Abstract
Autophagy targets various intracellular components ranging from proteins and nucleic acids to organelles for their degradation in lysosomes or vacuoles. In selective types of autophagy, receptor proteins play central roles in target selection. These proteins bind or localize to specific targets, and also interact with Atg8 family proteins on forming autophagosomal membranes, leading to the efficient sequestration of the targets by the membranes. Our recent study revealed that yeast cells actively degrade the endoplasmic reticulum (ER) and even part of the nucleus via selective autophagy under nitrogen-deprived conditions. We identified novel receptors, Atg39 and Atg40, specific to these pathways. Here, we summarize our findings on ‘reticulophagy’ (or ‘ER-phagy’) and ‘nucleophagy’, and discuss key issues that remain to be solved in future studies.
We recently identified novel Atg8-binding proteins, Atg39 and Atg40, in the budding yeast Saccharomyces cerevisiae. Both of them turned out to be receptors for autophagic degradation of the ER (hereafter, reticulophagy). These proteins cooperatively act in this pathway but not in a simply redundant manner. In S. cerevisiae, the ER consists of 3 subdomains, the cytoplasmic ER (cytoER), the cortical ER (cER), and the perinuclear ER (pnER), which is equivalent to the nuclear envelope (NE). Atg39 specifically localizes to the pnER/NE, and induces autophagic sequestration of double-membrane vesicles derived from the pnER/NE, which encapsulate intranuclear components (). Therefore, the Atg39-dependent pathway should also be called nucleophagy. Conversely, Atg40 predominantly localizes to the cytoER and cER, and loads fragments (tubules or sheets) of these ER subdomains into the phagophore (). Thus, our results delineated the fundamental mechanism of reticulophagy and nucleophagy.
Figure 1. Schematic model of reticulophagy and nucleophagy. Atg39 and Atg40 localize to the perinuclear ER (pnER)/nuclear envelope (NE) and the cytoplasmic ER (cytoER)/cortical ER (cER), respectively. These proteins also reside on pnER/NE-derived double-membrane vesicles or fragments (tubules or sheets) of cytoER/cER. It is still unclear whether these vesicles and fragments are formed in a manner coupled with autophagosome formation. The predicted topologies of Atg39 and Atg40 are also shown, in which Atg8 family-interacting motifs (AIMs) are located in the cytoplasmic regions. Atg39 and Atg40 are likely to bind to Atg8 on forming autophagosomal membranes via these motifs, thereby pnER/NE-derived vesicles and cytoER/cER fragments are respectively sequestered within autophagosomes.
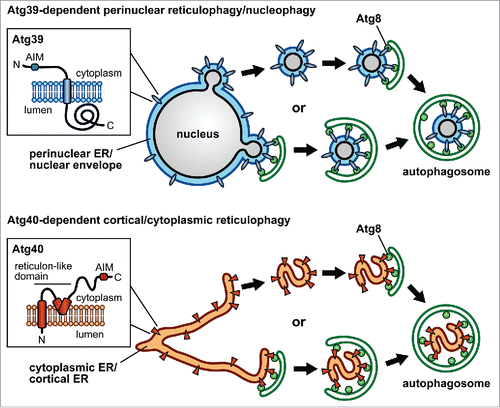
Precise mechanisms underlying these pathways should be elucidated by further analyses. One of the most interesting questions is how the ER and the nucleus are fragmented and sequestered by the phagophore. It is still unknown whether fragmentation of these organelles occurs prior to autophagosome formation, or if these events are intimately coupled (). Atg40 contains a reticulon-like domain (), which is found in a family of proteins that generate and maintain membrane curvature in the ER to shape its tubular and sheet structures. Atg40, using this domain, may evoke ER fragmentation, or fold ER fragments compactly. It is also interesting to investigate the mechanism of pnER/NE budding to form double-membrane vesicles. Atg39 is predicted to be a single membrane-spanning protein with its C-terminal region exposed to the pnER/NE lumen (). It is tempting to speculate that this Atg39 region interacts with some proteins and/or lipids in the inner nuclear membrane to induce the formation of double-membrane vesicles. Atg39 and Atg40 may be enriched in the organelle membranes via their interactions with Atg8 on forming autophagosomes. If these proteins have membrane-deforming functions, this can serve as a mechanism by which fragmentation of the ER or the nucleus is coupled with autophagosome formation. It is also intriguing to know how Atg39 and Atg40 localize to the specific ER subdomains/NE.
As with the case of other selective autophagy pathways, reticulophagy and nucleophagy should be tightly controlled. We found that the expression of Atg39 and Atg40 is strongly suppressed under nutrient-replete conditions, and induced by the removal of the nitrogen source. This is likely to be part of the mechanism that regulates reticulophagy and nucleophagy. Treating cells with rapamycin also increases the levels of Atg39 and Atg40, suggesting the involvement of Tor kinase complex I in the regulation of expression. In addition, as seen with other autophagy receptors, post-translational modifications may also regulate the functions of Atg39 and Atg40.
We showed that cells lacking Atg39 exhibit abnormal morphology in the nucleus and die earlier than wild-type cells during prolonged nitrogen starvation. Although deficiencies in bulk, nonselective autophagy result in a similar phenotype, this type of autophagy normally occurs in atg39 mutant cells. Therefore, a shortage of molecules produced by cytoplasm degradation cannot explain the phenotype of the atg39 mutants. Degradation of the pnER/nucleus may liberate some specific molecules, which are required to maintain cell viability under nitrogen-starvation conditions. Alternatively, the pnER/nucleus may accumulate deleterious material under these conditions without Atg39-dependent degradation. The relationship between cell death and aberrant nuclear morphology should also be clarified.
We observed highly reticulated cER in atg40 mutants compared with wild-type cells, consistent with the fact that Atg40 mediates degradation of this ER subdomain; however, these mutants survive nitrogen starvation. Thus, the physiological impact of Atg40-dependent reticulophagy remains to be explored. Recently, FAM134B was identified as a mammalian reticulophagy receptor. Atg40 and FAM134B do not show a significant similarity in their primary sequences. However, both of these proteins contain reticulon-like domains in the N-terminal halves and Atg8 family-interacting motifs (or LC3-interacting regions) in the vicinity of the C termini. These facts suggest that FAM134B is a functional counterpart of Atg40 in mammals. Conversely, Atg39 homologs can be found only in yeast species closely related to S. cerevisiae. However, phenomena that may represent nucleophagy have been reported in several organisms including mammals. Therefore, Atg39 counterparts may function in nucleophagy in these organisms.
We hope our findings will trigger studies on the molecular mechanisms and physiological roles of reticulophagy and nucleophagy, and their relevance to diseases in other organisms.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan to HN (25111003, 25711005, and 25111001) and KM (15J11855).
