, which indirectly inhibits the transfer of proteins from the endoplasmic reticulum to the Golgi apparatus by preventing the formation of SEC23A (Sec23 homolog A [S. cerevisiae])-coated transport vesicles. As brefeldin A structurally and functionally disrupts the Golgi apparatus, it also inhibits several manifestations of autophagy induced by cis-unsaturated (but not saturated) fatty acids, namely, the formation of GFP-MAP1LC3B+ puncta, the lipidation of endogenous MAP1LC3B detectable by immunoblotting, and the increased turnover of long-lived proteins. However, cis-unsaturated fatty acids do not cause any selective reduction in the expression of Golgi apparatus-associated proteins, and do not compromise the ability of the Golgi apparatus to catalyze protein glycosylation and to participate in exocytic and endocytic pathways, arguing against the idea that cis-unsaturated fatty acids promote the autophagic turnover of the Golgi apparatus itself.
Intrigued by these findings, we performed yet another screen in which we measured the capacity of fatty acids to induce the formation of intracellular vesicles containing PtdIns3P, which can be detected by means of a red fluorescent protein (RFP)-tagged variant of the FYVE zinc finger domain. While saturated fatty acids cause the accumulation RFP-FYVE+ puncta as do other autophagy inducers that we used as positive controls (e.g., rapamycin, nutrient deprivation), cis-unsaturated fatty acids fail to do so. RFP-FYVE-detectable PtdIns3P results from the phosphorylation of phosphatidylinositol by PIK3C3, which operates within a BECN1-containing multiprotein complex. In line with this notion, chemical inhibitors of PIK3C3 (such as 3-methyladenine or wortmannin) as well as small-interfering RNAs (siRNAs) targeting PIK3C3 or BECN1 fully inhibit all manifestations of autophagy elicited by saturated (but not cis-unsaturated) fatty acids. Importantly, such a functional heterogeneity could be detected in 4 different models, namely cultured human cancer cells, mice, the nematode Caenorhabditis elegans and the yeast Saccharomyces cerevisiae. In all cases, the knockdown or knockout of the BECN1 orthologs only blocks the induction of autophagy by saturated fatty acids, yet has no effect on autophagy elicited by cis-unsaturated fatty acids.
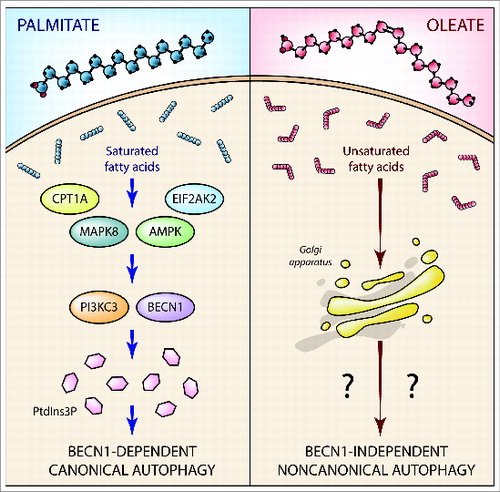
In addition, saturated fatty acids induce a canonical autophagic program that involves various nutrient sensors and proteins linked to lipid metabolism, including AMPK (AMP-activated protein kinase), EIF2AK2/PKR (eukaryotic translation initiation factor 2-α kinase 2), MAPK8/JNK1 (mitogen-activated protein kinase 8) and CPT1A (carnitine palmitoyltransferase 1A [liver]). In sharp contrast, none of these proteins is required for the induction of autophagy by cis-unsaturated fatty acids ().
Figure 1. Autophagy induction by saturated versus cis-unsaturated fatty acids. Saturated fatty acids, such as palmitate, elicit a conventional autophagic response that involves AMPK, EIF2AK2, MAPK8, CPT1A, and PIK3C3 as well as BECN1, and is accompanied by the production of PtdIns3P. Conversely, cis-unsaturated fatty acids, such as oleate, promote noncanonical autophagy, via a mechanism that relies on none of these proteins but requires an intact Golgi apparatus. The precise molecular mechanisms underlying noncanonical autophagy as induced by cis-unsaturated fatty acids remain to be determined.
Epidemiological studies indicate that cis-unsaturated fatty acids not only constitute essential nutrients but also are far superior to their saturated counterparts with respect to cardiovascular and metabolic health. Indeed, cis-unsaturated fatty acids have beneficial effects on obesity, metabolic syndrome, atherosclerosis and neurodegeneration. It is tempting to speculate that the capacity of cis-unsaturated fatty acids to stimulate noncanonical autophagy might promote a homeostatic program that is qualitatively different from, and perhaps quantitatively additive to, that ensured by conventional autophagic responses. If validated by future experiments, this hypothesis might have a major impact on our understanding of the relationship between diet and health span.
