Abstract
During xenophagy, pathogens are selectively targeted by autophagy receptors to the autophagy machinery for their subsequent degradation. In infected cells, the autophagy receptor CALCOCO2/NDP52 targets Salmonella Typhimurium to the phagophore membrane by concomitantly interacting with LC3C and binding to ubiquitinated cytosolic bacteria or to LGALS8/GALECTIN 8 adsorbed on damaged vacuoles that contain bacteria. We recently reported that in addition, CALCOCO2 is also necessary for the maturation step of Salmonella Typhimurium-containing autophagosomes. Interestingly, the role of CALCOCO2 in maturation is independent of its role in targeting, as these functions rely on distinct binding domains and protein partners. Indeed, to mediate autophagosome maturation CALCOCO2 binds on the one hand to LC3A, LC3B, or GABARAPL2, and on the other hand to MYO6/MYOSIN VI, whereas the interaction with LC3C is dispensable. Therefore, the autophagy receptor CALCOCO2 plays a dual function during xenophagy first by targeting bacteria to nascent autophagosomes and then by promoting autophagosome maturation in order to destroy bacteria.
Xenophagy is the process referring to the selective degradation of intracellular microorganisms by autophagy. Xenophagy is a very potent intrinsic cellular line of defense to fight pathogens and requires first the detection and targeting of microorganisms to growing phagophores prior to autophagosome maturation leading to microbial destruction. The targeting step can be achieved by cytosolic autophagy receptors, which bind on the one hand to the pathogen and on the other hand to LC3, a phagophore membrane-anchored protein. Once entrapped within an autophagosome, bacteria can survive or escape, unless they are rapidly destroyed. Therefore, autophagosome maturation allows the discharge of lysosomal enzymes in autolysosomes, allowing destruction of the bacteria. It is, however, not well known how autophagosomes mature, especially in the context of xenophagy. Recently, the endosomal membrane-bound protein TOM1 and the dynein motor MYO6 have been both shown to be implicated in the transport of endosomes into the vicinity of autophagosomes in order to ensure fusion of autophagosomes with vesicles of the endo/lysosomal pathway. Moreover, the concomitant absence of 3 autophagy receptors, CALCOCO2, TAX1BP1/T6BP, and OPTN/OPTINEURIN, impairs autophagosome biogenesis and maturation. As CALCOCO2 was already shown to have a MYO6 binding domain, we wondered whether CALCOCO2 could also be implicated in autophagosome maturation per se to promote bacterial degradation.
We first observed that the binding site of CALCOCO2 to MYO6 was required for cells to control Salmonella Typhimurium intracellular growth. Nevertheless, when the binding of CALCOCO2 to MYO6 was abolished, bacteria were still efficiently targeted to autophagosomes, but yet still able to replicate to levels similar to the one observed in CALCOCO2-depleted cells. Strikingly, in noninfected cells the absence of CALCOCO2 perturbs the autophagy flux, resulting in a strong accumulation of autophagosomes, suggesting a positive role for CALCOCO2 in the autophagosome-lysosome fusion process. Surprisingly, we found that CALCOCO2 binding to LC3C, through its noncanonical LC3 interacting region (CLIR), is not involved in the maturation of autophagosomes. Instead, we identified another motif in the primary sequence of CALCOCO2, which mediates binding to at least LC3A, LC3B, and GABARAPL2 (but not LC3C). We referred to this motif as “LIR-like” as it differs from the canonical LIR motif by the absence of a hydrophobic residue in position X3. This LIR-like motif was necessary for autophagosome maturation, along with the domain of CALCOCO2 responsible for its binding to MYO6. Eventually, mutation of this LIR-like motif also resulted in an increased Salmonella Typhimurium intracellular proliferation, whereas bacteria were still efficiently targeted within nondegradative autophagosomes. Interestingly, the absence of the autophagy receptor OPTN also led to the accumulation of nondegradative autophagosomes, suggesting that other autophagy receptors could share CALCOCO2 dual functions in xenophagy.
Having autophagy receptors ensuring both targeting and degradation of pathogens could be an important evolutionary advantage against infections. Indeed, this mechanism could help to reduce the delay necessary for maturation, thus avoiding adaptation of the pathogen to its new environment (as proposed for Coxiella burnetti, Listeria monocytogenes, and Legionella pneumophila) or its escape from the autophagosome. Conversely, pathogens could avoid autophagy entrapment or autophagic degradation by targeting CALCOCO2 or any other autophagy receptors, which could play similar roles. For instance Chikungunya virus was reported to target CALCOCO2 in human cells leading to increased virus replication. Nevertheless, redundancy among autophagy receptors could also ensure a selective immune advantage against pathogens targeting any one of these receptors.
Our results and those from others suggest for now that CALCOCO2 serves as a docking platform for MYO6-bound endosomes, thus facilitating autophagosome maturation (). How this action is coordinated with CALCOCO2 directing pathogens to the phagophore membranes remains unclear. During xenophagy against Salmonella Typhimurium, CALCOCO2 interaction first with LC3C is necessary to further recruit other ATG8 orthologs and ensure the final degradation of bacteria. Since the LIR-like motifs bind several ATG8s, whereas the CLIR motif only mediates binding to LC3C, it is possible that binding of CALCOCO2 to LC3C induces conformational changes and uncovers the LIR-like motif that can be then engaged with other ATG8 orthologs to trigger autophagosome maturation. Moreover, it is still unclear whether the action of CALCOCO2 in autophagosome maturation is coordinated with other partners, such as STX17/SYNTAXIN 17, which is recruited on the external membrane of autophagosomes and regulate fusion with lysosomes.
Figure 1. Schematic model for the dual role of CALCOCO2 in xenophagy. CALCOCO2 targets bacteria to the phagophore through its LC3C binding site (CLIR motif), and, independently, regulates autophagosome maturation through its LC3A, LC3B, or GABARAPL2 binding site (LIR-like motif) and its MYO6 interacting region.
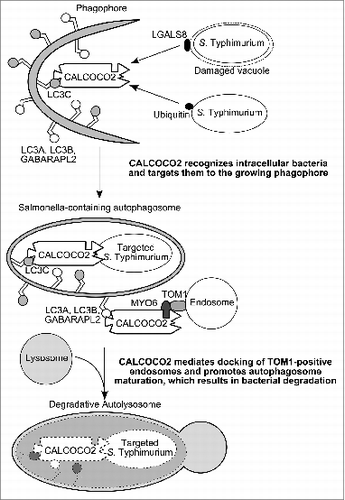
Our findings reveal a new role for the autophagy receptor CALCOCO2 in autophagosome maturation, unravelling another function for CALCOCO2 in cell autonomous defense against pathogens: CALCOCO2 not only targets pathogens to phagophore membranes, but also regulates subsequent maturation of pathogen-containing autophagosomes, thus assuring efficient degradation of autophagy-targeted pathogens.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
