ABSTRACT
To investigate the role of LC3 in bulk autophagy we compared its autophagic-lysosomal processing (using an improved quantitative immunoblotting method) with autophagic-lysosomal bulk cargo flux (measured by our established LDH [lactate dehydrogenase] sequestration assay) in amino acid-starved rat hepatocytes treated with cycloheximide to prevent new LC3 influx. Block-release experiments with the reversible autophagy inhibitors 3-methyladenine (3MA) and thapsigargin (TG) showed that while only 3MA suppressed phagophoric LC3 attachment (lipidation), both inhibitors prevented phagophore closure (cargo sequestration). Upon release from closure blockade, some autophagic-lysosomal LC3 flux was resumed even in the presence of 3MA, i.e., without an accompanying bulk cargo flux. Conversely, whereas the autophagic-lysosomal flux of LC3 halted within ∼100 min of cycloheximide treatment, the bulk cargo flux continued at a high rate. siRNA-mediated knockdown of LC3 family proteins in LNCaP prostate carcinoma cells confirmed that autophagy of cytoplasmic bulk cargo was completely LC3 independent also in these cells, and in the absence of cycloheximide. However, a strong requirement for GABARAP family proteins was evident. Since bulk autophagy of cytoplasm (macroautophagy) and autophagic-lysosomal LC3 processing may apparently be mutually independent, LC3 would seem to be unsuitable as a general indicator of autophagy.
Autophagy was originally defined by Christian de Duve as “the bulk segregation and digestion of portions of cytoplasm;”Citation1 later Glenn Mortimore suggested the designation macroautophagy to distinguish this bulk process from other autophagic mechanisms.Citation2 In recent years, the description of numerous forms of selective autophagy have made it all the more important to reserve the term “macroautophagy” for the bulk process.Citation3
The bulk nature of macroautophagy has been strikingly demonstrated by the fact that cytosolic enzymes with widely different half-lives are all sequestered into autophagic vacuoles at the same rate, which also make them potential probes for macroautophagic cargo sequestration.Citation4 We routinely use LDH (lactate dehydrogenase) for this purpose, since it is a cytosolic enzyme present in all cells, and is degraded exclusively by the autophagic-lysosomal pathway.Citation4 Our macroautophagic cargo sequestration assay, which can be adapted to any cell type,Citation5 measures the net transfer of LDH into sedimentable autophagic vacuoles in the presence of leupeptin or bafilomycin A1 to prevent LDH degradation in amphisomes and lysosomes. Cells are usually incubated in an amino acid- and serum-free buffer to ensure that the maximally attainable autophagic activity (autophagic capacity) is measured. For quantitative measurements of LC3, an immunoblotting procedure that separates the cytosolic (unlipidated) form LC3-I from the phagophore-attached (lipidated) form LC3-II is used. Since anti-LC3 antibodies stain LC3-II more efficiently than LC3-I, the latter values are, after blot scanning and band quantification, routinely multiplied by 4, a staining ratio that we derived empirically by analyzing ATG4-mediated delipidation of LC3-II.Citation6
We simplified the analysis of hepatocellular LC3 dynamics by blocking de novo influx of LC3 with the protein synthesis inhibitor cycloheximide. Under these conditions, total LC3 (LC3-I + LC3-II) declined rapidly (half-life ∼2 h);Citation6 this early LC3 disappearance could largely be accounted for by an autophagic-lysosomal degradation that was suppressed by inhibitors of autophagic sequestration (3MA, TG) or lysosomal function (NH4Cl, bafilomycin A1, leupeptin)Citation6 (, inhibitors added at 0 min). The reversibility of 3MA and TG effects allowed block-release experiments, which revealed that while only 3MA suppressed LC3 lipidation, both inhibitors blocked phagophore closure and thus bulk cargo sequestration.Citation7 Following release from a closure blockade, re-addition of TG prevented subsequent LC3 degradation, presumably by suppressing the fusion of phagophores/autophagosomes with amphisomes/lysosomes. In contrast, re-added 3MA, while still blocking cargo sequestration, allowed some degradative LC3 flux, suggesting that LC3-equipped phagophores could traverse the autophagic-lysosomal pathway without carrying bulk cytoplasmic cargo.Citation7
Figure 1. Experimental evidence for LC3-independent autophagy of cytosolic bulk cargo during starvation. (A) Turnover of LC3 in rat hepatocytes incubated for 200 min at 37°C with cycloheximide (100 μM) alone (CTR) or with 3-methyladenine (3MA, 10 mM), thapsigargin (TG, 5 μM) or NH4Cl (20 mM) added at 0 min (causing inhibition of degradative autophagic-lysosomal LC3 flux) or at 100 min (with no effect, indicating the absence of autophagic-lysosomal LC3 flux). Total LC3 remaining at 200 min is expressed as percent of the 0-min value (mean ±SE of the number of experiments given in parentheses). (B) Density gradient distribution of sedimentable hepatocytic organelles carrying autophagically sequestered LDH (open circles) or organelle-associated LC3-II (filled circles). The cells were harvested after 4 h of cycloheximide treatment, leupeptin (0.3 mM) having been added at 2 h to allow LDH accumulation during the period 2–4 h. (C) Effect of 52-h siRNA-mediated knockdown of the Atg8 homologs LC3A, LC3B, GABARAP (GAB), GABARAPL1 (GL1) and GABARAPL2 (GL2) on expression of the corresponding immunoblotted protein in LNCaP prostate cancer cells (siCTR, nontargeting siRNA; I, nonlipidated; II, lipidated form). The cells were incubated for 4 h in a complete medium or under amino acid- and serum-starved conditions (EBSS) in the presence of bafilomycin A1 (200 nM) as indicated. (D) Effects of the knockdowns on macroautophagic cargo (LDH) sequestration, expressed as percent of cellular LDH sequestered per h (mean ±SE of 3 experiments). Figures adapted from ref. Citation6.
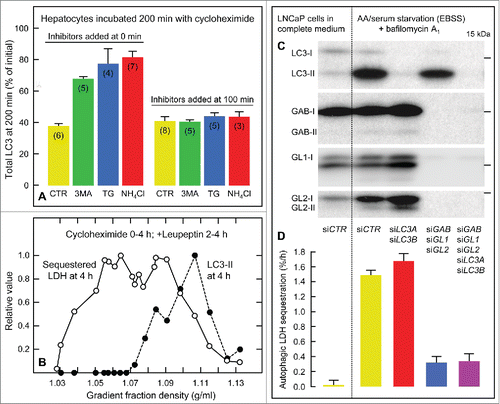
After ∼100 min of uninterrupted LC3 turnover during cycloheximide treatment, LC3 degradation was no longer sensitive to 3MA, TG or NH4Cl, indicating that its autophagic-lysosomal flux had stopped (, inhibitors added at 100 min). However, despite this absence of autophagic-lysosomal LC3 flux beyond 100 min, the cargo (LDH) sequestration capacity was surprisingly well maintained, and remained sensitive to autophagy inhibitors at all times.Citation6,7 Subcellular fractionation on density gradients confirmed an extensive accumulation of sequestered LDH 2–4 h after cycloheximide; strikingly, a substantial amount of this LDH was present in LC3-free fractions (1.03–1.07 g/ml; ). Furthermore, immunofluorescence microscopy of hepatocytes stained with an anti-LC3 antibody showed that large LC3 dots had disappeared beyond 2 h, probably reflecting that autophagic vacuoles were no longer equipped with amounts of LC3 sufficient for immunodetection.Citation6 The efficient sequestration and degradation of cytoplasmic cargo in the absence of any autophagic-lysosomal LC3 flux, taking place in large measure in autophagic vacuoles devoid of LC3, would be consistent with a macroautophagic process capable of operating independently of LC3. Although about one-half of the hepatocellular LC3 still remained after 100 min of cycloheximide treatment,Citation6 this LC3 population was clearly not engaged in the autophagic-lysosomal pathway (, inhibitors added at 100 min), but might conceivably serve other cellular functions.
To test if this conclusion could be extended to other cell types and to conditions without cycloheximide, macroautophagic cargo (LDH) sequestration was measured in LNCaP prostate carcinoma cells,Citation8 a cell type that (unlike hepatocytes) allows genetic knockdown experiments to be performed. We found that the LC3 family members expressed in these cells (LC3A1, LC3A2, LC3B1) could be collectively knocked down by a combination of siRNAs.Citation6 As shown in , no LC3-I was detectable by immunoblotting in the LC3-siRNA-treated LNCaP cells, and only a very faint band could be discerned in the LC3-II region. However, this very effective knockdown did not impair macroautophagic cargo sequestration as measured either under amino acid- and serum-depleted conditions (EBSS; ) or in a complete medium in the presence of the MTOR inhibitor Torin1.Citation6 We have obtained similar results with other cell lines (HeLa, PC3), suggesting the possibility that LC3-independence may be a general property of mammalian macroautophagy.
Although we cannot formally exclude the possibility that the minute amounts of LC3 that remain after siRNA-mediated knockdown may fully support macroautophagy, we find it very unlikely, both in light of the above-mentioned observations in hepatocytes,Citation6 and since the LC3-targeting siRNAs could completely abolish the increase in LC3-II levels observed upon treatment with EBSS and bafilomycin A1 (the LC3-II levels were actually lower than in fully fed cells; ). Furthermore, a genetic knockout of LC3B failed to affect starvation-induced morphological changes in the lysosome population of mouse embryonic fibroblasts,Citation9 consistent with an undisturbed macroautophagic-lysosomal function.
In contrast to the ineffectiveness of the LC3 family knockdown, a collective siRNA-mediated knockdown of the GABARAP family members expressed in LNCaP cells (GABARAP, GABARAPL1 and GABARAPL2; ) strongly suppressed macroautophagic cargo sequestration (). Macroautophagy thus seems to require an Atg8 homolog, but this requirement is apparently fulfilled by GABARAPs rather than by LC3s.Citation6 The fact that the starvation-induced macroautophagic activity remaining after knockdown of the GABARAPs was unaffected by the extent of LC3 expression () would further support a noninvolvement of LC3 in this process. It should be noted that in addition to the GABARAPs, we have found that macroautophagy in LNCaP cells requires the RB1CC1/FIP200 protein (and undoubtedly many more).Citation5
Although our density gradient fractionations showed some overlap between LC3 and sequestered LDH at the early hours after cycloheximide treatment,Citation6 they cannot really tell whether there is ever any physical association of LC3 with the phagophores that perform macroautophagic cargo sequestration. If there is, it is not implausible that LC3 could perform some auxiliary function,Citation10 e.g., picking up defective proteins, aggregates, or organelles, thus adding a selective element to an otherwise nonselective macroautophagy, given the well-established role of LC3 as a recruiter of cargo receptors in selective autophagy.Citation11
Since current autophagy research has used LC3 rather indiscriminately as a general autophagy indicator, there is clearly a need to reconsider and redefine the molecular requirements for LC3-independent macroautophagy relative to selective autophagy and other autophagic mechanisms.
Abbreviations
| 3MA | = | 3-methyladenine |
| GABARAP | = | GABA(A) receptor-associated protein |
| LC3 | = | microtubule-associated protein 1 light chain 3 |
| LDH | = | lactate dehydrogenase |
| TG | = | thapsigargin |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work has been generously supported by The Norwegian Cancer Society, the Norwegian Research Council, the University of Oslo, the Anders Jahre Foundation, and the Legacy in the memory of Henrik Homan.
References
- de Duve C, Wattiaux R. Functions of lysosomes. Annu Rev Physiol 1966; 28:435-92; PMID:5322983; http://dx.doi.org/10.1146/annurev.ph.28.030166.002251
- Mortimore GE, Hutson NJ, Surmacz CA. Quantitative correlation between proteolysis and macro- and microautophagy in mouse hepatocytes during starvation and refeeding. Proc Natl Acad Sci USA 1983; 80:2179-83; PMID:6340116; http://dx.doi.org/10.1073/pnas.80.8.2179
- Klionsky DJ, Cuervo AM, Dunn WA, Levine B, van der Klei I, Seglen PO. How shall I eat thee? Autophagy 2007; 3:413-6; PMID:17568180; http://dx.doi.org/10.4161/auto.4377
- Kopitz J, Kisen GØ, Gordon PB, Bohley P, Seglen PO. Non-selective autophagy of cytosolic enzymes by isolated rat hepatocytes. J Cell Biol 1990; 111:941-53; PMID:2391370; http://dx.doi.org/10.1083/jcb.111.3.941
- Seglen PO, Luhr M, Mills IG, Sætre F, Szalai P, Engedal N. Macroautophagic sequestration assays. Methods 2015; 75:25-36; PMID:25576638; http://dx.doi.org/10.1016/j.ymeth.2014.12.021
- Szalai P, Hagen LK, Sætre F, Luhr M, Sponheim M, Øverbye A, Mills IG, Seglen PO, Engedal N. Autophagic bulk sequestration of cytosolic cargo is independent of LC3, but requires GABARAPs. Exp Cell Res 2015, 333:21-38; PMID:25684710; http://dx.doi.org/10.1016/j.yexcr.2015.02.003
- Sætre F, Hagen LK, Engedal N, Seglen PO. Novel steps in the autophagic-lysosomal pathway. FEBS J 2015; 282:2202-14; PMID:25779646; http://dx.doi.org/10.1111/febs.13268
- Engedal N, Torgersen ML, Guldvik IJ, Barfeld SJ, Bakula D, Sætre F, Hagen LK, Patterson JB, Proikas-Cezanne T, Seglen PO, Simonsen A, Mills IG. Modulation of intracellular calcium homeostasis blocks autophagosome formation. Autophagy 2013; 9:1475-90; PMID:23970164; http://dx.doi.org/10.4161/auto.25900
- Cann GM, Guignabert C, Ying L, Deshpande N, Bekker JM, Wang L, Zhou B, Rabinovitch M. Developmental expression of LC3a and b: Absence of fibronectin or autophagy phenotype in LC3b knockout mice. Dev Dyn 2008; 237:187-195; PMID:18069693; http://dx.doi.org/10.1002/dvdy.21392
- Øverbye A, Fengsrud M, Seglen PO. Proteomic analysis of membrane-associated proteins from rat liver autophagosomes. Autophagy 2007; 3:300-22; PMID:17377489; http://dx.doi.org/10.4161/auto.3910
- Birgisdottir ÅB, Lamark T, Johansen T. The LIR motif – crucial for selective autophagy. J Cell Sci 2013; 126:3237-47; PMID:23908376
