Abstract
Macroautophagy is a conserved degradative pathway in which a double-membrane compartment sequesters cytoplasmic cargo and delivers the contents to lysosomes for degradation. Efficient formation and maturation of autophagic vesicles, so-called phagophores that are precursors to autophagosomes, and their subsequent trafficking to lysosomes relies on the activity of small RAB GTPases, which are essential factors of cellular vesicle transport systems. The activity of RAB GTPases is coordinated by upstream factors, which include guanine nucleotide exchange factors (RAB GEFs) and RAB GTPase activating proteins (RAB GAPs). A role in macroautophagy regulation for different TRE2-BUB2-CDC16 (TBC) domain-containing RAB GAPs has been established. Recently, however, a positive modulation of macroautophagy has also been demonstrated for the TBC domain-free RAB3GAP1/2, adding to the family of RAB GAPs that coordinate macroautophagy and additional cellular trafficking pathways.
Abbreviations
| ATG | = | autophagy related |
| BECN1 | = | Beclin 1, autophagy related |
| CALCOCO2 | = | calcium binding and coiled-coil domain 2 |
| ER | = | endoplasmic reticulum |
| GABARAP | = | GABA(A) receptor-associated protein |
| GDP | = | guanosine-5′-diphosphate |
| GTP | = | guanosine-5′-triphosphate |
| LRRK1 | = | leucine-rich repeat kinase 1 |
| MAP1LC3 | = | microtubule-associated protein 1 light chain 3 |
| NBR1 | = | neighbor of BRCA1 gene 1 |
| PAS | = | phagophore assembly site |
| PE | = | phosphatidylethanolamine |
| PIK3C3 | = | phosphatidylinositol 3-kinase, catalytic subunit type 3 |
| RAB GAP | = | RAB GTPase activating protein |
| RAB GEF | = | RAB GTPase guanine exchange factor |
| SQSTM1 | = | sequestosome 1 |
| TBC domain | = | TRE2-BUB2-CDC16 domain |
| TBCGAP | = | TBC domain-containing RAB GAP |
| ULK | = | unc-51 like autophagy activating kinase |
| WIPI | = | WD repeat domain, phosphoinositide interacting 1 |
| ZFYVE1 | = | zinc finger, FYVE domain containing 1 |
Macroautophagy is a membrane mobilization and vesicle trafficking system
Macroautophagy is an evolutionarily conserved eukaryotic process in which cytoplasmic contents are sequestered by phagophores, which mature into autophagosomes and deliver their cargo to lysosomes for degradation.Citation1 The pathway is induced under conditions of nutrient deprivation or stress and is an important functional component of the cellular homeostasis network. Deterioration of macroautophagy is associated with several disorders, including neurodegenerative diseases and cancer.Citation2
One main characteristic of macroautophagy is the double-membrane autophagosomes, which are generated at distinct cellular locations, the phagophore assembly sites (PAS). Upon macroautophagy induction, the activated ULK1/2 complex (including ATG13 and RB1CC1/FIP200) and phosphatidylinositol 3-kinase complex (including PIK3C3/Vps34, ATG14, and BECN1/Vps30/Atg6) are recruited to the PAS and initiate the formation of a phagophore by directing additional autophagic proteins to this site. These include WIPI1/Atg18, WIPI2/Atg18, ZFYVE1/DFCP1, ATG9, and the ATG12–ATG5-ATG16L1 complex.Citation3 The latter is part of a ubiquitin-like conjugation system and mediates the attachment of phosphatidylethanolamine to the C terminus of Atg8 family members. This protein family comprises the subfamilies of MAP1LC3 and GABARAP in mammals, and lipidation results in their binding to the growing phagophore membrane which is essential for phagophore expansion and maturation.Citation4
Phagophore formation and autophagosome maturation are dependent on the adequate supply of membranes and appropriate cellular membrane dynamics. Recently, the plasma membrane, the Golgi, the ER,Citation5,6 and lipid dropletsCitation7 have been recognized as lipid sources. In response to different regimens of macroautophagic activity they are considered to be selectively accessed to satisfy macroautophagic membrane requirements.Citation8 Interestingly, it is considered that the phagophore matures to an autophagosome by the addition of lipids via vesicular fusion rather than via lateral movement of membranes from existing cellular organelles.Citation5,9 Consequently, the resulting sophisticated and complex membrane acquisition system needs to be carefully coordinated, and proteins that control vesicle transport systems are important factors for macroautophagy.
The protein family of small RAB GTPases is specialized in the control of vesicle transport routes and ensures trafficking of vesicles to their appropriate target compartments.Citation10 RAB GTPases interact with effector proteins such as cargo sorting complexes, motor proteins, and tethering factors, which results in vesicle budding, transport, and fusion. The interactions with these effectors are precisely controlled by GDP/GTP exchange and hydrolysis of GTP. Since GDP is principally tightly bound by RAB GTPases and their intrinsic GTP hydrolysis rates are low, this cycle is regulated by guanine exchange factors (RAB GEFs) that catalyze the dissociation of GDP, and RAB GTPase activating proteins (RAB GAPs) that facilitate the hydrolysis of GTP.Citation11 Both regulators are required to coordinate the temporal-spatial activity of RAB GTPases. In recent years multiple RAB GTPases, RAB GEFs, and RAB GAPs have functionally been associated with macroautophagy.Citation12 This commentary will focus on RAB GAPs and briefly address their effects on this degradative pathway (schematically summarized in ) and vesicle trafficking systems.
Figure 1. Schematic representation of RAB GAPs established to function in macroautophagy. TBC1D5, TBC1D14, and RAB3GAP1/2 function during autophagosome formation, and TBC1D2 and TBC1D25 support autophagosome-lysosome fusion. The TBC domain is depicted by dark purple globules. Note that this domain is missing in the heterodimeric RAB3GAP complex.
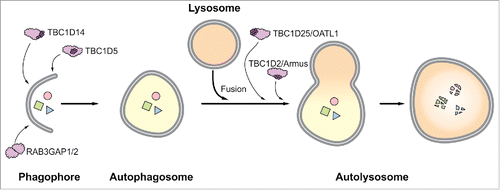
TBCGAPs: TBC domain-containing RAB GAPs that function in macro-autophagy
In approaches aiming to identify RAB GAPs that affect macroautophagy, several TBC domain-containing RAB GAPs have been characterized.Citation13-15 The TBC domain accelerates the hydrolysis of GTP by RAB GTPases and TBC domain-containing RAB GAPs (hereafter referred to as TBCGAPs) are linked to different trafficking routes, and are important factors that integrate diverse cellular pathways.Citation16
TBC1D25/OATL1 was identified in a study expressing 41 TBCGAPs in mouse embryonic fibroblasts and selecting proteins that colocalize with endogenous MAP1LC3.Citation13 TBC1D25/OATL1 targets the ATG16L1-interacting RAB GTPase RAB33B and is recruited to autophagosomes by direct binding to Atg8 family members. Increased levels of TBC1D25/OATL1 inhibit the fusion of autophagosomes with lysosomes and prevent autophagosomal maturation.
In an approach overexpressing 38 TBCGAPs in HEK293 cells and analyzing their ability to inhibit autophagosome formation upon nutrient deprivation, 11 TBCGAPs were shown to negatively regulate macroautophagy.Citation14 The TBCGAP TBC1D14 was analyzed in detail and was shown to modify the trafficking of ULK1-containing recycling endosomes and to interfere with the activity of the RAB GTPase RAB11A/B. The function of RAB11 is required to transport recycling endosomes to the PAS and, thus, TBC1D14 and RAB11 regulate starvation-induced formation of autophagosomes.
In another study employing GST affinity isolation techniques, 14 TBCGAPs were identified to interact with Atg8 family members.Citation15 Subsequently, the colocalization of these TBCGAPs with MAP1LC3 and SQSTM1 was analyzed, resulting in 4 promising candidates. The TBCGAP TBC1D5 was further characterized and was shown to have 2 binding motifs for Atg8 family members. During basal macroautophagy conditions TBC1D5 binds to the retromer complex and influences retrograde transport routes. Upon macroautophagy induction, TBC1D5 dissociates from the retromer, associates with MAP1LC3, and directs ATG9 and active ULK1 from the retromer to the PAS.Citation17 This rerouting of ATG9 is additionally regulated by the clathrin adaptor complex (AP2) and requires functional clathrin-mediated endocytosis. Thus, the dynamic translocation of TBC1D5 to autophagosomes is central for the trafficking of ATG9 from the retromer complex to the site of autophagosome biogenesis.
The protein TBC1D2/Armus is an additional TBCGAP that interacts with MAP1LC3 and integrates trafficking pathways and macroautophagy.Citation18 Overexpression of TBC1D2 results in the accumulation of enlarged autophagosomes, and its deficiency delays macroautophagic flux. Upon macroautophagy induction, TBC1D2 is recruited to autophagosomes by binding to Atg8 family members and regulates the activity of the RAB GTPase RAB7, which is essential for the fusion of autophagosomes and lysosomes.Citation12 Interestingly, TBC1D2 is also an effector of the small GTPase RAC1, which is a negative regulator of macroautophagy. Nutrient deprivation inactivates RAC1, which allows the association of TBC1D2 with autophagosomes and results in regulation of RAB7. Thus, the interplay of TBC1D2, RAC1, and RAB7 underlines the coordinate character of macroautophagy and other cellular trafficking pathways mediated by RAB GTPases and RAB GAPs.
In these studies a multitude of TBCGAPs were linked to macroautophagy, which are summarized in with respect to their substrate RAB GTPases and their nonautophagic functions, if characterized. Although the influence on macroautophagy of the majority of these RAB GAPs needs to be confirmed, the large number of potential candidates highlights the complexity of the coordination of membrane or vesicle trafficking and the macroautophagic pathway.
Table 1. Summary of macroautophagy-associated RAB GAPs.
RAB3GAP1 and RAB3GAP2 as non-TBCGAPs and their function in macroautophagy and beyond
The introduced TBCGAPs function in macroautophagy and contribute to the reorganization of membrane trafficking routes according to the cellular requirements. This coordinate property has been well established for TBCGAPs that are ideally placed for such a role, as one TBCGAP can act as an effector of different RAB GTPases. Interestingly, according to sequence homology the human TBCGAP family includes 44 proteins and is complemented by the RAB3GAP complex, which is the only described RAB GAP without a TBC domain.Citation16 The heterodimeric complex consists of the catalytic subunit RAB3GAP1 and the noncatalytic subunit RAB3GAP2Citation19 and has been well established to regulate the name-giving RAB GTPase RAB3A-D and to modify neurotransmitter release at the neuronal synapse. In a RAB3GAP1 knockout mouse model, GTP-bound RAB3 accumulates in the brain and Ca2+-dependent glutamate release from cerebrocortical synaptosomes is inhibited.Citation20 Indeed, by regulating the activity of RAB3, the RAB3GAPs are essential for maintenance of synaptic homeostasis.Citation21 Recently, we showed that the TBC domain-free RAB3GAP1/2 also modulate macroautophagy and are essential factors of autophagosome formation.Citation22 Deficiency of both proteins in human primary fibroblasts deteriorates autophagosomal biogenesis and reduces macroautophagic activity at basal and induced macroautophagy conditions, whereas their overexpression enhances this process. The positive modulation of macroautophagy is dependent on the GAP activity of RAB3GAP1 but independent of RAB3, suggesting that RAB3GAP1/2 access an alternative RAB GTPase, which has not been identified yet. Interestingly, the RAB3GAP complex was recently shown to be a RAB GEF for the RAB GTPase RAB18 and provokes localization of RAB18 to the ER, which is necessary for maintenance of ER structure.Citation23 Excitingly, mutations in RAB3GAP1/2 and RAB18 cause the Warburg Micro syndrome, a devastating developmental disorder.Citation24 The molecular mechanisms of this disease are not clarified yet but a functional association of RAB3GAP1/2 and RAB18 might support the identification of responsible pathogenetic pathways. Next to RAB3 regulation and its involvement in macroautophagy, RAB3GAP1 interacts with LMAN1/ERGIC53Citation25 and mediates the exocytosis of CLDN1,Citation26 which highlights the coordinative character of this TBC domain-free RAB GAP in cellular trafficking systems.
As indicated above, several macroautophagy-modifying TBCGAPs were identified by their interaction with Atg8 family members and this interaction is counteracted by other interacting proteins that compete for binding sites. The ability of Atg8 family members to direct RAB GAPs to phagophores indicates that they might act as scaffolding molecules and, thus, are central partners for the activity of RAB GAPs in macroautophagy. This mechanism is comparable to the interaction of Atg8 family members with cargo receptors involved in selective macroautophagy, such as SQSTM1, NBR1, or CALCOCO2.Citation27 MAP1LC3 serves as a binding partner and recruits cargo receptors to phagophores, which mediates substrate-specificity to macroautophagy. Interestingly, an interaction with Atg8 family members has also been indicated for RAB3GAP1/2 based on a proteomic approach,Citation28 although a direct physical interaction awaits confirmation.Citation22
Relevance of RAB GAPs in macroautophagy and compensatory mechanisms for membrane mobilization
The formation and transport of autophagosomes is one of the major challenges for the entire macroautophagy process and needs to be carefully controlled to reduce interference with other cellular trafficking pathways. The activity of RAB GTPases, RAB GEFs, and RAB GAPs positions these proteins as central factors for this coordination and their relevance for macroautophagy has been shown in multiple studies.Citation12 However, the selection of macroautophagy-deficient yeast strains resulted in the characterization of at least 40 Atg proteins, most of which do not appear to be involved in membrane mobilization or vesicle transport. An exception (although not an “Atg” protein) is the ortholog of RAB1, Ypt1,Citation29 and its RABGEF, the TRAPPIII complex,Citation9 which have been defined as important factors for autophagosome formation in yeast and possess a likewise important role for macroautophagy also in mammalian cell lines.Citation12 Interestingly, several RAB GAPs modulate macroautophagy particularly under induced conditions when macroautophagic membrane requirements are increased, which underlines the need for a stringent control, and some RAB GAPs seem to function in overlapping pathways. For example, TBC1D14 and TBC1D5 appear to be important both for the coordination of endosomal trafficking and autophagosome biogenesis.Citation14,15,17 Recently, TBC1D2, which effects the RAB GTPase RAB7 and modulates autophagosome-lysosome fusion, was shown to be activated by LRRK1 upon macroautophagy induction.Citation30 Therefore, the characterization of upstream factors that modulate the activity of RAB GAPs and the identification of target RAB GTPases will help to dissect the precise pathways that are modulated by these proteins and allow the identification of possible compensatory mechanisms. This will increase our understanding of the reorganization and the condition-dependent plasticity of cellular trafficking systems that are necessary to keep macroautophagy going.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors would like to thank Anne Feldmann and Fazilet Bekbulat for helpful discussions and Michael Plenikowski for artwork.
Funding
The work of Christian Behl is supported by the Corona Foundation and Collaborative Research Center CRC 1080 of the Deutsche Forschungsgemeinschaft, DFG. Ivan Dikic is supported by grants from the DFG (DI 931/3-1), the Cluster of Excellence “Macromolecular Complexes” of the Goethe University Frankfurt (EXC115), LOEWE grant Ub-Net and LOEWE Centrum for Gene and Cell therapy Frankfurt and the European Research Council / ERC grant agreement n° 250241-LineUb.
References
- Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science 2000; 290:1717-21; PMID:11099404; http://dx.doi.org/10.1126/science.290.5497.1717
- Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell 2008; 132:27-42; PMID:18191218; http://dx.doi.org/10.1016/j.cell.2007.12.018
- Wilson MI, Dooley HC, Tooze SA. WIPI2b and Atg16L1: setting the stage for autophagosome formation. Biochem Soc Trans 2014; 42:1327-34; PMID:25233411; http://dx.doi.org/10.1042/BST20140177
- Weidberg H, Shvets E, Shpilka T, Shimron F, Shinder V, Elazar Z. LC3 and GATE-16/GABARAP subfamilies are both essential yet act differently in autophagosome biogenesis. EMBO J 2010; 29:1792-802; PMID:20418806; http://dx.doi.org/10.1038/emboj.2010.74
- McEwan DG, Dikic I. Not all autophagy membranes are created equal. Cell 2010; 141:564-6; PMID:20478247; http://dx.doi.org/10.1016/j.cell.2010.04.030
- Rubinsztein DC, Shpilka T, Elazar Z. Mechanisms of autophagosome biogenesis. Curr Biol 2012; 22:R29-34; PMID:22240478; http://dx.doi.org/10.1016/j.cub.2011.11.034
- Dupont N, Chauhan S, Arko-Mensah J, Castillo EF, Masedunskas A, Weigert R, Robenek H, Proikas-Cezanne T, Deretic V. Neutral lipid stores and lipase PNPLA5 contribute to autophagosome biogenesis. Curr Biol 2014; 24:609-20; PMID:24613307; http://dx.doi.org/10.1016/j.cub.2014.02.008
- Puri C, Renna M, Bento CF, Moreau K, Rubinsztein DC. Diverse autophagosome membrane sources coalesce in recycling endosomes. Cell 2013; 154:1285-99; PMID:24034251; http://dx.doi.org/10.1016/j.cell.2013.08.044
- Lynch-Day MA, Bhandari D, Menon S, Huang J, Cai H, Bartholomew CR, Brumell JH, Ferro-Novick S, Klionsky DJ. Trs85 directs a Ypt1 GEF, TRAPPIII, to the phagophore to promote autophagy. Proc Natl Acad Sci U S A 2010; 107:7811-6; PMID:20375281; http://dx.doi.org/10.1073/pnas.1000063107
- Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol 2009; 10:513-25; PMID:19603039; http://dx.doi.org/10.1038/nrm2728
- Cherfils J, Zeghouf M. Regulation of small GTPases by GEFs, GAPs, and GDIs. Physiol Rev 2013; 93:269-309; PMID:23303910; http://dx.doi.org/10.1152/physrev.00003.2012
- Szatmari Z, Sass M. The autophagic roles of Rab small GTPases and their upstream regulators: a review. Autophagy 2014; 10:1154-66; PMID:24915298; http://dx.doi.org/10.4161/auto.29395
- Itoh T, Kanno E, Uemura T, Waguri S, Fukuda M. OATL1, a novel autophagosome-resident Rab33B-GAP, regulates autophagosomal maturation. J Cell Biol 2011; 192:839-53; PMID:21383079; http://dx.doi.org/10.1083/jcb.201008107
- Longatti A, Lamb CA, Razi M, Yoshimura S, Barr FA, Tooze SA. TBC1D14 regulates autophagosome formation via Rab11- and ULK1-positive recycling endosomes. J Cell Biol 2012; 197:659-75; PMID:22613832; http://dx.doi.org/10.1083/jcb.201111079
- Popovic D, Akutsu M, Novak I, Harper JW, Behrends C, Dikic I. Rab GTPase-activating proteins in autophagy: regulation of endocytic and autophagy pathways by direct binding to human ATG8 modifiers. Mol Cell Biol 2012; 32:1733-44; PMID:22354992; http://dx.doi.org/10.1128/MCB.06717-11
- Frasa MA, Koessmeier KT, Ahmadian MR, Braga VM. Illuminating the functional and structural repertoire of human TBC/RABGAPs. Nat Rev Mol Cell Biol 2012; 13:67-73; PMID:22251903; http://dx.doi.org/10.1038/nrm3364
- Popovic D, Dikic I. TBC1D5 and the AP2 complex regulate ATG9 trafficking and initiation of autophagy. EMBO Rep 2014; 15:392-401; PMID:24603492; http://dx.doi.org/10.1002/embr.201337995
- Carroll B, Mohd-Naim N, Maximiano F, Frasa MA, McCormack J, Finelli M, Thoresen SB, Perdios L, Daigaku R, Francis RE, et al. The TBC/RabGAP Armus coordinates Rac1 and Rab7 functions during autophagy. Dev Cell 2013; 25:15-28; PMID:23562278; http://dx.doi.org/10.1016/j.devcel.2013.03.005
- Nagano F, Sasaki T, Fukui K, Asakura T, Imazumi K, Takai Y. Molecular cloning and characterization of the noncatalytic subunit of the Rab3 subfamily-specific GTPase-activating protein. J Biol Chem 1998; 273:24781-5; PMID:9733780; http://dx.doi.org/10.1074/jbc.273.38.24781
- Sakane A, Manabe S, Ishizaki H, Tanaka-Okamoto M, Kiyokage E, Toida K, Yoshida T, Miyoshi J, Kamiya H, Takai Y, et al. Rab3 GTPase-activating protein regulates synaptic transmission and plasticity through the inactivation of Rab3. Proc Natl Acad Sci U S A 2006; 103:10029-34; PMID:16782817; http://dx.doi.org/10.1073/pnas.0600304103
- Muller M, Pym EC, Tong A, Davis GW. Rab3-GAP controls the progression of synaptic homeostasis at a late stage of vesicle release. Neuron 2011; 69:749-62; PMID:21338884; http://dx.doi.org/10.1016/j.neuron.2011.01.025
- Spang N, Feldmann A, Huesmann H, Bekbulat F, Schmitt V, Hiebel C, Koziollek-Drechsler I, Clement AM, Moosmann B, Jung J, et al. RAB3GAP1 and RAB3GAP2 modulate basal and rapamycin-induced autophagy. Autophagy 2014; 10:2297-309; PMID:25495476; http://dx.doi.org/10.4161/15548627.2014.994359
- Gerondopoulos A, Bastos RN, Yoshimura S, Anderson R, Carpanini S, Aligianis I, Handley MT, Barr FA. Rab18 and a Rab18 GEF complex are required for normal ER structure. J Cell Biol 2014; 205:707-20; PMID:24891604; http://dx.doi.org/10.1083/jcb.201403026
- Handley MT, Morris-Rosendahl DJ, Brown S, Macdonald F, Hardy C, Bem D, Carpanini SM, Borck G, Martorell L, Izzi C, et al. Mutation spectrum in RAB3GAP1, RAB3GAP2, and RAB18 and genotype-phenotype correlations in warburg micro syndrome and Martsolf syndrome. Hum Mutat 2013; 34:686-96; PMID:23420520; http://dx.doi.org/10.1002/humu.22296
- Haines DS, Lee JE, Beauparlant SL, Kyle DB, den Besten W, Sweredoski MJ, Graham RL, Hess S, Deshaies RJ. Protein interaction profiling of the p97 adaptor UBXD1 points to a role for the complex in modulating ERGIC-53 trafficking. Mol Cell Proteomics 2012; 11:M111 016444; http://dx.doi.org/10.1074/mcp.M111.016444
- Youssef G, Gerner L, Naeem AS, Ralph O, Ono M, O'Neill CA, O'Shaughnessy RF. Rab3Gap1 mediates exocytosis of Claudin-1 and tight junction formation during epidermal barrier acquisition. Dev Biol 2013; 380:274-85; PMID:23685254; http://dx.doi.org/10.1016/j.ydbio.2013.04.034
- Stolz A, Ernst A, Dikic I. Cargo recognition and trafficking in selective autophagy. Nat Cell Biol 2014; 16:495-501; PMID:24875736; http://dx.doi.org/10.1038/ncb2979
- Behrends C, Sowa ME, Gygi SP, Harper JW. Network organization of the human autophagy system. Nature 2010; 466:68-76; PMID:20562859; http://dx.doi.org/10.1038/nature09204
- Lipatova Z, Belogortseva N, Zhang XQ, Kim J, Taussig D, Segev N. Regulation of selective autophagy onset by a Ypt/Rab GTPase module. Proc Natl Acad Sci U S A 2012; 109:6981-6; PMID:22509044; http://dx.doi.org/10.1073/pnas.1121299109
- Toyofuku T, Morimoto K, Sasawatari S, Kumanogoh A. LRRK1 regulates autophagy through turning on the TBC1D2-dependent Rab7 inactivation. Mol Cell Biol 2015; 35(17):3044-58; PMID:26100023
- Chavez JA, Roach WG, Keller SR, Lane WS, Lienhard GE. Inhibition of GLUT4 translocation by Tbc1d1, a Rab GTPase-activating protein abundant in skeletal muscle, is partially relieved by AMP-activated protein kinase activation. J Biol Chem 2008; 283:9187-95; PMID:18258599; http://dx.doi.org/10.1074/jbc.M708934200
- Stockli J, Meoli CC, Hoffman NJ, Fazakerley DJ, Pant H, Cleasby ME, Ma X, Kleinert M, Brandon AE, Lopez JA, et al. The RabGAP TBC1D1 plays a central role in exercise-regulated glucose metabolism in skeletal muscle. Diabetes 2015; 64:1914-22; PMID:25576050; http://dx.doi.org/10.2337/db13-1489
- Frasa MA, Maximiano FC, Smolarczyk K, Francis RE, Betson ME, Lozano E, Goldenring J, Seabra MC, Rak A, Ahmadian MR, et al. Armus is a Rac1 effector that inactivates Rab7 and regulates E-cadherin degradation. Curr Biol 2010; 20:198-208; PMID:20116244; http://dx.doi.org/10.1016/j.cub.2009.12.053
- Cartee GD. AMPK-TBC1D4-dependent mechanism for increasing insulin sensitivity of skeletal muscle. Diabetes 2015; 64:1901-3; PMID:25999533; http://dx.doi.org/10.2337/db15-0010
- Di Chiara M, Glaudemans B, Loffing-Cueni D, Odermatt A, Al-Hasani H, Devuyst O, Faresse N, Loffing J. The Rab-GAP TBC1D4 (AS160) is dispensable for the control of sodium and water homeostasis but regulates GLUT4 in mouse kidney. Am J Physiol Renal Physiol 2015; 309(9):F779-90:ajprenal 00139 2015; PMID:26336159
- Seaman MN, Harbour ME, Tattersall D, Read E, Bright N. Membrane recruitment of the cargo-selective retromer subcomplex is catalysed by the small GTPase Rab7 and inhibited by the Rab-GAP TBC1D5. J Cell Sci 2009; 122:2371-82; PMID:19531583; http://dx.doi.org/10.1242/jcs.048686
- Yoshimura S, Egerer J, Fuchs E, Haas AK, Barr FA. Functional dissection of Rab GTPases involved in primary cilium formation. J Cell Biol 2007; 178:363-9; PMID:17646400; http://dx.doi.org/10.1083/jcb.200703047
- Dibble CC, Elis W, Menon S, Qin W, Klekota J, Asara JM, Finan PM, Kwiatkowski DJ, Murphy LO, Manning BD. TBC1D7 is a third subunit of the TSC1-TSC2 complex upstream of mTORC1. Mol Cell 2012; 47:535-46; PMID:22795129; http://dx.doi.org/10.1016/j.molcel.2012.06.009
- Gallo LI, Liao Y, Ruiz WG, Clayton DR, Li M, Liu YJ, Jiang Y, Fukuda M, Apodaca G, Yin XM. TBC1D9B functions as a GTPase-activating protein for Rab11a in polarized MDCK cells. Mol Biol Cell 2014; 25:3779-97; PMID:25232007; http://dx.doi.org/10.1091/mbc.E13-10-0604
- Li W, Hu Y, Jiang T, Han Y, Han G, Chen J, Li X. Rab27A regulates exosome secretion from lung adenocarcinoma cells A549: involvement of EPI64. APMIS 2014; 122:1080-7; PMID:24673604
- Reczek D, Bretscher A. Identification of EPI64, a TBC/rabGAP domain-containing microvillar protein that binds to the first PDZ domain of EBP50 and E3KARP. J Cell Biol 2001; 153:191-206; PMID:11285285; http://dx.doi.org/10.1083/jcb.153.1.191
- Hsu C, Morohashi Y, Yoshimura S, Manrique-Hoyos N, Jung S, Lauterbach MA, Bakhti M, Grønborg M, Möbius W, Rhee J, et al. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J Cell Biol 2010; 189:223-32; PMID:20404108; http://dx.doi.org/10.1083/jcb.200911018
- Hou Y, Chen X, Tolmachova T, Ernst SA, Williams JA. EPI64B acts as a GTPase-activating protein for Rab27B in pancreatic acinar cells. J Biol Chem 2013; 288:19548-57; PMID:23671284; http://dx.doi.org/10.1074/jbc.M113.472134
- Patino-Lopez G, Dong X, Ben-Aissa K, Bernot KM, Itoh T, Fukuda M, Kruhlak MJ, Samelson LE, Shaw S. Rab35 and its GAP EPI64C in T cells regulate receptor recycling and immunological synapse formation. J Biol Chem 2008; 283:18323-30; PMID:18450757; http://dx.doi.org/10.1074/jbc.M800056200
- Bisserier M, Berthouze-Duquesnes M, Breckler M, Tortosa F, Fazal L, de Regibus A, Laurent AC, Varin A, Lucas A, Branchereau M, et al. Carabin protects against cardiac hypertrophy by blocking calcineurin, Ras, and Ca2+/calmodulin-dependent protein kinase II signaling. Circulation 2015; 131:390-400; discussion; PMID:25369805; http://dx.doi.org/10.1161/CIRCULATIONAHA.114.010686
- Goueli BS, Powell MB, Finger EC, Pfeffer SR. TBC1D16 is a Rab4A GTPase activating protein that regulates receptor recycling and EGF receptor signaling. Proc Natl Acad Sci U S A 2012; 109:15787-92; PMID:23019362; http://dx.doi.org/10.1073/pnas.1204540109
- Vaibhava V, Nagabhushana A, Chalasani ML, Sudhakar C, Kumari A, Swarup G. Optineurin mediates a negative regulation of Rab8 by the GTPase-activating protein TBC1D17. J Cell Sci 2012; 125:5026-39; PMID:22854040; http://dx.doi.org/10.1242/jcs.102327
- Miserey-Lenkei S, Couedel-Courteille A, Del Nery E, Bardin S, Piel M, Racine V, Sibarita JB, Perez F, Bornens M, Goud B. A role for the Rab6A' GTPase in the inactivation of the Mad2-spindle checkpoint. EMBO J 2006; 25:278-89; PMID:16395330; http://dx.doi.org/10.1038/sj.emboj.7600929
- Cuif MH, Possmayer F, Zander H, Bordes N, Jollivet F, Couedel-Courteille A, Janoueix-Lerosey I, Langsley G, Bornens M, Goud B. Characterization of GAPCenA, a GTPase activating protein for Rab6, part of which associates with the centrosome. EMBO J 1999; 18:1772-82; PMID:10202141; http://dx.doi.org/10.1093/emboj/18.7.1772
- Fukui K, Sasaki T, Imazumi K, Matsuura Y, Nakanishi H, Takai Y. Isolation and characterization of a GTPase activating protein specific for the Rab3 subfamily of small G proteins. J Biol Chem 1997; 272:4655-8; PMID:9030515; http://dx.doi.org/10.1074/jbc.272.8.4655
