Abstract
WDR45/WIPI4, encoding a WD40 repeat-containing PtdIns(3)P binding protein, is essential for the basal autophagy pathway. Mutations in WDR45 cause the neurodegenerative disease β-propeller protein-associated neurodegeneration (BPAN), a subtype of NBIA. We generated CNS-specific Wdr45 knockout mice, which exhibit poor motor coordination, greatly impaired learning and memory, and extensive axon swelling with numerous axon spheroids. Autophagic flux is defective and SQSTM1 (sequestosome-1)/p62 and ubiquitin-positive protein aggregates accumulate in neurons and swollen axons. Nes-Wdr45fl/Y mice recapitulate some hallmarks of BPAN, including cognitive impairment and defective axonal homeostasis, providing a model for revealing the disease pathogenesis of BPAN and also for investigating the possible role of autophagy in axon maintenance.
Abbreviations:
- ACTB, β-actin
- AMC, aminomethylcoumarin
- Atg, autophagy-related
- BPAN, β-propeller protein-associated neurodegeneration
- CALB, calbindin
- CNS, central nervous system
- DCN, deep cerebellar nuclei
- Ei24, etoposide-induced gene 24
- epg, ectopic P granule
- fEPSP, field excitatory postsynaptic potential
- GFAP, glial fibrillary acid protein
- H&E, hematoxylin and eosin
- KO, knockout
- LC3, microtubule-associated protein 1 light chain 3
- LTP, long-term potentiation
- MBP, myelin basic protein
- NBIA, neurodegeneration with brain iron accumulation
- RBFOX3, RNA binding protein, fox-1 homolog (C. elegans) 3
- rpm, rotations per min
- SENDA, static encephalopathy of childhood with neurodegeneration in adulthood
- SQSTM1, sequestosome-1
- WDR5/WIPI4, WD repeat domain 45
- WT, wild type.
Introduction
Autophagy is a lysosome-mediated degradation system, in which a portion of cytosol is engulfed in an enclosed double-membrane vesicle, termed the autophagosome, and subsequently delivered to the lysosome for degradation.Citation1 A group of evolutionarily conserved Atg (autophagy-related) genes and metazoan-specific epg (ectopic P granule) genes, including epg-3, -4, -5, and -6, has been identified from yeast and worm genetic studies that act at distinct steps of the autophagy pathway in higher eukaryotes.Citation1-3 The basal constitutive level of autophagy functions as a quality control mechanism by removing misfolded proteins and/or damaged organelles.Citation4 Under physiological conditions, autophagy is crucial for maintaining neuron homeostasis. Mice deficient in autophagy genes essential for autophagosome formation, such as Atg5, Atg7, and Ei24 (etoposide-induced gene 24, the mammalian ortholog of Caenorhabditis elegans epg-4), exhibit massive neuron loss and axonal degeneration, accompanied by dramatic accumulation of ubiquitinated protein aggregates.Citation5-7
Recent human genetic studies reveal that de novo mutations in WDR45 (WD repeat domain 45) cause BPAN, previously known as SENDA (static encephalopathy of childhood with neurodegeneration in adulthood), which is a subtype of neurodegeneration with brain iron accumulation (NBIA).Citation8-10 Mammalian WDR45 is the ortholog of worm epg-6 and one of the orthologs of yeast Atg18 and is essential for an early step of autophagosome formation.Citation3 NBIA encompasses a heterogeneous group of progressive extrapyramidal disorders characterized by parkinsonism, intellectual deterioration, deposition of iron in the basal ganglia, and pathologically, the presence of axonal spheroids in the CNS (central nervous system).Citation11,12 BPAN patients have static encephalopathy in childhood, characterized by psychomotor retardation with or without concurrent spasticity, and then develop sudden-onset dystonia-parkinsonism and dementia in adulthood.Citation12 Samples from affected BPAN patients display lower autophagic activity and accumulation of aberrant early autophagic structures, suggesting that WDR45 functions in maintaining neural homeostasis through its role in autophagy.Citation10
To study the relationship between WDR45 and neurodegeneration, we generated CNS-specific Wdr45 knockout (KO) mice. These mice displayed learning and memory defect and axonal swelling. Wdr45 deficiency impaired the autophagic flux with accumulation of SQSTM1- and ubiquitin-positive aggregates in neurons and swollen axons. Our study indicates that the Nes-Wdr45fl/Y mice exhibit hallmarks of BPAN, providing a model for understanding the neurodegeneration process underlying the pathogenesis of BPAN.
Results
Nes-Wdr45fl/Y mice develop learning and memory impairments
We generated conditional KO mice by flanking exons 8 to 14 of Wdr45 with 2 Loxp sequences (). Wdr45 locates on the X chromosome. Wdr45fl/fl mice were crossed with Nes-Cre mice to produce CNS-specific Wdr45 knockout mice. Real-time PCR confirmed the absence of Wdr45 mRNA in various parts of the CNS in Nes-Wdr45fl/Y mice (). Nes-Wdr45fl/Y mice were born normally, and showed no obvious growth retardation. Neither motor dysfunctions, such as tremor, hypokinesia, rigidity and dystonia, nor limb-clasping reflexes were evident in Nes-Wdr45fl/Y mice. Rotarod experiments were performed to examine motor coordination. Nes-Wdr45fl/Y mice at 7 to 8 mo showed no significant difference at 20 rotations per min (rpm) (), but Nes-Wdr45fl/Y mice at 11 to 13 mo spent less time on the rotarod compared to their wild-type (WT) littermates when assessed at higher than 5 rpm (), indicating that aged Nes-Wdr45fl/Y mice showed impaired motor coordination.
Figure 1. For figure legend, see page 884.Figure 1. (See previous page) Nes-Wdr45fl/Y mice show cognitive impairment and LTP attenuation. (A) Scheme for generating Wdr45 conditional knockout mice. (B) Southern blot analysis of genomic DNA from +/+ and F/+ ES clones. After digestion with SpeI, the WT and flox alleles were detected as 12- and 7.7-kb bands with a 5′ probe, respectively. After digestion with NdeI, the WT and flox alleles were detected as 14- and 8.4-kb bands with 3′ probe, respectively. (C) Wdr45 mRNA level in different tissues of WT and Nes-Wdr45fl/Y mice at 13 mo. Results are representative of at least 3 experiments. Compared to WT mice, the Wdr45 mRNA level is slightly decreased in the heart of Nes-Wdr45fl/Ymice, probably due to nonspecific expression of Nes-Cre (http://cre.jax.org/Nes/Nes-CreNano.html). (D) Rotarod performance of Nes-Wdr45fl/Y and Nes-Wdr45fl/+ mice (7 to 8 mo) at rolling speed of 20 rpm. Mean±SEM of 11 mice is shown. (E) Rotarod performance of Nes-Wdr45fl/Y and Nes-Wdr45fl/+ mice at rolling speeds of 5, 10, 20, and 40 rpm. *, P < 0.05; **, P < 0.01. (F–H) In the Morris water maze test, Nes-Wdr45fl/Ymice show decreased learning and memory ability in the learning and probe test, respectively. The swimming speeds show no obvious difference between Nes-Wdr45+/Y and Nes-Wdr45fl/Y mice. (I) Nes-Wdr45fl/Y mice display a reduced percentage of correct alternations, compared with Nes-Wdr45+/Y mice in a Y-maze test. No obvious difference in the total number of alternations was detected between WT and Nes-Wdr45fl/Y mice. (J) In a contextual fear conditioning test, the percentage of the test time taken up by a freezing response is significantly lower in Nes-Wdr45fl/Y mice. In a cued test, the freezing time is slightly reduced in Nes-Wdr45fl/Ymice. Mean±SEM of 11 mice (11 to 13 mo) is shown (C–H). (K and L) Impaired LTP in Nes-Wdr45fl/Y mice. The upper part in (K) shows averaged fEPSPs comparing baseline and the last 5 min in Nes-Wdr45+/Y and Nes-Wdr45fl/Y mice. LTP was not induced in 7 out of 11 hippocampal slices from 3 Nes-Wdr45fl/Y mice, while LTP was normally induced in all of 5 slices from 2 WT mice. Only slices with LTP induction were analyzed here. Averaged responses from the last 5 min in control and Nes-Wdr45fl/Y mice (L).
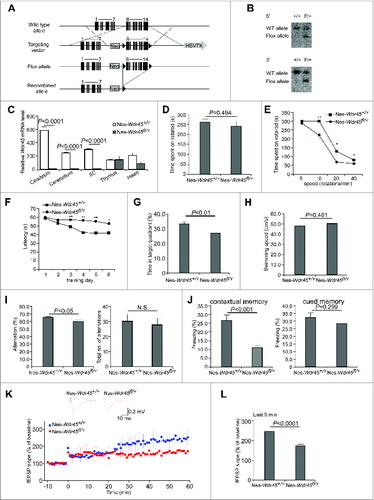
The hallmark neurological defect associated with BPAN is intellectual deterioration.Citation12 We thus performed a series of behavioral tests to determine the learning and memory ability of Nes-Wdr45fl/Y mice. Since the performances of female animals change during various phases of the estrous cycle,Citation13 we analyzed only males. The Morris water maze test involves recognizing and remembering visual cues to locate a hidden platform, thus measuring learning and memory function. In training sessions, Nes-Wdr45fl/Y mice showed significantly prolonged latency to find the hidden platform compared to controls (). Furthermore, Nes-Wdr45fl/Y mice spent significantly less time in the target quadrant than control littermates without the platform during the probe sessions, suggesting impaired retention of spatial memory (). Swimming speeds were similar for Nes-Wdr45fl/Y and control mice (). In a Y-maze test for spontaneous alternation behavior, Nes-Wdr45fl/Y mice were less likely than controls to explore alternate arms of the Y-maze within an 8-min test period, reflecting impaired immediate spatial working memory performance (). In a fear conditioning test, on the training day, mice were placed in a chamber and received paired conditioned stimulus (a 70 dB tone) and adverse stimulus (an electric foot shock). On the testing day, in the contextual test, Nes-Wdr45fl/Y mice showed a significant decrease in freezing time compared to controls when the mice were put in the same chamber, indicating that Wdr45 deficiency causes impaired fear recall in the same context. In the cued test, Nes-Wdr45fl/Y mice displayed no significant difference in the freezing response with the conditioned stimulus, compared with WT mice (). Thus, Nes-Wdr45fl/Y mice show significant deficits in cognitive function.
The hippocampus plays a crucial role in the consolidation of information from short-term memory to long-term memory and spatial navigation.Citation14 Long-term potentiation (LTP), a well-characterized form of synaptic plasticity, has been postulated as a cellular correlate of learning and memory.Citation15 To investigate whether the behavioral deficits in Nes-Wdr45fl/Y mice were associated with altered hippocampal synaptic plasticity, LTP was evaluated at Schaffer Collateral (SC)-CA1 synapses in hippocampal slices induced by θ-burst stimulation. LTP was significantly attenuated in Nes-Wdr45fl/Y mice, as indicated by the decreased field excitatory postsynaptic potential (fEPSP) amplitude (). This is consistent with the defect in learning and memory in Nes-Wdr45fl/Y mice.
Axonal swelling in Nes-Wdr45fl/Y mice
To investigate the neural defect in Nes-Wdr45fl/Y mice, we performed histological analyses on sections from CNS of control and Nes-Wdr45fl/Y mice at 13 mo. No extensive or selective neuron loss was observed, unlike in mice deficient in several other autophagy genes.Citation5-7,16 The number of Purkinje cells in the cerebellum and hippocampus pyramidal cells in the CA1 region showed no significant change in Nes-Wdr45fl/Y mice compared to controls (). GFAP (glial fibrillary acid protein) staining showed mild reactive astrogliosis in various brain regions of Nes-Wdr45fl/Y mice, including the cortex, hippocampus, thalamus, hypothalamus, caudate nucleus, deep cerebellar nuclei (DCN), and pons (, and data not shown). Thus, Wdr45 deficiency causes mild neural damage.
Figure 2. Nes-Wdr45fl/Ymice show axon swelling. (A) The number of Purkinje cells was quantified and divided by the total length of the lobules III, IV and V. (B) The number of CA1 hippocampal pyramidal cells of Nes-Wdr45+/Y and Nes-Wdr45fl/Y mice at 13 mo was quantified and divided by the length of the layer. Mean ± SEM of 3 mice is shown (A and B). (C–E) Compared to control mice, the GFAP signals (red) in sections of hippocampus (C) and cortex (D) are stronger in Nes-Wdr45fl/Y mice. Quantification data are shown as mean±SEM of 3 mice (E). Bar: 50µm. (F) H&E staining reveals the presence of eosinophilic spheroids (arrow) in the cortex. (G) H&E staining shows vacuolated structures (arrows) in thalamus. (H and I) H&E staining reveals the presence of eosinophilic spheroids (arrows) in the DCN of Nes-Wdr45fl/Y mice at 13 mo. Bar: 20 µm (F–I). (J and K) Costaining of CALB (green) and MBP (red) shows that Nes-Wdr45fl/Y mice at 13 mo exhibit dilated CALB-positive bulbs (arrows) in the DCN region. Bar: 10 µm. (L to Q) EM pictures of DCN in Nes-Wdr45+/Y (L and N) and Nes-Wdr45fl/Y (M, O, P and Q) mice at 13 mo. Arrows in (M) indicate swollen axons. Swollen mitochondria accumulate in these axons (arrows in O).The arrow in (P) indicates a demyelinated axon. Degenerated axons were also occasionally detected (Q). Bar: 2 µm (L and M), 500 nm (N–Q). (R and S) EM pictures of medulla in Nes-Wdr45+/Y (R) and Nes-Wdr45fl/Y (S) mice at 13 mo. Arrows in (S) indicate degenerated axons. Bar: 500 nm. (T–X) H&E staining of the DCN from Wdr45 mutant mice and controls at 6 wk and 4 mo. Arrows indicate eosinophilic spheroids. Quantification data are shown as mean±SEM of 3 mice (X). Bar: 20µm.
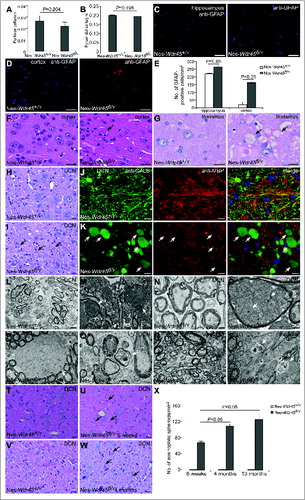
White matter changes are pathological features associated with BPAN.Citation12 We next determined whether homeostasis of myelinated axons is affected by Wdr45 deficiency. Hematoxylin and eosin (H&E) staining revealed that eosinophilic spheroids, representing axon swellings, were scattered in various cerebral regions, including cortex, thalamus, and hypothalamus (, and data not shown). Clusters of vacuolated structures were found in the thalamus, inferior colliculus, and medulla of Nes-Wdr45fl/Y mice ( and data not shown). We also examined the cerebellar white matter. Numerous large eosinophilic spheroid structures were observed in the DCN, indicative of axonal swellings (). We further stained the DCN region with anti-CALB/calbindin antibody, which specifically labels Purkinje cell axons, and found that a large number of huge bulging CALB-positive structures (some of them surrounded by MBP (myelin basic protein)-labeled myelin) were present in Nes-Wdr45fl/Y mice, indicating Purkinje cell axonal swellings (). Ultrastructural study further confirmed accumulation of swollen axons in the DCN of Nes-Wdr45fl/Y mice (), and abundant swollen mitochondria were found in these axons (). Demyelinated axons were also detected, as shown in . We occasionally found degenerated axons in the DCN () and medulla of Nes-Wdr45fl/Y mice (). We analyzed brain sections at 6 wk and 4 mo and found that eosinophilic spheroids were formed progressively (). Thus, Wdr45 plays a critical function in axon homeostasis.
Accumulation of SQSTM1- and ubiquitin-positive aggregates in Nes-Wdr45fl/Y mice
To assess whether the autophagic flux is impaired in Nes-Wdr45fl/Y mice, we performed immunostaining of brain sections to detect SQSTM1 and ubiquitin, 2 well-characterized autophagy substrates that are elevated after inhibition of autophagy.Citation17 Compared to Nes-Wdr45+/Y mice, in some brain regions of Nes-Wdr45fl/Y mice, including thalamus, DCN, and medulla, both SQSTM1- and ubiquitin-positive aggregates accumulated, and these aggregates largely colocalized with each other ( and data not shown). Accumulation of ubiquitin-positive aggregates was also found in the caudate nucleus and the molecular layer of the dentate gyrus of the hippocampus in Nes-Wdr45fl/Y mice, without evident SQSTM1 accumulation (). Sqstm1 mRNA levels were not upregulated in Nes-Wdr45fl/Y mice (). Proteasome activity was not altered in brain extracts of Nes-Wdr45fl/Y mice (). Our previous study shows that loss of function of epg-6 causes accumulation of ATG-9 puncta.Citation3 Immunostaining assays demonstrated that ATG9-positive structures were also moderately increased in the DCN region of Nes-Wdr45fl/Y mice ().
Figure 3. Nes-Wdr45fl/Y mice exhibit defective autophagic flux. (A–D) Coimmunostaining with anti-SQSTM1 (red) and anti-ubiquitin (green) antibodies in DCN (A), thalamus (B), hippocampus (C) and caudate nucleus (D) of Nes-Wdr45fl/Y mice at 13 mo. (E) No increase in the Sqstm1 mRNA level is detected in Nes-Wdr45fl/Y mice. (F) Compared to control littermates, proteasome activity is not altered in brain extracts of Nes-Wdr45fl/Y mice at 13 mo. Proteasome activity was measured using AMC-linked substrate peptides. A: Ac-Gly-Pro-Leu-Asp-AMC; B: Suc-Leu-Leu-Val-Tyr-AMC; C: Ac-Arg-Leu-Arg-AMC; D: Boc-Leu-Arg-Arg-AMC. Results are representative of 2 experiments. (G and H) Anti-ATG9 staining shows that ATG9 puncta accumulate in the DCN of Nes-Wdr45fl/Y mice at 13 months. Quantification data are shown as mean±SEM of 3 mice (1 unit = 104 μm2) (H). (I and J) Anti-RBFOX3 (green) and anti-SQSTM1 (red) costaining shows that SQSTM1 aggregates are located in pyramidal neurons in the cortex (I) and hippocampus (J) of Nes-Wdr45fl/Y mice at 13 mo. (K) Anti-ubiquitin (green) and anti-CALB (red) costaining in the DCN of Nes-Wdr45fl/Y mice at 13 mo (arrows indicate dilated axonal terminals). Bar: 10 µm (A–D, G, and I–K). (L and M) Immunoblotting assays show that levels of LC3-I, LC3-II and SQSTM1 are increased in Wdr45-deficient neurons. Data are relative to ACTB level and representative of 3 independent experiments. (N and O) Immunoblotting assays show that levels of LC3-I and LC3-II are comparable between WT and Wdr45-deficient neurons after 6 h treatment with 20 μM bafilomycin A1. Data are relative to ACTB level and representative of 3 independent experiments.
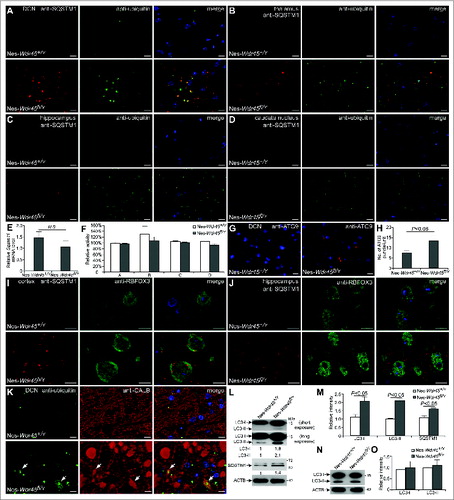
We costained SQSTM1 with different neural cell markers to determine which type of neural cell is affected by Wdr45 deficiency. In Nes-Wdr45fl/Y mice, neurons detected by anti-RBFOX3/NeuN (RNA binding protein, fox-1 homolog [C. elegans] 3), including pyramidal cells in hippocampus and cortex, contained SQSTM1 aggregates (), while SQSTM1 accumulation was hardly observed in oligodendrocytes (data not shown). In the DCN region, ubiquitin and CALB costaining assays revealed that ubiquitin-positive aggregates were present in small axon spheroids but were mostly absent from large ones (), suggesting that the ubiquitin inclusions became diffuse or were removed during later axon swelling.
To directly detect whether Wdr45 deficiency blocks neuronal autophagic flux, we cultured primary neurons from WT and Nes-Wdr45fl/Y mice. Immunoblotting assays revealed that levels of LC3 (Microtubule-associated protein 1 light chain 3)-I, LC3-II, and SQSTM1 increased in Wdr45 KO neurons (). After treatment with bafilomycin A1, a potent inhibitor of the vacuolar H+ ATPase that inhibits the lysosomal degradation of LC3-II, there is no difference of LC3-II level between control and mutant cells (), suggesting that autophagic flux is impaired in Wdr45-deficient neurons.
Axonal swelling and autophagy defects in female Nes-Wdr45fl/fl mice
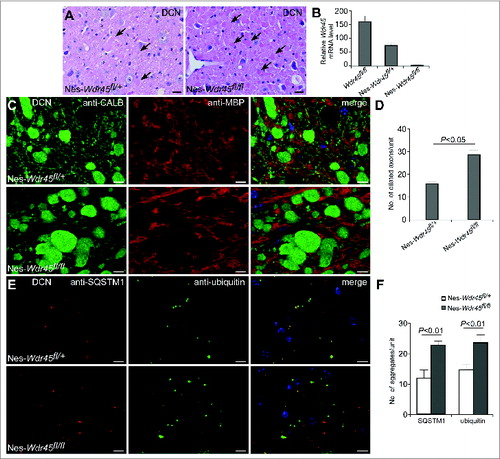
As Wdr45 locates on the X chromosome, we examined whether there is a gender-dependent difference in the axonal swelling and autophagy defects. Numerous eosinophilic spheroids accumulated in female Nes-Wdr45fl/fl mice, just as in males (). Real-time PCR confirmed the absence of Wdr45 mRNA in the cerebrum of Nes-Wdr45fl/fl mice (). The Wdr45 mRNA level in the cerebrum of Nes-Wdr45fl/+ mice was also reduced compared to WT mice (). Dilated axons, detected by anti-CALB and anti-MBP costaining, were present, and SQSTM1- and ubiquitin-positive aggregates accumulated in the DCN of female Nes-Wdr45fl/+ mice and Nes-Wdr45fl/fl mice. The defects in the heterozygous mice are less severe than in the homozygous mice ().Therefore, similar to male Wdr45mutant mice, female Wdr45-deficient mice also displayed axon swelling and autophagy defects.
Figure 4. Axon swelling and autophagy defects in Nes-Wdr45fl/fl and Nes-Wdr45fl/+ female mice. (A) H&E staining of cerebellar sections shows accumulation of eosinophilic spheroids (arrows) in both Nes-Wdr45fl/+ and Nes-Wdr45fl/fl female mice at 13 mo. Bar: 20 µm. (B) The Wdr45 mRNA level in the brain of WT, Nes-Wdr45fl/+ mice and Nes-Wdr45fl/fl mice. Results are representative of at least 3 experiments. (C and D) Costaining of CALB (green) and MBP (red) in DCN of Nes-Wdr45fl/+ and Nes-Wdr45fl/fl female mice at 13 mo. Quantification data are shown as mean±SEM of 3 mice (1 unit = 104 μm2) (D). (E and F) SQSTM1 (red) and Ubiquitin (green) costaining in DCN of Nes-Wdr45fl/+ and Nes-Wdr45fl/fl female mice at 13 mo. Quantification data are shown as mean ± SEM of 3 mice (1 unit = 104 μm2) (F). Bar: 10 µm (C and E).
Discussion
Here we show that CNS-specific Wdr45 KO mice develop axonal swelling and behavioral abnormalities, including motor deficits and learning and memory impairment. The cognitive defects in Nes-Wdr45fl/Y mice may be associated with impaired axon homeostasis and/or accumulation of ubiquitin-positive inclusions in the hippocampus and caudate nucleus. Previous study has reported that lesions of the hippocampus affect fear conditioning to the context but not to the cue, while damage of the amygdala interferes with the conditioning to both the cue and the context.Citation18 Nes-Wdr45fl/Y mice display obvious impairment in the contextual fear conditioning test, but not in the cued test, supporting that Wdr45 deficiency mainly influences the function of hippocampus. This result is also in consistent with the attenuated LTP at SC-CA1 synapses in the hippocampus of Nes-Wdr45fl/Y mice. The poor motor coordination in Nes-Wdr45fl/Y mice is probably caused by the lesion in the DCN region of the cerebellum, which contributes to coordination, precision, and accurate timing of movements. Nes-Wdr45fl/Y mice show no signs of iron deposition in the basal ganglia. In NBIA, iron accumulation is variable and sometimes occurs later in the course of the disease after symptoms have already become evident. Moreover, iron accumulation is sometimes absent even in patients with demonstrable mutations in the causative genes associated with NBIA.Citation12
Mice deficient for autophagy genes that function at distinct steps show distinct neuropathological deficits. Neural-specific depletion of Atg5, Atg7, and Ei24 causes massive neuron loss, while Epg5-deficient mice exhibit selective vulnerability of motor neurons.Citation5-7,16Epg5 acts at a late step of autophagy and loss of function of Epg5 causes accumulation of nondegradative autolysosomes.Citation16 epg-6 acts at an early step of autophagosome formation.Citation3 However, compared to Atg5-, Atg7-, and Ei24-deficient mice, both the autophagy defect and the neural damage are much milder in Nes-Wdr45fl/Y mice, which could be because the other 3 Wipi-family genes, Wipi1, Wipi2, and Wdr45b/Wipi3, function redundantly in the autophagy pathway.Citation19Axon spheroids represent swollen or distended axons, possibly secondary to defects in axonal transport. Autophagy is required for normal axon terminal membrane trafficking and turnover, and plays an essential role in the maintenance of axonal homeostasis and prevention of axonal degeneration.Citation20,21 Accumulation of axonal spheroid structures in Wdr45 mice indicates that axoN-terminals are more vulnerable to autophagy impairment than dendrites and neurons. Purkinje cell-specific deletion of Atg7 shows that ablation of autophagy leads to abnormal swellings and dystrophy of axon terminals in the DCN, preceding the dentritic atrophy, neuron loss, and behavioral deficits.Citation22 Another possibility is that Wdr45 may have some functions independent of autophagy, which are important for maintaining neural homeostasis and learning and memory ability. Autophagy proteins also function in autophagy-independent processes.Citation23 For example, the essential autophagy gene Atg5 is required for cellular immunity to intracellular pathogens via autophagosome-independent processes such as GTPase trafficking.Citation24 The Nes-Wdr45fl/Y mice provide a valuable model for understanding the neurodegeneration process underlying the pathogenesis of BPAN, and also for preclinical testing and exploration of therapeutic interventions.
Materials and Methods
Mice
The Wdr45 targeting vector was constructed by flanking exons 8 to 14 of Wdr45 by 2 loxP sequences. After electroporating the linearized vector into 129 R1 embryonic stem cells, positive clones with homologous recombinants were identified by Southern blot analysis, as shown in . Southern blot analysis was performed by digestion of genomic DNA with SpeI and hybridization with the 5′ probe (or NdeI and hybridization with 3′ probe). The positive clone was then microinjected into C57BL/6N blastocysts. Chimeric males were mated with wild-type C57BL/6N females, and heterozygous and hemizygous mutant offspring were backcrossed to C57BL/6 mice.
All mice were kept under specific pathogen-free conditions in the animal facility at the Institute of Biophysics, Chinese Academy of Sciences, Beijing. All animal experiments were approved by the institutional committee of the institute.
Antibodies
The following antibodies were used: rabbit anti-SQSTM1 (MBL, PM045), mouse anti-SQSTM1 (Abcam, ab56416), mouse anti-ubiquitin (Cell Signaling Technology, 3936), rabbit anti-GFAP (Bioss, bs-0199R), mouse anti-RBFOX3/NeuN (Millipore, MAB377), rabbit anti-LC3 (Cell Signaling Technology, 2775S), mouse anti-CALB/calbindin (Sigma, C9848), rabbit anti-CALB/calbindin (Abcam, ab25085), rabbit anti-ATG9 (MBL, PD042), and rabbit anti-MBP (Abcam, ab40390).
Rotarod test
Motor coordination was measured as described previously with some modifications.Citation7 After trained on the rolling rod at 5 rpm for 5 min for one d, mice were tested for their ability to remain on the rotarod at 5, 10, 20, and 40 rpm. The time when the mice fell from the rod was recorded, with a maximum observation time of 5 min.
Morris water maze test
A round tub (diameter 120 cm, height 45 cm) was filled with opaque water (20°C) to a depth of 25 cm. A platform (diameter 9 cm, height 24 cm) was located in the center of one quadrant. In the learning test, the mouse was placed in the maze starting from one of 4 predetermined spots and was given 60 s to find the fixed platform. The time taken to reach the platform (escaping latency) was recorded by a Smart 2.0 video tracking system (Panlab, Harvard Apparatus, Cornellà (Barcelona), Spain). Each mouse received 4 trials per day for 6 consecutive d. On the 7th d, the probe test was performed without the platform. The mouse was allowed to search for 60 s, and the percentage of time spent in the target quadrant was recorded to monitor spatial memory ability.
Y-maze test
Mice were individually placed into the center of a symmetrical Y-maze with 3 arms, and allowed to freely enter the arms during an 8-min session. The series of arm entries was recorded visually. The percentage of alternation was calculated as the ratio of correct alternations (successive entry into the 3 arms on overlapping triplet sets) to total alternations.
Fear conditioning test
The fear conditioning test was performed using the Startle and Fear combined system (Panlab, Harvard Apparatus, Cornellà (Barcelona), Spain). For training, after 10-min acclimation, mice were placed in a chamber, and a 70 dB tone was delivered for 30 s as a conditioned stimulus. During the last 2 s, a foot shock of 0.4 mA was delivered through a shock generator. This procedure was repeated 6 times at 15-s intervals. Animal movement was recorded through a high-sensitivity weight transducer system by PACKWIN software. On the second d, for the contextual test, mice were placed in the chamber, and the freezing response was measured for 2 min without the conditioned stimulus. For the cued test, the freezing response was measured with the conditioned stimulus.
Electrophysiology
Mice were sacrificed, and transverse hippocampal slices (400 μm) were prepared using a vibratome (VT1200S, Leica, Wetzlar, Germany) in oxygenated (95% O2 and 5% CO2) ice-cold artificial cerebrospinal fluid (ACSF, 126 mM NaCl, 3 mM KCl, 26 mM NaHCO3, 1.2 mM NaH2PO4, 10 mM D-glucose, 2.4 mM CaCl2 and 1.3 mM MgCl2). The slices were kept in an incubating chamber filled with oxygenated ACSF at 28 to 30°C. After a recovery period of at least 60 min, an individual slice was transferred to a recording chamber and was continuously superfused with oxygenated ACSF (5 ml/min) at 30°C.
fEPSPs were recorded using ACSF-filled glass pipettes (1 to 3 MΩ) placed at the stratum radiatum of area CA1. fEPSPs were evoked using a concentric bipolar electrode (WPI; FHC, CBBEB75) which was used to stimulate the SC fibers with a brief current pulse (50 μs), and the current pulse was delivered by a stimulus isolation unit (ISO-Flex,A.M.P.I., Jerusalem, Israel) every 30s. The distance between stimulating electrode and recording pipette was 200 to 300 μm. An input-output curve was used to set the stimulating strength, which yielded 30 to 50% of the maximal slope. After obtaining baseline measurements for 20 min, LTP was induced using θ-burst stimulation (each burst contained 5 pulses at 100 Hz repeated at 5 Hz and 3 10-burst trains separated by 20 s). Evoked fEPSPs were recorded for 1 h after tetanization. Signals were filtered at 2 kHz and digitized at 100 kHz using Digidata 1440A (Molecular Devices). Data acquisition and slope measurement were carried out using pClamp 10.2 (Molecular Devices). Pulse generation was achieved using a Master 8 stimulator (A.M.P.I., Jerusalem, Israel).
Primary neuron culture Primary cortical neurons were harvested from embryonic d 18 pups. Cerebral cortices were collected and dissociated by incubation in TypLEEM express enzymes (Gibco, 12605010) for 30 min at 37°C. After termination with FBS, cells were centrifuged at 217 g for 2 min, and plated with Neurobasal medium (Invitrogen, 10888022) supplemented with B27 (Invitrogen, 17504044), penicillin/streptomycin, Glutamax (Invitrogen, 35050061) and 5% FBS (HyClone Laboratories, SH3008403) on 35-mm dishes (BD Falcon, 353001) precoated with poly-d-lysine (100 μg/ml, Sigma, P1524). After incubation at 37°C for at least 4 h, the plating medium was changed to Neurobasal medium containing B27, penicillin/streptomycin, and Glutamax. Half the volume of culture medium was changed every 3 d For lysosome function inhibition, cells were treated with 20 μM bafilomycin A1 (Sigma, B1793) for 6 h before harvest.
Histology and immunofluorescence staining
After transcardial perfusion with 10% neutral buffered formalin (Sigma, HT501128), CNS tissues were post-fixed, dehydrated, embedded in paraffin, and sectioned at 5 µm. Sections were stained with hematoxylin and eosin for histological examination and signals were acquired by light microscopy (Imager A1, Zeiss, Göttingen, Germany) with a 40×/0.75 objective lens (Plan-NeoFluar, Zeiss, Göttingen, Germany) and a camera (AxioCam MRc5, Zeiss, Göttingen, Germany) at RT. Images were processed and viewed using AxioVision 40v4.6.3.0 software.
For immunostaining, after deparaffinization and rehydration, heat-induced epitope retrieval (0.1 M citrate buffer) was performed on sections. Samples were then blocked with normal goat serum and incubated with primary antibodies at 4°C overnight in a humidity chamber. After incubation, sections were washed in phosphate-buffered saline (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2.0 mM KH2PO4, pH 7.2) 3 times, and incubated with fluorescently-labeled secondary antibodies (Jackson ImmunoResearch Laboratories, 111–095–003, 111–025–003, 115–025–003, 115–095–003) for 1 h at room temperature. Finally, sections were counterstained with DAPI, mounted and examined under a confocal microscope (Zeiss LSM 710 Meta plus Zeiss Axiovert zoom, Göttingen, Germany) with a 63×/1.40 oil-immersion objective lens (Plan-Apochromatlan, Zeiss, Göttingen, Germany) and a camera (AxiocamHRm, Zeiss, Göttingen, Germany) at RT. Images were processed and viewed using ZEN 2011 software.
Immunoblotting
Cells were lysed with RIPA buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 0.1% SDS [SERVA, 20765.03], 1% NP40 [Amresco, E109]) supplemented with protease inhibitor cocktail (Roche, 04693116001), and incubated on ice for 30 min. Homogenates were centrifuged at 13,000 rpm for 10 min at 4°C. Supernatant fractions were collected and protein concentrations were determined by Bradford protein assay (Genstar, E161–01). Equal amounts (20 to 30 µg) of proteins were subjected to SDS-PAGE electrophoresis, transferred to a PVDF membrane and then blocked with 5% nonfat milk for 1 h at room temperature, membranes were incubated with primary antibodies at 4°C overnight. After washing with PBST (0.5% Tween 20 [Amresco, 0777] in PBS) 3 times, the membranes were incubated with HRP-labeled secondary antibodies (Sigma, A0545, A9044) for 1 h at room temperature. Immunoreactivity was determined using an Enhanced Chemiluminescent kit (GE, RPN2232).
Proteasome activity assay
Mice cerebral tissues were homogenized in lysis buffer (50 mM HEPES, pH 7.5, 5 mM EDTA, 150 mMNaCl, 1% Triton X-100 [Amresco, 0694] and 2 mM ATP). 250 µl lysate containing equal amounts of protein (2 to 4 µg) were incubated for 30 min at 37°C in the dark with 2.5 µl of each substrate (final concentration: 50 nM). Aminomethylcoumarin (AMC)-linked synthetic peptide substrates, Ac-Gly-Pro-Leu-Asp-AMC, Suc-Leu-Leu-Val-Tyr-AMC or Ac-Arg-Leu-Arg-AMC (and Boc-Leu-Arg-Arg-AMC), were used for detecting caspase-like, chymotrypsin-like, or trypsin-like activity, respectively (Proteasome Substrate Pack, Enzo Life Sciences, PW9905–0001). The reaction was stopped by adding 252.5 µl precooled 96% ethanol solution. Proteasome activity was measured by detecting fluorescence from AMC hydrolysis (380 nm excitation and 460 nm emission).
Transmission electron microscopy
Mice were transcardially perfused, and cerebral tissues were dissected and postfixed in 2.5% glutaraldehyde. Then samples were fixed with 1% OsO4 for 2 h, followed by dehydration with graded ethanol solutions, and embedded in Embed812 (Electron Microscopy Sciences, 14120). Ultrathin sections were cut at 80 nm, stained with 2% uranyl acetate for 30 min and lead citrate for 10 min. The samples were examined using a 120 kV electron microscope (FEI, Tecnai Spirit, Oregon, USA) at 80 kV and images were captured with a CCD camera (FEI, Eagle, Oregon, USA) using Digital Micrograph software at RT.
Quantitative RT-PCR
Total RNA was extracted using TRIzol (Invitrogen, 15596018) and cDNA was synthesized by Super Script® III First-Strand Kit (Invitrogen, 11752050). Quantitative PCR was performed on a StepOnePlus™ Real-Time PCR system (Applied Biosystems, CA, USA) using SYBR® Premix Ex Taq™ (TaKaRa, RR820A).
The following primers were used:
F-Wdr45: 5′-CATCTTGACCACGAGCAGGT-3′
R-Wdr45: 5′-GAAGGTGAACTCCAGCACCA-3′
F-Sqstm1: 5′-GCTGCCCTATACCCACATCT-3′
R-Sqstm1: 5′-CGCCTTCATCCGAGAAAC-3′
F-Actb (β-actin): 5′-CTGGCTCCTAGCACCATGAAGAT-3′
R-Actb: 5′-GGTGGACAGTGAGGCCAGGAT-3′
Statistical analysis
Statistical significance was calculated by the Student t test. A P value less than 0.05 was considered significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are grateful to the National Institute of Biological Sciences Transgenic Research Center for generating Wdr45 conditional KO mice, and Dr. Isabel Hanson for editing work. We also thank the animal facility at the Institute of Biophysics, Chinese Academy of Sciences for mice maintenance. Electron microscopy work was performed at the Center for Biological Imaging (http://cbi.ibp.ac.cn), Institute of Biophysics.
Funding
This work was supported by the National Natural Science Foundation of China (31421002, 31225018), grants from the National Basic Research Program of China (2013CB910100, 2011CB910100) to HZ, and also a grant from the National Natural Science Foundation of China (31401184) to YGZ. The research of Hong Zhang was supported in part by an International Early Career Scientist grant from the Howard Hughes Medical Institute.
References
- Nakatogawa H, Suzuki K, Kamada Y, Ohsumi Y. Dynamics and diversity in autophagy mechanisms: lessons from yeast. Nat Rev Mol Cell Biol 2009; 10:458-67; PMID:19491929; http://dx.doi.org/10.1038/nrm2708.
- Tian Y, Li Z, Hu W, Ren H, Tian E, Zhao Y, Lu Q, Huang X, Yang P, Li X, et al. C. elegans screen identifies autophagy genes specific to multicellular organisms. Cell 2010; 141:1042-55; PMID:20550938; http://dx.doi.org/10.1016/j.cell.2010.04.034.
- Lu Q, Yang P, Huang X, Hu W, Guo B, Wu F, Lin L, Kovács AL, Yu L, Zhang H. The WD40 repeat PtdIns(3)P-binding protein EPG-6 regulates progression of omegasomes to autophagosomes. Dev Cell 2011; 21:343-57; PMID:21802374; http://dx.doi.org/10.1016/j.devcel.2011.06.024.
- Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell 2008; 132:27-42; PMID:18191218; http://dx.doi.org/10.1016/j.cell.2007.12.018.
- Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 2006; 441:885-9; PMID:16625204; http://dx.doi.org/10.1038/nature04724.
- Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 2006; 441:880-4; PMID:16625205; http://dx.doi.org/10.1038/nature04723.
- Zhao YG, Zhao H, Miao L, Wang L, Sun F, Zhang H. The p53-induced gene Ei24 is an essential component of the basal autophagy pathway. J Biol Chem 2012; 287:42053-42063; PMID:23074225; http://dx.doi.org/10.1074/jbc.M112.415968.
- Haack TB, Hogarth P, Kruer MC, Gregory A, Wieland T, Schwarzmayr T, Graf E, Sanford L, Meyer E, Kara E, et al. Exome sequencing reveals de novo WDR45 mutations causing a phenotypically distinct, X-linked dominant form of NBIA. Am J Hum Genet 2012; 91:1144-9; PMID:23176820; http://dx.doi.org/10.1016/j.ajhg.2012.10.019.
- Hayflick SJ, Kruer MC, Gregory A, Haack TB, Kurian MA, Houlden HH, Anderson J, Boddaert N, Sanford L, Harik SI, et al. β-Propeller protein-associated neurodegeneration: a new X-linked dominant disorder with brain iron accumulation. Brain 2013; 136:1708-17; PMID:23687123; http://dx.doi.org/10.1093/brain/awt095.
- Saitsu H, Nishimura T, Muramatsu K, Kodera H, Kumada S, Sugai K, Kasai-Yoshida E, Sawaura N, Nishida H, Hoshino A, et al. De novo mutations in the autophagy gene WDR45 cause static encephalopathy of childhood with neurodegeneration in adulthood. Nat Genet 2013; 45:445-9; PMID:23435086; http://dx.doi.org/10.1038/ng.2562.
- Gregory A, Polster BJ, Hayflick SJ. Clinical and genetic delineation of neurodegeneration with brain iron accumulation. J Med Genet 2009; 46:73-80; PMID:18981035; http://dx.doi.org/10.1136/jmg.2008.061929.
- Schneider SA, Bhatia KP. Syndromes of neurodegeneration with brain iron accumulation. Semin Pediat Neurol 2012; 19:57-66; http://dx.doi.org/10.1016/j.spen.2012.03.005.
- D'Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Research Reviews. Brain Res Rev 2001; 36:60-90; PMID:11516773; http://dx.doi.org/10.1016/S0165-0173(01)00067-4.
- Packard MG, Cahill L. Affective modulation of multiple memory systems. Curr Opin Neurobiol 2001; 11:752-6; PMID:11741029; http://dx.doi.org/10.1016/S0959-4388(01)00280-X.
- Malenka RC. The long-term potential of LTP. Nat Rev Neurosci 2003; 4:923-6; PMID:14595403; http://dx.doi.org/10.1038/nrn1258.
- Zhao H, Zhao YG, Wang X, Xu L, Miao L, Feng D, Chen Q, Kovács AL, Fan D, Zhang H. Mice deficient in Epg5 exhibit selective neuronal vulnerability to degeneration. J Cell Biol 2013; 200:731-41; PMID:23479740; http://dx.doi.org/10.1083/jcb.201211014.
- Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell 2010; 140:313-26; PMID:20144757; http://dx.doi.org/10.1016/j.cell.2010.01.028.
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci 1992;106:274-85; PMID:1590953; http://dx.doi.org/10.1037/0735-7044.106.2.274.
- Dooley HC, Razi M, Polson HE, Girardin SE, Wilson MI, Tooze SA.WIPI2 links LC3 conjugation with PI3P, autophagosome formation, and pathogen clearance by recruiting Atg12-5-16L1. Mol Cell 2014; 55:238-52; PMID:24954904; http://dx.doi.org/10.1016/j.molcel.2014.05.021.
- Lee S, Sato Y, Nixon RA. Lysosomal proteolysis inhibition selectively disrupts axonal transport of degradative organelles and causes an Alzheimer-like axonal dystrophy. J Neurosci 2011; 31:7817-30; PMID:21613495; http://dx.doi.org/10.1523/JNEUROSCI.6412-10.2011.
- Maday S, Wallace KE, Holzbaur EL. Autophagosomes initiate distally and mature during transport toward the cell soma in primary neurons. J Cell Biol 2012; 196:407-17; PMID:22331844; http://dx.doi.org/10.1083/jcb.201106120.
- Komatsu M, Wang QJ, Holstein GR, Friedrich VL Jr, Iwata J, Kominami E, Chait BT, Tanaka K, Yue Z. Essential role for autophagy protein Atg7 in the maintenance of axonal homeostasis and the prevention of axonal degeneration. Proc Natl Acad Sci USA 2007; 104:14489-94; PMID:17726112; http://dx.doi.org/10.1073/pnas.0701311104.
- Subramani S, Malhotra V. Non-autophagic roles of autophagy-related proteins. EMBO Rep 2013; 14(2):143-51; PMID:23337627; http://dx.doi.org/10.1038/embor.2012.220.
- Zhao Z, Fux B, Goodwin M, Dunay IR, Strong D, Miller BC, Cadwell K, Delgado MA, Ponpuak M, Green KG, et al. Autophagosome-independent essential function for the autophagy protein Atg5 in cellular immunity to intracellular pathogens. Cell Host Microbe 2008;4:458-69; PMID:18996346; http://dx.doi.org/10.1016/j.chom.2008.10.003.
